Abstract
Our study examined whether human bone marrow-derived MSCs are able to differentiate, in vitro, into functional epithelial-like cells. MSCs were isolated from the sternum of 8 patients with different hematological disorders. The surface phenotype of these cells was characterized.To induce epithelial differentiation, MSCs were cultured using Epidermal Growth Factor, Keratinocyte Growth Factor, Hepatocyte Growth Factor and Insulin-like growth Factor-II. Differentiated cells were further characterized both morphologically and functionally by their capacity to express markers with specificity for epithelial lineage. The expression of cytokeratin 19 was assessed by immunocytochemistry, and cytokeratin 18 was evaluated by quantitative RT-PCR (Taq-man). The data demonstrate that human MSCs isolated from human bone marrow can differentiate into epithelial-like cells and may thus serve as a cell source for tissue engineering and cell therapy of epithelial tissue.
Keywords: human mesenchymal stem cells, differentiation, epithelial-like cells, cytokeratin 18, cytokeratin 19
Introduction
All body surfaces and cavities, such as the skin, gastrointestinal tract, urogenital system and breast ducts are lined by epithelial tissue, providing a protective barrier. Cell loss from this tissue barrier must be precisely balanced by cell production, maintaining epithelial homeostasis. The source of the cells involved in tissue repair after an injury remains controversial and poorly defined. One possible cell source is stem-like progenitor cells, probably the most important, but rare components of the proliferative compartment of an epithelial tissue. These cell types have been identified in a variety of tissues including neural [1], vascular [2], hepatic [3], pancreatic [4] and epidermal [5]. A second possible source of stem-like progenitor cells, which migrate to the injured tissue, is bone marrow that contains at least two major kinds of stem cells: hematopoietic stem cells (HSCs), which give rise to the red cells and white cells of the blood, and mesenchymal stem cells (MSCs), which can be reproducibly isolated and expanded in vitro, and that can differentiate in vitro into cells with properties of cartilage, bone, adipose, and muscle cells [6]. MSCs can be isolated from various sources and were characterized by their ability to proliferate in culture with an attached well-spread morphology, and by the presence of a consistent set of marker proteins on their surface [7,8]. Several studies have shown that MSCs, HSCs and also unfractionated bone-marrow derived cells can give rise, in vivo, to epithelial cell types in lung and other tissues [9–12].
The aim of this study was to examine the differentiation potential of human MSCs toward the epithelial lineage. We suggested that a pure population of MSCs isolated from the bone marrow are capable of in vitro differentiation to functional epithelial-like cells.
Materials and methods
Isolation and culture of human MSCs
The marrow aspirates for the isolation of human MSC were obtained from the sternum of eight patients with different hematological disorders. Signed, informed consent was obtained from each patient and the protocol was accepted by the local Ethics Committee of the University of Medicine and Pharmacy “Victor Babes” Timisoara. Human MSCs were isolated by adherence on plastic [6]. The mononuclear cell (MNC) fraction from the bone marrow was isolated by density gradient centrifugation. After isolation, the MNC were cultivated at a density of 5 × 104 cells/cm2 in uncoated flasks on DMEM low-glucose medium (1 g/l, Stemcell Technologies, Inc.), supplemented with 10% FBS (Promocell, Germany). The media were changed every 3 days. After 14–21 days of plating, cells were harvested with 0.25% trypsin and 1 mM EDTA (Invitrogen Life Technologies, Scotland) and replated in uncoated flasks, at a density of 3 × 103 cells/cm2, using the same culture media. After another 7–14 days, when cells reached 50–70% confluence, the cells were analysed for the following markers: CD45, CD34, CD29, CD73 and CD105 and CD14. For flowcytometric analysis, 50 × 103 cells were incubated with 10 μl fluorescence-conjugated antibodies (CD45, CD34, CD73, CD90, GlyA from BD Biosciences, Heidelberg, Germany, and CD105 from Serotec, UK) for 20 min in the dark. After 2 washing steps with PBS, cells were acquired using a FACSCalibur (Becton Dickinson) flow cytometer using CellQuest software and analysed with Paint- A-Gate software.
These cells were self-regenerating cells, and were able to differentiate to various cell lines. If necessary two passages were performed in order to obtain a pure population of MSC. Morphologically, in the cell culture flask, a purity of 95% of the MSC population was considered satisfactory, a number also confirmed by flow cytometry. Contamination with other cells was not an issue: the only two other cell types besides the MSCs that could be accidentally found in the cell culture system were monocytes/macrophages (characterized by CD14 expression) and/or hematopoietic stem cells (characterized by CD34 expression). Both these markers were expressed below 1%, as revealed by flow-cytometry.
Differentiation of MSCs to epithelial-like cells
Human MSCs obtained as described above were plated in uncoated flasks and entered into the differentiation process at the second passage (usually 35–42 days from the initial isolation). The culture medium was DMEM low-glucose (1 g/l) with 10% FBS, initially supplemented with 10 ng/ml keratinocyte growth factor (KGF, Peprotech Inc., Rocky Hill, NJ), and 20–30 ng/ml epidermal growth factor (EGF, Peprotech Inc., Rocky Hill, NJ, USA). After 3 days of culture, 10 ng/ml hepatocyte growth factor (HGF, Peprotech Inc., Rocky Hill, NJ, USA), and 60 ng/ml insulin-like growth factor-2 (IGF-2; Human Recombinant, Peprotech Inc., Rocky Hill, NJ, USA) were added. After 7–14 days from the initial plating, when the cells acquired a rounded or polygonal shape, the expression of two epithelial markers, cytokeratin 19 and 18, was assessed.
Characterization of epithelial-like cells by immunocytochemistry–cytokeratin 19 expression
DAKO-EPOS (Dako Corporation, Sweden) method was performed in order to detect the expression of cytokeratin 19 in differentiated cells. Before detection the cells were washed twice with PBS and fixed with 4% paraformaldehyde for 10 min. The fixed cells were washed twice with PBS and then incubated for a 5–10 min period in 0.1% hydrogen peroxide in PBS, to quench endogenous peroxidase activity. Incubation at room temperature with primary antibodies was performed for 60 min. The specimens were washed with 0.01 mol/l PBS for 15 min. The reaction product was developed with 0.5 g/l 3′3-diaminobenzidine tetrahydrochloride (DAB). After counterstaining with hematoxylin, the result was observed microscopically. Pure MSCs were used as negative controls, and a spontaneously immortalized, nontumourigenic human skin keratinocyte cell line (HaCaT) as positive control.
Relative quantification of epithelial marker cytokeratin 18 mRNA using real-time RT-PCR (Taq-man)
Detection of cytokeratin 18 transcripts in MSC differentiated to epithelial phenotype was performed by reverse transcriptase-polymerase chain reaction (RT-PCR). Total RNA from the cultured cells was extracted using TRIzol reagent (Invitrogen, Karlsruhe, Germany). RNA was reverse transcribed by using the Omniscript Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. RT was carried out at 42°C for 60 min. Thermal cycling involved 50 cycles of 95°C for 15 s, 60°C for 30 s, after initial denaturation for 10 min at 95°C in an ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Germany). Fluorescence was measured after each cycle of PCR. To standardize the amount of RNA applied, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control. Primers (Biospring, Germany) were as follows: GAPDH (sense) 5′-GAAGGTGAAGGTCGGAGTC-3′;(antisense), 5′-GAAGCCCATCACCATCTTC-3′ (amplicon length: 226 bp), Cytokeratin 18 (sense) 5′-CAAGGAGGAGCTGCTCTTCATG-3′, (antisense), 5′-TGGTGCTCTCCTCAATCTGCTG-3′. For positive control, cDNA from human liver was used. To confirm the quality of isolated RNA and to standardize the amount of RNA applied, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control. Relative cytokeratin-18 expression levels were measured by ΔΔCT method (PE-Applied Biosystems; Sequence Detector User Bulletin) (Table 1). The “Primer Express” software, supplied by Perkin-Elmer, Weiterstadt, Germany, was used for oligonucleotide design.
1.
Cytokeratin 18 expression levels were measured by δδ CT method and normalized to GAPDH expression
| probe 1 | probe 2 | probe 3 | pos. contr. | |
|---|---|---|---|---|
| GAPDH | 28.9903 | 28.45 | 28.0668 | 36.4619 |
| CK18 | 20.2139 | 20.0308 | 26.454 | 24.5209 |
| δ CT | −8.7764 | −8.4192 | −1.6128 | −11.941 |
| δδ CT | 3.1646 | 3.5218 | 10.3282 | 0 |
| 2-δδ CT | 1.12E-01 | 8.71E-02 | 7.78E-04 | 1.00E+00 |
Results
Biological properties of bone marrow-derived human MSCs
The flow cytometric analysis of the mononuclear cell suspension showed a heterogeneous mixture of cells with different forward and sideward scatter profiles. No staining with anti-CD34 antibody was noted, suggesting a primitive population of hematopoietic cells. Most of the cells expressed CD45 and Glycophorin A. The typical MSC markers, CD105 and CD73 (SH-2 and SH-3) were not expressed (Fig. 1A).
1.
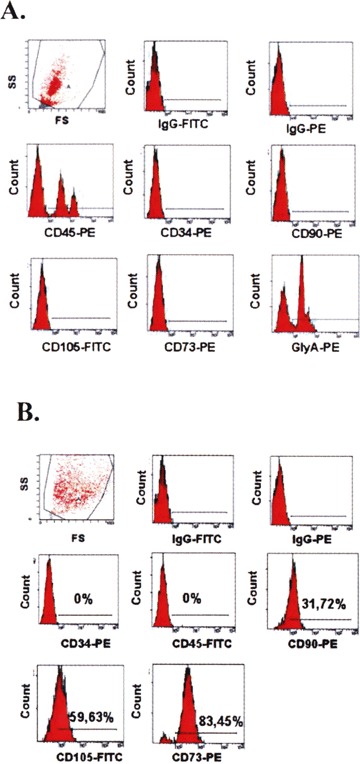
The flow cytometric analysis of the mononuclear cell suspension: (A) after density gradient centrifugation and (B) after 21 days of culture.
After 14–21 days of culture, the cells had acquired three different phenotypes: (1) spindleshaped cells, which were the most abundant, (2) thin star-shaped cells and (3) large flattened cells. After passage, almost all cells displayed a fibroblastic elongated phenotype (Fig. 2A and B).
2.
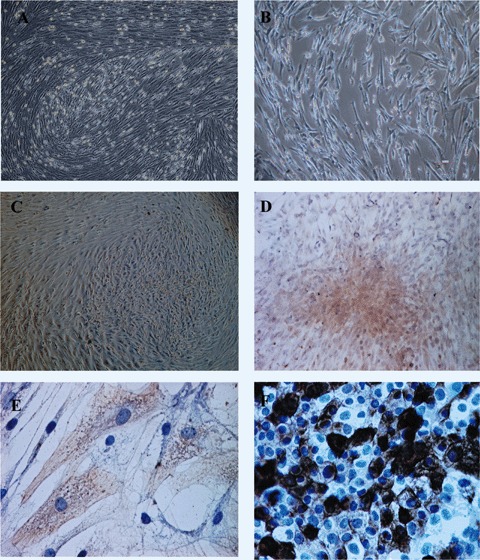
(A and B) Bone marrowderived human MSCs–microscopical analysis at day 21. Magnification: 40x (A) and 200x (B). (C) Adherent cells with a rounded or polygonal shape placed on the top of a mesenchymal stem cells’layer. Magnification: 400x. (D and E) Cytokeratin 19-positive cells with an epithelial-like morphology. Magnification: 40x (D) and 400x (E). (F) Cytokeratin 19-positive imunohistochemical reaction on HaCaT cell lines, used as positive control. Magnification: 200x.
In this phase, the flow cytometric analysis revealed no positive staining for CD45 or Glycophorin A. The SH-2 (CD105) and SH-3 (CD73) markers were now expressed at higher levels. CD34 was not expressed, and CD90 became positive (Fig. 1B).
Differentiation of human MSCs to epithelial-like cells
After 14 days of MSC culture in a differentiating medium containing 10 ng/ml KGF, 20-30 ng/ml EGF, 10 ng/ml HGF and 60 ng/ml IGF-2, 20–30% of the cells acquired a rounded/polygonal shape. These cells were proliferating, forming an adherent monolayer and were organized in cobblestone pattern clusters (Fig. 2C).
While pure populations of MSCs did not express cytokeratin 19 (CK19), the immunohystochemical analysis of the differentiated cells from cobblestone pattern clusters showed that they were positive for CK 19 (Fig. 2D and E), in a proportion of about 40% of the total cells, as revealed by microscopic analysis using Lucia software (Nikon Corporation Co., Kanagawa, Japan). Comparative to our positive controls using HaCaT cells, the expression of this marker was higher in the middle of the clusters, while in the peripheral regions was reduced (Fig. 2F).
During the epithelial differentiation of the MSCs, the level of some specific markers determined by flow cytometric analysis was decreased (Fig. 3) and this could be due to a phenotypic alteration of the MSCs entering the epithelial-like differentiation program.
3.
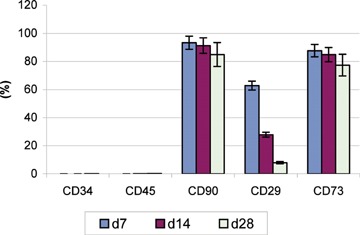
Flow cytometric assessment of different MSCs surface antigens during their differentiation (day 7, 14 and 28).
The RT-PCR assessed the expression of cytokeratin 18 (CK18, an epithelial cell marker) in two samples of cultured epithelial cells. For positive control, cDNA from human liver was used. A strong expression of CK18 was detected in the cultured cells (Fig. 4 and Table 1).
4.
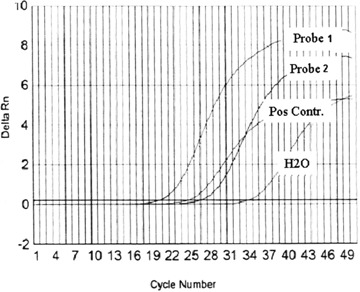
Expression of cytokeratin 18 assessed by quantitative RT-PCR (Taq-man) performed on epithelial-like cells derived from human MSCs.
To confirm the epithelial nature of these cells, RT-PCR amplification of cytokeratin 18 was performed with RNA isolated from induced and non-induced MSC.
Discussion
Many reports have raised the idea of the pluripotecy of adult stem cells that might be used for the regeneration and repair of damaged tissues. Although this property of adult stem cells may be controversial, bone marrow derived cells are reported to have potential to contribute to tissue repair in both physiological and pathological conditions, in different non-hematopoietic tissues, including various types of epithelia.
Some reports explored the capacity of adult stem cells to promote restoration of airway epithelium: Bone marrow-derived cells have been detected in the lung tissue as fibroblast-type cells [12], and as differentiated bronchial epithelium and alveolar type II pneumocytes [9, 13]. Bone-marrow derived cells were detected in the recipient's lung parenchyma as cells with morphological and molecular phenotype of type I pneumocytes of the alveolar epithelium [10]. In another experimental setting, human MSCs were cocultured with heat-shocked small airway epithelial cells. A subset of the hMSCs rapidly differentiated into epithelium-like cells, and restored the epithelial monolayer. Immunocytochemistry and microarray analyses show that the cells had the phenotypic profile and gene expression of normal small airway epithelial cells [14]. All these data raise the possibility of utilizing marrow to generate lung epithelium for the therapy of lung diseases associated with extensive bronchial and alveolar damage.
Other studies examined the potency of mouse bone marrow cells to differentiate into cells comprising skin tissuesusing a skin reconstitution assay. Bone marrow cells from adult GFP-transgenic mice were transplanted in a mixture of embryonic mouse skin cells onto skin defects made on the backs of nude mice. Within 3 weeks, fully differentiated skin with hair was reconstituted. The localization and morphology of the GFP-positive cells confirmed that the bone marrow cells had differentiated into epidermal keratinocytes, sebaceous gland cells, follicular epithelial cells, dendritic cells, and endothelial cells [15].
Since the endometrium is an epithelial tissue that regenerates after each menstrual cycle, the possibility of repopulating the endometrium with cells of extrauterine origin was studied by evaluating for donor HLA expression the endometrium from 4 HLA-mismatched bone marrow transplant recipients. Donor-derived endometrial cells were detected in endometrial biopsy samples from all recipients and accounted for 0.2–48% of epithelial cells and 0.3–52% of stromal cells [16].
The potential of adult stem cells to differentiate into renal epithelial cells has been studied extensively. Bone marrow derived cells expressing epithelial cell markers were detected in histologically normal mouse kidneys [17]. In men who received a kidney transplant from female donors, Y chromosome containing cells expressing renal tubular cell markers were observed in the kidneys after injury. Similarly, in men who received female kidneys, 1% of the tubular cells were Y chromosome positive after the kidneys had recovered from acute tubular necrosis [18]. Lin-Sca-1+ bone marrow cells differentiate into renal proximal tubular cells in ischemically injured mouse kidneys [19]. Injection of mesenchymal stem cells of male bone marrow origin remarkably protected cisplatin-treated syngeneic female mice from renal function impairment and severe tubular injury. In fact, mesenchymal stem cells markedly accelerated tubular proliferation in response to cisplatin-induced damage, whereas hematopoietic stem cells failed to exert such beneficial effects [20].
In addition to tubular epithelial cells, differentiation of bone marrow cells into glomerular mesangial cells has been reported in a rat model of glomerulonephritis [21].
Several lines of evidence suggest that bone marrow cells may represent a source for digestive epithelial regeneration. In human beings, bone marrow-derived epithelial cells contributed to the regeneration of the damaged epithelia after graft versus-host disease (GVHD) [22]. Also, the contribution of bone marrow-derived epithelial cells was observed to accelerate regeneration in the epithelia of gastric ulcer [22]. In fact, this was the first report showing clinical evidence that bone marrow cells might be a source of epithelial regeneration in human beings. In mice, similar results have been reported, using experimental colitis [23]. These reports support the strategy of using bone marrow cells for regeneration of damaged intestinal epithelia. Other investigations supported the hypothesis that bone marrow could represent a potential source of hepatic oval cells [11], and esophageal squamous epithelium [24].
Most of the above presented data are the result of systemically injected marrow-derived cells, indicating indicating the importance of local environmental factors for homing, engraftment and further differentiation of these cells.
Our experiment demonstrates that human bone marrow-derived MSCs, under appropriate conditions, in vitro, may differentiate into epithelial cells. One criticism of papers describing the plasticity of adult stem cells is that of heterogenous cells’ preparations. In our study, we performed two passages of the isolated MSCs in order to obtain a pure, homogenous cellular population.
In our experimental setup, human MSCs were cultured in a media containing several growth factors, namely KGF, EGF, HGF, and IGF-2. These four factors, gradually added in the basal medium, without any gene manipulation or co-culture with epithelial cells, are responsible for MSCs differentiation to the epithelial-like lineage. This mixture could be considered the appropriate environment required for the activation of MSCs differentiation into epithelial-like cells.
The fibroblastic morphology of MSCs gradually progressed toward the rounded or polygonal shape of epithelial-like cells in a time-dependent manner and became apparent after 10–14 days post-induction within the differentiation medium. The functional analysis of the differentiated cells revealed a higher expression of epithelial markers in the central region of cobblestone-pattern clusters that decreased at the periphery, suggesting an asymmetric division and differentiation of the cells. Therefore, we suggest that only cells from the ‘inner zone’ differentiate toward the epithelial lineage, probably due to paracrine effects [25], perhaps mediated directly by the growth factors [26]
This study provides preliminary results indicating that the acquisition of an epithelial phenotype may be possible starting with human MSCs and using a specific differentiation protocol. These epithelial cells are likely to be amenable to further genetical engineering to fit different medical or research needs. Another possibility is to investigate the de-differentiation/trans-differentiation potential of these cells.
Acknowledgments
Part of this work was supported by VIASAN grant no. 314/2004 from the Romanian Ministry of Education and Research.We thank Professor Lajos Kemeny, Department of Dermatology and Allergology, University of Szeged, Hungary for the HaCaT cells donation.
References
- 1.Gage HH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–39. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alison MR, Poulsom R, Forbes SJ. Update on hepatic stem cells. Liver. 2001;21:367–73. doi: 10.1034/j.1600-0676.2001.210601.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Sharma A. Pancreatic stem cells. J Pathol. 2002;197:519–26. doi: 10.1002/path.1158. [DOI] [PubMed] [Google Scholar]
- 5.Janes SM, Lowell S, Hutter C. Epidermal stem cells. J Pathol. 2002;197:479–91. doi: 10.1002/path.1156. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–84. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 8.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 9.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multiorgan, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 10.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–8. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 11.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 12.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Marrow stromal cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA. 1995;92:4857–61. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA. 2003;100:2397–402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, Huh N. Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol. 2003;163:1227–31. doi: 10.1016/S0002-9440(10)63482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–5. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–35. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–90. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- 19.Kale SKA, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–9. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Suzuki A, Imai E, Okabe M, Hori M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12:2625–35. doi: 10.1681/ASN.V12122625. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto R, Watanabe M. Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J Gastroenterol. 2004;39:1–6. doi: 10.1007/s00535-003-1259-8. [DOI] [PubMed] [Google Scholar]
- 23.Komori M, Tsuji S, Tsujii M, Murata H, Iijima H, Yasumaru M, Nishida T, Irie T, Kawano S, Hori M. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–18. doi: 10.1111/j.1067-1927.2005.130114.x. [DOI] [PubMed] [Google Scholar]
- 24.Epperly MW, Guo H, Shen H, Niu Y, Zhang X, Jefferson M, Sikora CA, Greenberger JS. Bone marrow origin of cells with capacity for homing and differentiation to esophageal squamous epithelium. Radiat Res. 2004;162:233–40. doi: 10.1667/rr3224. [DOI] [PubMed] [Google Scholar]
- 25.Alexanian AR. Neural stem cells induce bone-marrow-derived mesenchymal stem cells to generate neural stem-like cells via juxtacrine and paracrine interactions. Exp Cell Res. 2005;310:383–91. doi: 10.1016/j.yexcr.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–93. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]


