Abstract
It was shown that IgGs from the sera of 2–7-month-old control non-autoimmune (CBA x C57BL)F1 and BALB/c mice and 2–3-month-old autoimmune prone MRL-lpr/lpr mice (conditionally healthy mice) are catalytically inactive. During spontaneous development of deep systemic lupus erythematosus (SLE)-like pathology a specific reorganization of immune system of these mice leads to conditions associated with a production of IgGs hydrolyzing DNA, ATP and polysaccharides with low catalytic activities (conditionally pre-diseased mice).A significant increase in DNase, ATPase and amylase IgG relative activities associated with a transition from pre-diseased to deep diseased mice is correlated with additional changes in differentiation and proliferation of mice bone marrow haematopoietic stem cells (HSCs) and lymphocyte proliferation in different organs.The highest increase in all abzyme activities was found in mice immunized with DNA, which in comparison with pre-diseased and diseased mice are characterized by a different profile of HSC differentiation and by a suppression of cell apoptosis. Abzyme activities in the serum of pregnant females were comparable with those for pre-diseased mice, but the profile of HSC differentiation and cell apoptosis levels in pregnant and pre-diseased mice were quite different. Right after the beginning of lactation (4 days after delivery) and in a late time of lactation (14 days after delivery) there was an observed increase in cell apoptosis and two different stages of significant change in the HSC differentiation profiles; the first stage was accompanied with a significant increase and the second with a remarkable decrease in abzyme activities. Overall, all mouse groups investigated are characterized by a specific relationship between abzyme activities, HSC differentiation profiles, levels of lymphocyte proliferation, and cell apoptosis in different organs. From our point of view, the appearance of ATPase, DNase activities may be considered the earliest statistically significant marker of mouse spontaneous SLE and a further significant increase in their activities correlates with the appearance of SLE visible markers and with an increase in concentrations of anti-DNA Abs and urine protein. However, development of autoimmune (AI)-reactions and the increase in the sera anti-DNA antibodies (Abs) and in the abzyme activities in pregnant and lactating mice do not associate with SLE visible markers and proteinuria. The possible differences in immune system reorganizations during pre-disease, disease, pregnancy and lactation leading to production of different auto-antibodies and abzymes are discussed.
Keywords: autoimmune-prone MRL-lpr/lpr mice, catalytic antibodies, colony formation of haematopoietic progenitors
Introduction
Antibodies (Abs) against transition states of reactions and natural abzymes (Abzs) catalysing more than 100 distinct chemical reactions are novel biological catalysts that attracted much interest in the last years [1–5]. Natural catalytic Abzs hydrolyzing DNA, RNA, polysaccharides, oligopeptides, and proteins exist in the sera of patients with many autoimmune (AI) and viral diseases [2–5] Abzs hydrolyzing some proteins were found not only in the organisms of AI patients, but also in norm [6–7] and in patients with diseases like sepsis, which causes many deaths in intensive care units and results from a deleterious systemic host response to infection [8]. Ab amylase activity in healthy donors was ∼40–100-fold lower than in AI patients [9–11]. Healthy humans can develop Abzs with low DNase and RNase activities, their levels usually on a borderline of the sensitivity of detection methods [2–5, 12–16]. In addition, there was no confirmed nuclease Abzs in the sera of patients with many different diseases with insignificant AI reactions [2–5, 13–14].
Although Abzs with low activity can sometimes be detected in healthy people, the enzymic relative activity (RAs) of AI patients are usually significantly higher and from our point of view different Abzs can present a convenient diagnostic marker of some AI pathologies [2–5]. We have shown that appearance of Abzs specific for various substrates is among the earliest and clear signs of AI reactions in a number of AI diseases (systemic lupus erythematosus (SLE),Hashimoto's thyroiditis, polyarthritis, multiple sclerosis) and viral diseases with strong immune system disturbances (AIDS, hepatitis) [2–5]. According to our data, catalytic activity of nuclease Abzs is usually very easily detectable at the beginning of AI diseases when concentrations of Abs to DNA or other auto-antigens are not yet increased significantly and correspond to their ranges for healthy donors [2–5].
Anti-VIP Abzs of patients with asthma are cyto-toxic. Since mice immunized with these IgGs develop asthma, they can have an important effect in pathogenesis decreasing concentrations of VIP, which plays a major role in the asthma pathophysiology [17]. DNase Abzs from SLE, lymphoproliferative diseases [18] and multiple sclerosis patients [4], and DNA-hydrolyzing Bence–Jones proteins from multiple myeloma patients [19] are cytotoxic, cause nuclear DNA fragmentation and induce cell death by apoptosis. Serine protease-like and metal-dependent proteolytic IgGs, IgMs, and IgAs from patients with multiple sclerosis hydrolyse myelin basic protein of the myelin-proteolipid shell of axons, and therefore can play an important role in pathogenesis of this AI pathology ([20] and refs therein). Proteolytic IgGs from patients with sepsis may participate in the control of disseminated microvascular thrombosis and play a role in recovery from the disease [8]. Obviously, the study of mechanisms of Abzs production and their biological role is very important for understanding the pathogenesis of AI diseases.
During pregnancy and immediately after delivery, women are very often characterized by immune processes similar to those for AI patients ([2–5, 21] and refs therein). sIgA and/or IgG possessing DNase and RNase, amylase or ATPase activities were found in the sera and in the milk of pregnant and lactating females [9, 22–25]. We have discovered that the milk of clinically healthy human mothers contains very unusual sIgA and/or IgG possessing protein [26], lipid [27] and polysaccharide kinase activities [28]. Analysis of published data suggests that pregnant women may be directly immunized through specific response of their immune system to certain compounds of viral, bacterial or food origin that can efficiently stimulate production of different Abs and Abzs ([2–4, 21, 25] and refs therein). Many different AI pathologies can be ‘activated’ or ‘triggered’ in clinically healthy women during pregnancy and soon after childbirth [29–30]. Interestingly, DNase and ATPase activities are increased 4–5-fold in the sera of women with Hashimoto's thyroiditis stimulated by pregnancy [21]. Thus, pregnancy and especially the beginning of lactation may be considered important periods associated with the production not only of different Abs and auto-Abs, but also of Abzs.
The mother's milk sIgA is active at the mucosal surfaces protecting them from the invasion of pathogenic microorganisms and limiting the access of environmental antigens [31–32]. Milk IgGs can penetrate into blood through intestinal epithelium, thus protecting newborns. Therefore, one cannot exclude that in contrast to auto-Abs of AI patients, Abzs of mother milk could contribute to the protective role of Abs through hydrolysis of different nucleic acids, polysaccharides, and proteins. Possible differences and/or similarities in the roles of Abzs as well as in mechanisms of their production in AI patients and in lactating mothers are very interesting. Many questions concerning Abzs can be answered only using experimental animals to model certain immune states, but they have not yet been widely used for studying the mechanisms of Abzs accumulation.
MRL-lpr/lpr mice spontaneously developing a SLE-like disorder is a very promising model to study the mechanisms of natural Abzs generation and their role in pathogenesis of deep AI disturbances. SLE is one of several AI diseases with increased level of anti-DNA Abs, DNase and RNase Abzs possessing highest catalytic activities and broad substrate specificity ([2–5, 11, 12, 15] and refs therein). Many SLE anti-DNA Abs are directed against histone-DNA nucleosomal complexes appearing as a result of internucleosomal cleavage during apoptosis. Apoptotic cells are the primary source of antigens and immunogens in SLE, and these features in recognition, perception, processing and/or presentation of apoptotic auto-antigens by antigen-presenting cells can cause AI processes [33].
MRL-lpr/lpr mice are characterized by marked hypergammaglobulinemia, production of numerous auto-Abs, circulating immune complexes, glomeru-lonephritis and severe lymphadenopathy. A mutation in the lpr gene of these mice leads to a deficit in functional Fas ligand and dysregulation of apoptosis in homozygotes [34–35]. As a result, the mice develop SLE-like phenotype, including accumulation of double-negative T cells (CD4− CD8− B220+ TCR+) in peripheral lymphoid organs.
Although many questions concerning SLE Abzs could be answered using experimental animals for modelling certain states of the immune system, they have not yet been widely employed in studies of the mechanisms of Abzs accumulation. MRL/MPJ-lpr and SJL mice were used to obtain abzymes with amidase/esterase activity in the expanded sequence space of Ab repertoire using haptenic transition-state analogues with a phosphonate and/or phosphoimidate moiety ([36] and refs therein). A surprising result in this study was that the Abzs was obtained with a dramatically higher incidence in these autoimmune AI mouse strains than in conventionally used normal mouse strains. Testing the ability of several strains of mice to elicit esterolytic antibodies Abs after immunization with a p-nitrobenzyl phosphonate hapten revealed that the occurrence of catalytic Abs in SJL and MRL/lpr AI mice is dramatically higher than in normal mouse strains (e.g. the wild-type MRL/++ or BALB/c) [37].
Polyclonal auto-Abs purified from sera of NZB/W, MRL-lpr/lpr and SJL/J mice show DNase activity, as opposed to those harvested from non-AI BALB/c mice [38], although no standard criteria were checked in this study to attribute this activity to IgGs.
The levels of the catalytic activity was strongly dependent on the age of the animal, with the highest levels of catalytic activity found in the sera from mice between 8 and 12 months of age.
An affinity-linked oligonucleotide nuclease assay was successfully applied to screen a large number of hybridoma clones derived from non-immunized (NZB x NZW) F1 mice with spontaneous SLE [39]. Three clones producing DNase Abs were found. It was shown that the DNase centre of a monoclonal DNase IgG from AI-prone MRL-lpr/lpr mice is located at the interface between the light and heavy chains, and both L- and H- chains are able to hydrolyzehydrolyse DNA when separated [40].
Recently, IgGs were isolated from the sera of MRL-lpr/lpr mice [41]. Convincing evidence was provided using different approaches including several strict standard criteria that, similarly to SLE patients, DNase and amylase activities are intrinsic to mouse polyclonal IgGs [41–42]. Here, we have analysed for the first time a possible correlation of the RAs of mouse IgGs in the hydrolysis of DNA, ATP, and oligosaccharides with some visible and biochemical markers of AI pathologies (proteinuria, Ab titers to native and denatured DNA) during various stages of mouse SLE, pregnancy and lactation. An ever-growing number of observations suggest that AI diseases originate from defects in haematopoietic stem cells (HSC) [43]. Therefore, lymphocyte proliferation and apoptosis at different stages of the AI disorder development in MRL-lpr/lpr mice were studied for the first time. Haematopoietic progenitors colony formation in the course of spontaneous pathology, pregnancy, lactation and after immunization of mice was characterized.
Materials and methods
Reagents and sorbents used in this work were obtained mainly from Sigma or Pharmacia.
Experimental animals
AI-prone MRL-lpr/lpr mice (originated from Harlan, UK) and control non-AI BALB/c and (CBAxC57BL)F1 at 1–12 months of age (mainly 1–7 months-old in this study) used were housed in the colonies under the same standard pathogen-free conditions including a system for protection from bacterial and viral infections at the Institute of Cytology and Genetics mouse breeding facility. MRL-lpr/lpr mice have a primary defect in the Apo-1/Fas gene, which regulates programmed cell death (apoptosis) in lymphoid cells; the result is lymphoproliferation. The mice develop lympoadenopathy, indicating an important role for Fas antigen in the negative selection of autoreactive T cell in thymus. The MRL-lpr/lpr mice are characterized by spontaneous appearance of high-level proteinuria and Abs to double and single stranded DNA [41–42] with maximal DNase RAs and AI markers at 8–12 months of age similar to those published in [38]. Some characteristics of MRL-lpr/lpr mice are given below in the text.
Immunization of mice, proteinuria assay, ELISA of anti-DNA Abs
Mice were immunized three times with 40 μg of DNA per mouse using a conjugate of calf thymus DNA with methylated bovine serum albumin (BSA) as in [41]. Blood of the mice was used for the Abs analysis 1.3–1.5 months after the first immunization. Proteinuria (relative concentration of total proteins in urine, mg/ml) analysis was performed as in [41]. The concentrations of serum anti-DNA Abs was determined using standard ELISA plates with immobilized double- (native) or single-stranded (denatured) DNA as described in [41]. After a consecutive treatment of samples with the blood serum and rabbit anti-mouse, Abs conjugated with horseradish peroxidase, the reaction mixtures were incubated with tetraethyl benzidine and H2O2. The reaction was stopped with H2SO4 and optical density (A450) was determined. The relative concentrations of anti-DNA Abs in the samples was expressed as a difference in the relative absorption at 450 nm between experimental and control samples; controls with DNA, but without Abs and with Abs not interacting with DNA gave the same results.
IgG purification
Electrophoretically and immunologically homogeneous mouse IgGs were obtained by sequential chromatography of the serum proteins on protein A-Sepharose and FPLC gel filtration in 0.1 M glycine-HCl (pH 2.6) [21, 24, 41]. The type of Abs (sIgA, IgG or IgM) in the fractions during different chromatographies was determined by Western blotting [24]. In order to protect Ab preparations from bacterial and viral contaminations, they were filtered through a Millex syringe-driven filter units (0.2 μm) and kept in sterilized tubes. Incubation of standard bacterial medium with stored Ab preparations did not lead to a formation of colonies.
Sodium dodecyl sulphate– polyacrylamide gel electrophoresis (SDS–PAGE) analysis of the Ab fractions for homogeneity under non-reducing conditions was done in 4–15% gradient gels; for polypeptide separation it was performed in a reducing 12.5% gel (0.1% SDS and 10 mM DTT and the polypeptides were visualized by silver staining [24, 41]. To exclude possible artefacts due to hypothetical traces of contaminating enzymes, the IgG was separated by SDS–PAGE and its DNase and amylase activities were detected using in a gel assay as in [12, 15, 25, 41, 42]. The activities were revealed only in the band corresponding to intact IgGs and there were no other peaks of proteins, DNase or amylase activities [41–42].
DNA-hydrolyzing activity assay
DNase activity was analysed similarly to [41]. The reaction mixture (20 μl) for analysis of IgG DNase activity contained 10–20 μg/ml supercoiled pBluescript, 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 20 mM Tris-HCl, pH 7.5, and 0.001–0.2 mg/ml of Abs, and was incubated for 2–12 hrs (standard time 2 hrs) at 30°C. The cleavage products were analyzedanalysed by electrophoresis in 0.8% agarose gels. Ethidium bromide-stained gels were photographed and the films were scanned. The activities of IgG preparations were determined from the scanning data (Gel-Pro Analyser v9.11) as a relative percentage of DNA corresponding to an initial band of supercoiled DNA and its relaxed form, a distribution of DNA between these bands in the case of control experiment (incubation of pBluescript in the absence of Abs) was taken into account. All measurements (initial rates) were taken within the linear regions of the time courses (15–40% of DNA hydrolysis) and a complete transition of supercoiled to nicked DNA for 2 hrs was taken as 100 % of the activity. If the activity was low (< 5–10% of supercoiled DNA disappearance), the time of incubation was increased to 3–12 hrs, depending on the sample. If the degradation of supercoiled DNA during 2 hrs of incubation exceeded 50%, the concentration of Abs was decreased 2–100-fold, depending on the sample analysed. Finally, the relative activities were normalized to: 0.1 mg/ml IgGs and 2 hrs of incubation.
ATPase activity assay
ATPase activity was analysed as in [25]. Reaction mixtures (10–20 μl) containing standard components: 5 mM MgCl2, 1 mM EDTA, 50 mM Tris-HCl, pH 7.5, 0.2 mM [γ−32P]ATP and 0.001–0.2 mg/ml IgG were incubated for 2 hrs at 30°C. The products of ATP hydrolysis were analysed by TLC in 0.25 M KH2PO4 buffer, pH 7.0 on PEI cellulose plates (Merck). The relative amount of 32P radioactivity of products (cpm) was calculated using the densities of the spots corresponding to [32P]orthophosphate and control spots of [32P]orthophosphate applied on plates before chromatography. Finally, the enzymic RAs were normalized to 0.1 mg/ml IgGs and 2 hrs of incubation.
Amylase activity assay
Amylase activity was analysed as in [9–11]. The reaction mixture (20 μl) containing 50 mM Tris-HCl, pH 7.5, 1 mM NaN3, 1.5 mM MHO and 0.001–0.2 mg/ml of IgGs was incubated for 12 hrs at 30°C. Products of hydrolysis were identified by TLC on Kieselgel plates (Merck) using 1-butanol-acetic acid-H2O (12:4:4). The activities of IgGs were determined from the scanning data as a relative percentage of oligosaccharide in the spots of MHO and its hydrolysed forms. All measurements were taken within the linear regions of the time courses and Ab concentration curves. If the Ab activity was low (< 5–10 % of hydrolysis) the incubation time was increased to 18–24 hrs. If MHO hydrolysis during 12 hrs of incubation exceeded 40 %, the concentration of Abs was decreased 2–100-fold depending on the sample analysed. Finally, the catalytic RAs were normalized to 0.1 mg/ml IgGs and 12 hrs of incubation.
Analysis of bone marrow progenitor cells in culture and lymphocyte proliferation
Bone marrow was flushed out of the mice's femurs. To assess bone marrow cells colony-forming ability, 2 × 104 cells/dish (Four dishes for each animal) were cultured in a standard methylcellulose-based M3434 medium for mouse cells (StemCell Technologies, Canada) containing SCF, eosinophil peroxidase (EPO), interleukin (IL-3), and IL-6. Colonies (granulocytic-macrophagic colony-forming unit (CFU-GM), erythroid burst-forming unit (BFU-E), granulocytic-erythroid-megacaryocytic-macrophagic colony-forming unit (CFU-GEMM) were scored after 14 days of incubation at 37°C and 5% CO2 in a humidified incubator according to [44].
All in vitro assays of lymphocyte proliferation were performed in complete RPMI (50 μM β-mercaptoethanol, 100 UI/ml penicillin) supplemented with 10% foetal calf serum (FCS) as in [44]. Cells (1×105) were isolated and cultured in 0.15 ml of the medium with or without 2 μg/ml concanavalin A. Proliferation assays were performed for 3 days, 3H incorporation was measured over the last 18 hrs of culture.
Analysis of DNA fragmentation (apoptosis assay)
Cells (1×105/ml) were washed in phosphate-buffered saline containing 0.02% ethylenediaminetetraacetic acid (EDTA) and 0.1% NaN3 [44, 45]. The cells were fixed in 1 ml of 1% paraformaldehyde for 1 hr at 4°C. The fixed cells were incubated in 1 ml of propidium iodide (PI) solution (50 μg/ml PI and 20 μg/ml RNase A) at 20°C. PI fluorescence of individual nuclei was measured using an Epics Profile flow cytometer. A minimum of 10,000 events was counted per sample. Results are reported as the percentage of hypodiploid (fragmented) nuclei reflecting the fraction of apoptotic cells.
Statistical analysis
The results are reported as the mean and the standard deviation of at least 3–4 independent experiments for each mouse, averaged over at least five different animals. The number of preparations assayed for each age is shown in the Tables. Differences between the samples were analysed by Student's t-test, P £ 0.05 was considered statistically significant.
Results
It was shown that the sera of MRL-lpr/lpr mice characterized by spontaneous development of a lupus-like AI disorder with visual symptoms of AI pathology (pink spots, baldness of head and parts of the back, general health deterioration etc.), contain Abzs with DNase and amylase activities [41–42]. Appearance of pronounced visual symptoms correlated well with proteinuria (<3-mg/ml concentration of protein in urine) [42]. The highest levels of anti-DNA Abs, DNase Abz activity, proteinuria and visible markers of SLE were observed at 8–12 months of age, which agrees with previously reported data for MRL-lpr/lpr mice [38], but we have used spontaneously diseased mice with all visible symptoms no older than 7 months (see below). Although the state of ‘health’ in the case of AI-prone mice may be considered very provisional, the mouse SLE pathology is never-theless spontaneous and AI reactions leading to deep pathology develop gradually. In order to distinguish different levels of the pathology development, MRL-lpr/lpr mice demonstrating no typical SLE indices and abzyme activities (similar to healthy control non-AI mice) were conditionally designated (independently of age) as healthy MRL-lpr/lpr mice, whereas the animals demonstrating no visual or biochemical SLE indices, but having detectable abzyme activities were conditionally designated as pre-diseased mice.
The beginning of the lactation may also be regarded as an important period associated with the production of Abzs [2–5]. Taking this into account, we have analysed 12 groups of non-AI mice and AI-prone MRL-lpr/lpr mice with and without several pronounced SLE indices mentioned above, as well as pregnant and lactating mice (Tables 1 and 2) and assayed RAs of their IgGs in the hydrolysis of DNA, ATP, and MHO:
1.
Autoimmune characteristics of autoimmune-prone MRL-lpr/lpr and control non-autoimmune mice
| Group description | Group number | Number of mice | Urine protein, mg/ml** | Abs to native DNA, A450* | Abs to denatured DNA, A450* | DNase activity, %* | ATPase activity, %* | Amylase activity, %* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Control males and females | ||||||||||
| (CBA × C57BL) F1 (3–7 months) | 1 | 8 (4 f + 4 m) | 0.12 ± 0.07 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0*** | 0*** | 1.0 ± 0.5*** | ||
| BALB/c (3–7 months) | 2 | 8 (4 f + 4 m) | 0.1 ± 0.08 | 0.03 ± 0.01 | 0.017 ± 0.004 | 0 | 0 | 1.1 ± 0.5 | ||
| MRL-lpr/lpr males | ||||||||||
| Healthy (2–3 months)ζ | 3 | 5 | 0.38 ± 0.1 | 0.032 ± 0.01 | 0.09 ± 0.07 | 0 | 0 | 1.9 ± 1.2 | ||
| Healthy, pre-diseased (7 months)ζ | 5 | 5 | 0.8 ± 0.3 | 0.1 ± 0.05+ | 0.16 ± 0.05 | 3.0 ± 1.0φ | 0.4 ± 0.25 | n.d.ξ | ||
| Diseased (7 months) | 7 | 8 | 8.0 ± 3.1** | 0.2 ± 0.05+ | 0.23 ± 0.11 | 22.0 ± 24.0 | 68.3 ± 98.0 | 3.7 ± 1.0 | ||
| Immunized | 9 | 6 | 9.5 ± 1.7** | 0.6 ± 0.17 | 1.1 ± 0.16 | 360.0 ± 230.0 | 1333 ± 530 | 17.6 ± 7.5 | ||
| MRL-lpr/lpr females | ||||||||||
| Healthy (2–3 months)ζ | 4 | 5 | 0.31 ± 0.03 | 0.08 ± 0.03 | 0.12 ± 0.06 | 0 | 0 | 1.8 ± 1.1 | ||
| Healthy, pre-diseased (7 months)ζ | 6 | 5 | 0.9 ± 0.2 | 0.18 ± 0.1+ | 0.08 ± 0.04 | 6.1 ± 2.8 | 2.4 ± 1.7 | n.d. | ||
| Diseased (7 months) | 8 | 5 | 5.0 ± 3.8** | 0.23 ± 0.1+ | 0.21 ± 0.12 | 20.0 ± 21.0 | 65.0 ± 93.0 | 9.2 ± 5.4 | ||
| Pregnant (2–3 months) | 10 | 5 | 0.31 ± 0.2 | 0.24 ± 0.05 | 0.25 ± 0.07 | 7.3 ± 6.0 | 39.3 ± 42.8 | 3.9 ± 3.6 | ||
| Lactating (3 months), 4 days after delivery | 11 | 5 | 0.32 ± 0.1 | 0.54 ± 0.3 | 0.35 ± 0.21 | 44.4 ± 40.6 | 367 ± 548 | 31.7 ± 27.3 | ||
| Lactating (3 months), 14 days after delivery | 12 | 5 | 0.70 ± 0.3 | 0.57 ± 0.28 | 0.39 ± 0.18 | 19.0 ± 24.0 | 191 ± 173 | 13.7 ± 11.0 | ||
For each mouse, the mean of three repeats is used.** Proteinuria corresponds to ≥ 3 mg of total protein/ml of urine *** 100 % relative activity corresponds to a complete transition of the substrate to its products of hydrolysis in the presence of
0.1 mg/ml IgGs (see ‘Methods’). ξ Not determined. MRL-lpr/lpr mice demonstrating no SLE indexes and showing these indexes similar to healthy control non-autoimmune mice were conditionally designated (independently of age) as healthy MRL-lpr/lpr mice; similar animals demonstrating detectable levels of abzymes were conditionally designated as pre-diseased. φ Cohorts with statistically significant (P ≤ 0.05) differences in the parameters in comparison with conditionally healthy MRL-lpr/lpr males and females are given in boldface. +Values obtained using 10–12 mice.
2.
Formation of bone marrow progenitor colonies in from AI-prone MRL-lpr/lpr and control non-autoimmune mice
| Group description | Visual symptoms | Group number | Number of mice | Number of colonies* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFU-E | CFU-GM | CFU-GEMM | |||||||||||||||
| CBA (3–7 months) | no | 1 | 8 | 3.0 ± 0.5** | 7.3 ± 1.0** | 0.25 ± 0.05** | |||||||||||
| MRL-lpr/lpr males | |||||||||||||||||
| Healthy (2–3 months)ζ | no | 3 | 5 | 6.5 ± 1.5 | 7.0 ± 1.0 | 0.5 ± 0.1 | |||||||||||
| Healthy, pre-diseased (7 months)ζ | no | 5 | 5 | 12.7 ± 1.4 | 30.0 ± 1.3 | 9.2 ± 1.9 | |||||||||||
| Diseased (7 months) | yes | 7 | 5 | 25.3 ± 9.8 | 7.4 ± 0.4 | 3.9 ± 2.0 | |||||||||||
| Immunized (3 months) | yes, weak | 9 | 5 | 7.0 ± 2.1 | 6.0 ± 2.6 | 0.9 ± 0.7 | |||||||||||
| MRL-lpr/lpr females | |||||||||||||||||
| Healthy (2–3 months)ζ | no | 4 | 5 | 5.5 ± 0.5 | 11 ± 2.5 | 0.5 ± 0.2 | |||||||||||
| Healthy, pre-diseased (7 months)ζ | no | 6 | 5 | 11.5 ± 2.0 | 23.0 ± 3.0 | 8.2 ± 3.0 | |||||||||||
| Diseased (7 months) | yes | 8 | 5 | 22.1 ± 8.0 | 9.0 ± 3.9 | 2.4 ± 1.8 | |||||||||||
| Pregnant (3 months) | no | 10 | 5 | 6.8 ± 2.0 | 7.8 ± 1.5 | 0.1 ± 0.08 | |||||||||||
| Lactating (3 months), 4 days after delivery | no | 11 | 5 | 8.8 ± 2.0 | 19.1 ± 1.8 | 0.25 ± 0.2 | |||||||||||
| Lactating (3 months), 14 days after delivery | no | 12 | 5 | 21.0 ± 8.0 | 9.7 ± 0.5 | 2.1 ± 0.7 | |||||||||||
MRL-lpr/lpr mice demonstrating no SLE indexes and showing these indexes similar to healthy control non-autoimmune mice were conditionally designated (independently of age) as healthy MRL-lpr/lpr mice; similar animals demonstrating detectable levels of abzymes were conditionally designated as pre-diseased. ΦCohorts with statistically significant (P≤ 0.05) differences in the parameters in comparison with conditionally healthy MRL-lpr/lpr males and females are given in boldface.
control non-AI (CBA x C57BL) F1 or CBA mice (3–7 months of age) and
control non-AI BALB/c mice (3–7 months), which usually demonstrate no visual or biochemical or immunological AI markers mentioned above
control AI-prone MRL-lpr/lpr males (2–3 months) and
control AI-prone MRL-lpr/lpr females (2–3 months), demonstrating no pronounced visual or biochemical markers of SLE pathologies; however, some of them may be at a very early stage of SLE (‘pre-disease’), since sometimes pronounced markers of spontaneous pathology are observed in 3–4-months-old mice or later (see below).
Nearly all 7-months old AI-prone MRL-lpr/lpr mice are ill and demonstrate visual and biochemical markers of SLE (at least those markers that we have analysed). Among a number of MRL-lpr/lpr males (7 months) a special small group of males without visual markers of SLE pathologies was selected and
AI-prone MRL-lpr/lpr females (7 months of age) without visual SLE symptoms; since ∼85–90% of males and females at 7–8 months of age usually demonstrate all visible and biochemical markers of deep SLE, groups 5 and 6 may be considered and conditionally designated as pre-diseased mice
AI-prone MRL-lpr/lpr males (7 months of age) having spontaneously developed deep SLE with pronounced visual symptoms and high proteinuria (> 3 mg/ml), and
AI-prone MRL-lpr/lpr females (7 months of age) having spontaneously developed deep SLE with pronounced visual symptoms and high proteinuria (> 3 mg/ml),
AI-prone MRL-lpr/lpr initially conditionally healthy males (2–3 months of age), but after immunization with DNA demonstrating slight visual markers of SLE pathology, but high proteinuria (> 3 mg/ml) 1.5 months after first immunization,
pregnant AI-prone MRL-lpr/lpr females (initially conditionally healthy, 2–3 months of age) without visual markers of SLE,
lactating AI-prone MRL-lpr/lpr females (initially conditionally healthy, 2–3 months of age) without pronounced visual markers of SLE (4 days after delivery)
lactating AI-prone MRL-lpr/lpr females (initially conditionally healthy, 2–3 months of age) without pronounced visual markers of SLE (14 days after delivery).
The relationship between the relative Abzs activities, visual SLE symptoms, proteinuria and concentration of anti-DNA Abs was analysed for these mouse groups.
Determination of the relative DNase, ATPase and amylase activities
Figure 1 shows cleavage of supercoiled DNA by Abs (0.1 mg/ml) from various mice after 2 hrs of incubation. During this time, some Abzs cause only single breaks in one strand of supercoiled DNA (lanes 1–4), whereas others cause multiple breaks leading to further degradation of relaxed DNA (lanes 5–11). To quantify the DNase activity, we have found a concentration for each IgG preparation that converted supercoiled DNA (scDNA) to relaxed plasmid DNA during 0.2–4 hrs of incubation without formation of linear or fragmented DNA (for example, lanes 1–3, Fig. 1). The efficiency of DNA cleavage was calculated from the relative percentage of DNA in the band of sc and relaxed DNA taking into account the relative amounts of DNA in these two bands for control substrates incubated in the absence of IgGs or with Abs from healthy mice (for example, lanes 12–13, Fig. 1). Since all measurements (initial rates) were taken within the linear regions of the time courses and Ab concentration curves, the measured RAs for IgGs were normalized to standard conditions (0.1 mg/ml Abs, 2 hrs) and a complete transition of sc DNA to its relaxed form was taken as 100% of DNase activity. The data obtained are summarized in Table 1.
1.
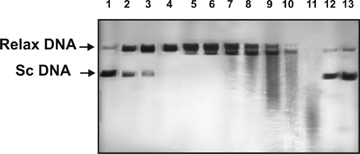
DNase activities of catalytic IgGs from different mice in the cleavage of supercoiled (sc) pBluescript DNA: lanes 1–11 correspond to 0.1 mg/ml IgGs from 11 different mouse sera incubated for 2 hrs at 30 °C; lane 12, DNA incubated with Abs from the serum of a healthy mouse; lane 13, DNA incubated alone.
The relative ATPase and amylase activities also varied greatly between different animals (Fig. 2). Therefore, we have used the same approach as described above for DNase analysis to choose the Ab concentrations and incubation time for estimating the ATPase and amylase RAs of IgGs from these mice. Complete hydrolysis of ATP after 2 hrs and MHO after 12 hrs at a standard concentration of IgGs (0.1 mg/ml) was taken for 100% of these activities. The results are summarized in Table 1. Since some IgGs hydrolysed DNA, ATP, and MHO completely at lower Ab concentrations (< 0.1 mg/ml) and shorter incubation times, the RAs of some IgGs in the hydrolysis of DNA, ATP, and MHO can be higher than 100% (Table 1). At 3–7 months of age, control non-AI BALB/c and CBA males and females (groups 1 and 2) demonstrated no proteinuria (0.1–0.12 mg/ml <3 mg/ml), very low and comparable concentrations of Abs to native and denatured DNA (0.017-0.04 A450), and non-detectable level of DNase or ATPase activities (Table 1). IgGs of some of these control mice did not possess detectable amylase activity, while other were characterised by low but detectable activity, average values of IgG amylase activity were ∼1% (Table 1).
2.
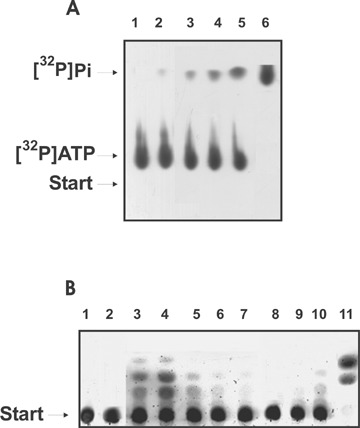
Analysis of ATPase (A) and amylase (B) activities of purified IgG by thin-layer chromatography on PEI cellulose (A) and on Kieselgel plates (B) and autoradiography. (A) Before the chromatography, standard reaction mixtures containing 0.1 mg/ml IgGs and 0.2 mM [γ-32P]ATP were incubated at 30°C for 2 hrs: lanes 1–6 correspond to IgGs from 6 different mice. (B) Before the chromatography, standard reaction mixtures containing 0.1 mg/ml IgGs and 0.15 mM MHO were incubated at 30°C for 12 hrs: lanes 2–11 correspond to IgGs from 10 different mice, lane 1, the substrate incubated alone.
The majority of 2-3-months-old MRL-lpr/lpr males and females demonstrated no visible signs of pathology and average biochemical markers similar to those for control healthy BALB/c and CBA mice; the absence of proteinuria (0.37-0.38 mg/ml), higher, but still relatively low concentrations of anti-DNA Abs (0.09 - 0.12 A450) and undetectable levels of DNase or ATPase activities (Table 1). Between these MRL-lpr/lpr mice there were individuals with somewhat higher amylase RAs than those for control non-AI mice, but on average there was no significant difference in this parameter between healthy controls (groups 1 and 2) and young MRL-lpr/lpr mice (groups 3 and 4; Table 1).
As we have shown previously, detection of Ab DNase and RNase activities in human serum may be considered a good indicator of beginning or a significant development of AI reactions associated with several AI pathologies [2–4]. Interestingly, detectable levels of nuclease activities of IgGs and/or IgMs can be revealed sometimes 1–6 months earlier than a statistically reliable increase in anti-DNA Ab concentrations. Some 7-months-old MRL-lpr/lpr males and females demonstrating no visual symptoms can be conditionally considered as animals soon to succumb to the disease (pre-diseased), since 80–95% of mice at 7–8 months usually develop very deep SLE. Interestingly, all conditionally healthy MRL-lpr/lpr males and females at 7 months of age (groups 5 and 6) demonstrated characteristic values of urine protein concentration (range 0.4–1.2 mg/ml), on average these values (∼0.8–0.9 mg/ml) were statistically significantly higher than in healthy mice (groups 1-4), but lower than ≤ 3 mg/ml (Table 1). These groups were on average characterized by increased concentrations of Abs against denatured (0.08–0.16 A450) and native (0.11–0.2 A450) DNA as compared with mice of 2–3-months of age (Table 1). A significant difference was observed between, respectively, male groups 3 and 5 and female groups 4 and 6 in the case of Abs to native DNA (Table 1). Interestingly, all 7-months-old MRL-lpr/lpr males and females with-out visible indices of SLE demonstrated detectable DNase (range 1–9%, on average 3.0–6.1%) and ATPase (range 0.1–6%, on average 0.4–2.4%) activ-ities (Table 1). Thus, like in the case of AI-patients, only mouse IgG DNase and ATPase activities can be considered statistically significant indicators of pre-disease conditions of spontaneous SLE.
The MRL-lpr/lpr males and females, also at 7 months of age, but with visual symptoms of spontaneous SLE, demonstrated very high proteinuria (∼5–8 mg/ml) and a moderate, statistically insignificant increase in the average concentrations of anti-DNA Abs (0.16–0.23 A450; groups 7 and 8) in comparison with 7–months-old pre-diseased mice (groups 5 and 6; Table 1). Spontaneous pathology in 7-months-old MRL-lpr/lpr mice (groups 7 and 8) is characterized by significant variations in Abz RAs. DNase and ATPase RAs of male Abzs (group 7) fell in the ranges ∼2–64% and ∼4–314%, respectively. Similar ranges of DNase and ATPase RAs were observed for group of ill 7-months-old females (group 8). The average values of DNase (∼20–22%) and ATPase (∼65–68.3%) activities for Abzs from different males and females (groups 7 and 8) were increased 3–7 and 27–171-fold, respectively, as compared with pre-diseased mice (groups 5 and 6; Table 1). The increase in the amylase RAs after the development of SLE by 7-months-old males and females (3.9 and 9.2%, respectively; groups 7 and 8) as compared with young mice (groups 3 and 4) was about ∼1.9–5.0-fold. Since IgGs from healthy control and young AI-prone mice possess amylase activity, this factor cannot be considered a very good marker of severe AI pathology. Thus, the most important markers of mouse severe SLE are very high protein-uria (5–8 mg/ml) and high activity of IgGs in the hydrolysis of DNA and ATP (Table 1).
We have immunized conditionally healthy MRL-lpr/lpr males (3 months of age) with DNA. This led to a development of slight visible markers of SLE 1.5 months after the first immunization, but to drastic effect on proteinuria (∼9.5 mg/ml, group 9), which was increased ∼25-fold as compared with young males (groups 3 and 4), but was comparable with those for males and females with spontaneous SLE (∼5–8 mg/ml, groups 7 and 8) (Table 1). Immunization of the males (group 9) led to the highest and statistically significant increase in the level of Abs against native (on average ∼0.6 A450) and denatured DNA (on average ∼1.0 A450) in comparison with healthy or ill mice (groups 1–8; Table 1). For immunized males, the highest increase in the relative DNase (range 50–510%) and especially ATPase (range 500–1887%) activities was observed, the average activities was estimated as ∼360% and ∼1333%, respectively (Table 1). As compared with the 7-month-old pre-diseased males (group 5) DNase activity of the immunized mice (group 9) was increased ∼120-fold, while ATPase, ∼3330-fold (Table 1). The amylase RA of immunized males was increased as compared with healthy males (group 3) by a factor of ∼8.8 (Table 1). Interestingly, development of SLE pathology stimulated by immunization is characterized mainly by a significant increase in anti-DNA Abs concentrations (12–19-fold) and Abz RAs (8.8–3330-fold), while indexes of proteinuria for spontaneous and stimulated pathologies are comparable (Table 1).
Ab catalytic activities in pregnant and lactating mice
We tried to understand possible differences in production of Abzs between spontaneous and stimulated development of SLE in MRL-lpr/lpr mice and during their pregnancy and lactation. In contrast to spontaneous or stimulated mouse SLE (5–9.5 mg/ml), pronounced proteinuria (≥ 3 mg/ml) was not detected in females during pregnancy (0.31 mg/ml), 4 days (0.32 mg/ml), or 2 weeks (0.7 mg/ml) after delivery and at the beginning of lactation (Table 1). The concentrations of anti-DNA Abs (0.24–0.25 A450, group 10) in pregnant females were on average ∼2.5–3.0-fold higher than in non-pregnant healthy females (0.08–0.12 A450, group 4) and comparable with these values for ill females and males (0.21–0.23 A450, groups 7 and 8; Table 1).
Interestingly, 4 days and 2 weeks after the beginning of lactation, the average values of the relative concentrations of Abs to native DNA were increased ∼1.8–2.4-fold (0.54–0.57 A450, groups 11 and 12) in comparison with those for pregnant mice (group 10) and became comparable with those for males after immunization with DNA (0.6 A450; Table 1). The average values of concentrations of Abs to denatured DNA for lactating females (0.35–0.39 A450) were ∼1.4–1.6 times higher than for pregnant females, but were remarkably lower than for immunized mice (1.1 A450; Table 1). Thus, similar to the ill mice (groups 7 and 8), or the immunized males (group 9), pregnant and especially lactating females (groups 10–11) are characterized overall by increased concentrations of anti-DNA Abs. However, in contrast to the ill and immunized mice, the high level of anti-DNA Abs for pregnant and lactating mice did not correlate with proteinuria (Table 1).
On average, 7-months-old pre-diseased females (group 4) demonstrated DNase (6.1%) and A TPase (24%) RAs comparable with those for pregnant animals (group 10; 6.3 and 39.3%, respectively; Table 1). The average amylase RA of pregnant females (3.9%) is also comparable with those for ill males and females (3.7–9.2%). After the beginning of lactation, the average DNase activity increased sharply to ∼44% (range 3–100%) and then after 2 weeks of lactation decreased to ∼19% (range 0.3–79%). Similar situation was observed for IgG amylase activity, which increased on average on going from pregnant to lactating mice from 3.9 to 31.7%, and then after 2 weeks of lactation decreased to 13.7%. The most significant increase in the activity was observed for A TPase Abzs in the females. The average relative A TPase activity reached 367% (range 20–1737%) in the mice 4 days after delivery and then decreased to 191% (range 1–567%) 2 weeks after the delivery (Table 1). Thus, 4 days after delivery, the lactating mice demonstrated 6–9-fold increases in average DNase, A TPase, and amylase RAs, and all these activities decreased ∼2-fold after two weeks of lactation (Table 1).
Haematopoietic progenitors colony formation
We have studied possible relationships between the Abz RAs and the colony formation of haematopoietic progenitors. In the bone marrow of young MRL-lpr/lpr males and females (groups 3 and 4) demonstrating no detectable Abz DNase and A TPase activities, we have found a normal distribution of committed progenitors similar to that for non-AI CBA mice (group 1; Table 2). The number of BFU-E and CFU-GEMM colonies increased respectively ∼2 and ∼16.4–28.4-fold in 7-months-old MRL-lpr/lpr males and females (groups 5 and 6) without SLE clinical manifestations and proteinuria, but possessing low detectable Abzs activities. In the spontaneously diseased males and females (groups 7 and 8) showing high Abz RAs, the HSC profile was changed remarkably as compared with pre-diseased mice: the number of BFU-E colonies increased ∼2-fold, while the number of CFU-GM and CFU-GEMM colonies decreased ∼2.6–4-fold and 2.4–3.4-fold in comparison with the pre-diseased mice (groups 5 and 6, respectively).
After the development of SLE induced by immunization, the mice (group 9) were characterized by the highest increase in proteinuria, anti-DNA Abs, Abz activities, and a very specific HSC differentiation profile was observed (Table 2). The numbers of BFU-E and CFU-GEMM colonies were 3.6- and 4.3-fold lower than for spontaneously diseased males (group 7), while these parameters for CFU-GM colonies were comparable.
Pregnancy (group 10) led to a remarkable decrease in the level of HSC proliferation and to a change in the differentiation profile: a 5–82-fold decrease in CFU-GEMM colonies was observed as compared with female groups 4, 6, and 8, while the number of BFU-E and CFU-GM cell colonies for pregnant and control healthy mice was comparable (Table 2). During pregnancy (group 10), the relative concentrations of anti-DNA Abs and Abz RAs are higher or, for some indexes, comparable with those for the pre-diseased and diseased mice (groups 6 and 8; Table 1), while the levels of all HSC colonies formation, especially BFU-E and CFU-GEMM, are significantly lower for pregnant females than for the pre-diseased and spontaneously diseased males and females (Table 2). Thus, the profile of HSC differentiation in the pregnant females (group 10) differs from those of the healthy non-pregnant, pre-diseased and diseased mice (groups 4, 6, and 8).
Four days after the beginning of lactation, a statistically significant 2.4-fold increase in the average number of CFU-GM colonies, the progenitors of neutrophils and macrophages, was observed, while the average increase in the formation of BFU-E and CFU-GEMM colonies was not statistically significant (Table 2). The HSC differentiation profile became formally comparable with that for young healthy males and females (groups 3 and 4, Table 2). Two weeks after the beginning of lactation, the average indexes of the differentiation profile again changed remarkably, the average number of CFU-GM colonies decreasing ∼2-fold, with a ∼2.4- and 8.4-fold increase observed in BFU-E and CFU-GEMM colonies, respectively (Table 2). Interestingly, the changes in HSC differentiation profiles occur in parallel with a significant increase in the IgG RAs at a transition from pregnancy to the beginning of lactation (4 days after delivery) and then to the remarkable decrease in the Abz activities 14 days after the delivery (Table 1).
Lymphocyte proliferation in different mouse organs
We have analysed relative levels of lymphocyte pro-liferation from different organs of MRL-lpr/lpr mice in the absence and in the presence of mitogen (Table 3). Spontaneous development of SLE with high proteinuria leads on average to a remarkable increase in lymphocyte proliferation in all analysed organs of males and females (groups 8 and 7) as compared with control young mice (groups 3 and 4). In spite of a significant difference in the Abz RAs for immunized males (group 9) and males or females spontaneously developing SLE (group 7 and 8), all these groups were characterized by comparable levels of lymphocyte proliferation, which on average were significantly higher than in control (groups 3 and 4; Table 3). Interestingly, pre-diseased males (group 5) on average demonstrated intermediate proliferation indexes between the control (group 3) and spontaneously diseased males (group 7). In the presence of mitogen, the level of proliferation of lymphocytes from all organs of these mouse groups increased 2–20-fold depending on the organ analysed, and the maximal increase was observed for cells from thymus and especially spleen (Table 3).
3.
Lymphocyte proliferation in different mouse organs
| Group description | Group number | Number of mice | Proliferation level × 10−2, cpm* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone marrow | Lymph nodes | Thymus | Spleen | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| - | +Con A | - | +Con A | - | +Con A | - | +Con A | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| CBA (3–7 months) | 1 | 8 | 28.1 ± 5.4 | n.d.*** | 11±3.2 | n.d. | 11±2.8 | n.d. | 40 ± 8.7 | n.d | |||||||||||||||||||||||||||||||||||||||||||||||||
| Groups of MRL-lpr/lpr males | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Healthy (2–3 months)ζ | 3 | 5 | nd | nd | 12 ± 7.3 | 24.7±16.3 | 5.6 ± 0.95 | 105 ± 22 | 13.7 ± 5.4 | 256 ± 59 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Healthy, pre-diseased (7 months)ζ | 5 | 5 | nd | nd | 17.5 ± 3.4 | 157 ± 63 | 10.1 ± 3.7 | 179 ± 53 | 38.6 ± 2.2 | 405 ± 109 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Diseased (7 months) | 7 | 5 | 41 ± 15.6 | 66.6 ± 45.7 | 14.6 ± 9.1 | 249 ± 154 | 15.6 ± 9.3 | 160 ± 81 | 29.3 ± 10.6 | 300 ± 82.5 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Immunized (3 months) | 9 | 5 | 37.6 ± 24.7 | 49.6 ± 35.7 | 20.4 ± 19.0 | 189 ± 144 | 7.0 ± 6.0 | 84 ± 61 | 33.0 ± 23.0 | 245 ± 163 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Groups of MRL-lpr/lpr females | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Healthy, pre-eased (2–3 months)ζ | 4 | 5 | 25.4 ± 12.2 | 24.8 ± 10.8 | 4.4 ± 1.8 | 254 ± 169 | 2.7 ± 1.0 | 106 ± 33 | 7.1 ± 4.4 | 215 ± 83 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Diseased (7 months) | 8 | 5 | 23 ± 9.1 | 35 ± 10 | 7.7 ± 6.0 | 196 ± 58 | 7.7 ± 4.5 | 186 ± 118 | 22 ± 11.8 | 246 ± 115 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Pregnant (2–3 months) | 10 | 5 | 36.8 ± 8.4 | 55.4 ± 23.7 | 10.3 ± 6.3 | 279± 85 | 18.2 ± 9.3 | 153 ± 57 | 5.1 ± 2.4 | nd | |||||||||||||||||||||||||||||||||||||||||||||||||
| Lactating (3 months) 4 days after delivery | 11 | 5 | 44.9 ± 11.4 | 93.1 ± 29.4 | 6.6 ± 3.9 | 340 ± 162 | 8.8 ± 0.9 | 108 ± 35 | 44.4 ± 18.4 | 525 ± 115 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Lactating (3 months) 14 days after delivery | 12 | 5 | 15.8 ± 5.4 | 29.6 ± 10.6 | 7.1 ± 4.4 | 137 ± 57 | 6.7 ± 5.1 | 69 ± 66 | 27.1 ± 18.4 | 310 ± 159 | |||||||||||||||||||||||||||||||||||||||||||||||||
For each mouse, the mean of three repeats is used. ξ Mean ± confidence interval. *** Not determined. ζ MRL-lpr/lpr mice demonstrating no SLE indexes and showing these indexes similar to healthy control non-autoimmune mice were conditionally designated (independently of age) as healthy MRL-lpr/lpr mice; similar animals demonstrating detectable levels of abzymes were conditionally designated as pre-diseased. Φ Cohorts with statistically significant (P ≤ 0.05) differences in the parameters in comparison with conditionally healthy MRL-lpr/lpr males and females are given in boldface.
Transition from the control (group 4) to pregnant females (group 10) associated with an appearance of detectable Abz activities (Table 1) led to a 6.7-fold statistically significant increase in the lymphocyte proliferation only in the thymus (group 10); this index for other organs of these mice was also on average higher, but the differences were not statistically significant (Table 3). The beginning of lactation (4 days after delivery, group 11) associated with the maximal increase in different Abzs RAs (Table 1) led to statistically significant 3.3- and 6.3-fold increases in cell proliferation from thymus and spleen, respectively, in comparison with the control females (group 4), while a 1.5–1.8-fold increase in this index for bone marrow and lymph nodes was statistically insignificant (Table 3). From a comparison of the pregnant (group 10) and lactating females (group 11), it can be seen that a statistically significant 6.3-fold increase in lymphocyte proliferation was observed only for cells from the spleen, while the cell proliferation from the thymus and lymph nodes decreased for lactating mice ∼1.6– 2.0-fold on average. Interestingly, a 1.9–2.3-fold decrease in RAs of Abzs (Table 1) in the mice lactating for 14 days in comparison with females 4 days after delivery occurs in parallel with a 2.9- and 1.6-fold decrease in the lymphocyte proliferation in marrow and spleen, respectively, while this index for thymus and spleen cells were comparable (Table 3). Activation of the cells from pregnant and lactating females with mitogen led to a minimal ∼2-fold increase in bone marrow cell proliferation, while for cells from other organs this factor was significantly higher (10–44-fold, Table 3).
Cell apoptosis assay
We have analyzed relative levels of cell apoptosis in different organs of various groups of MRL-lpr/lpr mice (Table 4). In control young mice (groups 3 and 4) characterized by the absence of DNase and ATPase Abzs (Table 1), the level of cell apoptosis on average was comparable with that for pre-diseased males (group 5), but was remarkably higher than in diseased males and females demonstrating Abz activities (groups 7 and 8). The maximal and statistically significant 2–3-fold decrease in the apoptosis was observed for bone marrow, thymus and spleen of the immunized males (group 9), while the pregnant females (group 10) demonstrated a ∼1.8-fold decrease in cell apoptosis in bone marrow, lymph nodes, and thymus, and a 13-fold decrease in spleen lymphocyte apoptosis (Table 4). For other groups of mice with spontaneously developed SLE (groups 8 and 9) or for lactating females (groups 11 and 12), the average apoptosis indexes were remarkably lower, but there was no statistically significant difference between them. In the presence of mitogen, the level of apoptosis was increased ∼1.2–3-fold depending on the group of mice and the organs analysed (Table 4).
4.
Cell apoptosis in different mouse organs
| Group description | Group number | Number of mice | Apoptosis level, fluorescence (relative units)* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone marrow | Lymph nodes | Thymus | Spleen | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| - | +Con A | - | +Con A | - | +Con A | - | +Con A | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| CBA (3–7 months) | 1 | 8 | 19.8 ± 6.9ξ | nd | 11.8 ± 6.50ξ | nd | 12.7 ± 6.4ξ | nd | 11.2 ± 7.5ξ | nd | |||||||||||||||||||||||||||||||||||||||||||||||||
| Groups of MRL-lpr/lpr males | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Healthy (2–3 months)ζ | 3 | 5 | 19.0 ± 5.2 | nd | 11.5 ± 0.5 | 18.5 ± 1.0 | 12.2 ± 2.0 | 23.0 ± 3.0 | 10.0 ± 1.0 | 13.2 ± 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Healthy, pre-diseased (7 months)ζ | 5 | 5 | 15.0 ± 4.2 | nd | 12.7 ± 2.0 | 18.5 ± 2.5 | 8.7 ± 1.2 | 24.3 ± 3.2 | 17.0 ± 2.1 | 21.3 ± 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Diseased (7 months) | 7 | 5 | 9.8 ± 2.3 | 14.6 ± 6.0 | 7.6 ± 2.6 | 9.1 ± 1.9 | 7.9 ± 4.2 | 14.6 ± 7.8 | 11.2 ± 1.9 | 15.6 ± 5.5 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Immunized (3 months) | 9 | 5 | 6.1 ± 2.3 | 8.7 ± 3.2 | 5.7 ± 2.6 | 9.8 ± 3.7 | 6.1 ± 4.2 | 11.2 ± 6.4 | 4.8 ± 1.9 | 10.8 ± 1.7 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Groups of MRL-lpr/lpr females | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Healthy (2–3 months)ζ | 4 | 5 | 17.8 ± 5.1 | 24.6 ± 6.1 | 15.2 ± 4.3 | 26.1 ± 8.7 | 14.4 ± 5.2 | 22.2 ± 7.3 | 19.5 ± 5.6 | 26.6 ± 8.4 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Diseased (7 months) | 8 | 5 | 15.8 ± 3.4 | 22.8 ± 5.2 | 12.2 ± 5.1 | 18.2 ± 4.5 | 11.2 ± 4.3 | 20.8 ± 4.9 | 13.2 ± 2.6 | 15.8 ± 4.6 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Pregnant (3 months) | 10 | 5 | 9.2 ± 2.7 | 16.2 ± 6.8 | 7.0 ± 2.3 | 9.5 ± 7.5 | 8.2±1.9 | 13.2 ± 2.7 | 1.5 ± 0.5 | nd | |||||||||||||||||||||||||||||||||||||||||||||||||
| Lactating (3 months), 4 days after delivery | 11 | 5 | 14.8 ± 4.4 | 17.3 ± 3.5 | 10.8 ± 4.5 | 9.2 ± 2.7 | 10.8 ± 4.6 | 14.0 ± 3.1 | 15.3 ± 2.8 | 20.0 ± 4.2 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Lactating (3 months), 14 days after delivery | 12 | 5 | 12.2 ± 6.5 | 13.3 ± 7.2 | 7.0 ± 2.5 | 9.2 ± 5.0 | 10.5 ± 4.0 | 11.7 ± 4.3 | 9.4 ± 4.3 | 11.8 ± 4.4 | |||||||||||||||||||||||||||||||||||||||||||||||||
For each mouse, the mean of three repeats is used.ξ Mean ± confidence interval. *** Not determined. ζ MRL-lpr/lpr mice demonstrating no SLE indexes and showing these indexes similar to healthy control non-autoimmune mice were conditionally designated (independently of age) as healthy MRL-lpr/lpr mice; similar animals demonstrating detectable levels of abzymes were conditionally designated as pre-diseased. Φ Cohorts with statistically significant (P ≤ 0.05) differences in the parameters in comparison with conditionally healthy MRL-lpr/lpr males and females are given in boldface.
Discussion
In this paper, we have analysed for the first time possible correlations between a subset of biochemical markers of SLE, Abz RAs, HSC differentiation profiles, apoptosis and lymphocyte proliferation in several organs of different mice. It was shown that, in contrast to control young mice, spontaneous SLE and pathology initiated by mouse immunization with DNA leads to statistically significant increase in protein-uria, concentration of anti-DNA Abs, and especially RAs of Abzs (Table 1). The data in Table 1 and the analysis of time-dependent changes in mouse pro-teinuria, anti-DNA Abs, and Abz RAs before and after the development of visible pathological markers permit us to conclude that MRL-lpr/lpr mice of any age 0.5–1.5 months before developing deep spontaneous pathology are characterized by a moderate increase in concentrations of urine proteins, anti-DNA Abs, and enzymic RAs. Interestingly, only the increase in DNase and ATPase activities at a pre-disease stage of MRL-lpr/lpr mice (groups 5 and 6) was a decisive and unambiguously significant indicator of the beginning of specific AI-reactions (Table 1).
Pregnancy and especially lactation, associated with a remarkable increase in anti-DNA Abs and with an appearance of Abzs catalytic activities, should be considered special female conditions leading to specific AI reactions without a development of protein-uria and visible SLE markers (Table 1). These findings are in agreement with the literature data that clinically healthy pregnant women may be effectively immunized by components of various viruses and bacteria, and autoimmunization of mothers may occur during pregnancy similar to that in AI patients ([2–5, 26] and refs therein).This leads to appearance of anti-DNA Abs and different Abzs in the serum of pregnant women and then to a significant elevation of these markers in the serum and especially in the milk after the beginning of lactation. Interestingly, the level of DNase and ATPase activities of IgGs in the milk of lactating women did not change significantly in the first month after delivery and then slowly decreased by 40–80% during 2–6 months of the postnatal period [21, 25]. The level of Abz RAs after 14 days of mouse lactation was ∼2-fold lower than for mice lactating for 4 days, but the mouse lactation period is ∼7–10 times shorter than in human females.
Although the mice were immunized with DNA, the ATPase and amylase activities of their Abs also increased greatly (Table 1). This was not surprising and is in agreement with previously published data that immunization of AI mice produces an unexpectedly high increase in the number of clones secreting various auto-Abs, including Abzs, compared with normal mice [2–5, 36-37]. A lower level of anti-DNA Abs and different Abzs in the diseased and pre-diseased mice as compared with the immunized mice (Table 1) may be due to a wider repertoire of different auto-Abs including DNase, ATPase, and amylase Abzs in immunized mice.
Table 2 shows a significant difference in HSC differentiation profiles for all types of mouse groups analysed. Our findings support the existence of specific and diverse relationships between the profile of HSC differentiation, lymphocyte proliferation, apoptosis and the Abz RAs in the case of various mouse groups (Tables 1–4). Interestingly, the maximal difference in lymphocyte proliferation in all mouse groups demonstrating high level of Abz activities (groups 7–10, 12) in comparison with control mice (groups 3 and 4) is observed in the thymus and especially in the spleen, where B-lymphocyte progenitors differentiate into plasmocytes producing Abs (Table 3).
Since immunized males demonstrate the maximal levels of anti-DNA Abs and very high DNase and ATPase activities (Table 1), one can suppose that cell apoptosis suppression in these males can lead to an increased production of B-lymphocytes secreting anti-DNA Abs and Abzs. The Abzs production in all other mouse groups may most probably result from specific combinations of different contribution of HSC proliferation and differentiation, proliferation of lymphocytes and cell apoptosis in different mouse organs. Taking into account these three factors one can see two specific groups, immunized and pregnant mice, significantly deviating from controls and other mouse groups analysed (Tables 2-4). Thus, immunization of males leads to the maximal increase in Abz activities, which occurs in parallel with a significant decrease in cell apoptosis, especially in bone marrow, thymus and spleen (Table 4). A similar situation is observed in pregnant females (group 10), demonstrating a maximal decrease in apoptosis in all organs and especially in the spleen (Table 4). Overall, for all other mouse groups there is a tendency to a decrease in the average levels of apoptosis in comparison with control groups, but more often there is no statistically significant difference in these values. Thus, a significant decrease in apoptosis in the immunized and pregnant mice can be an important factor providing the increased level of specific lymphocytes producing auto-Abs and Abzs, which are eliminated in norm and in different organs of other mouse groups. As a consequence, in contrast to control conditionally healthy young mice (groups 1–4), the serum of pregnant females is characterized by detectable levels of DNase and ATPase RAs, while immunized mice demonstrate the maximal level of these activities.
The pregnant and immunized mice (groups 9 and 10) differ from other groups in the HSC differentiation. Interestingly, upon transition from young males or females (groups 3 and 4) to conditionally pre-diseased 7-months-old mice without proteinuria, but demonstrating significant level of Abz RAs (groups 5 and 6; Table 1), a significant change in HSC differentiation profile occurred: a 2.1–4.3-fold and a 16.4–18.4-fold increase in the number of GFU-GM and CFU-GEMM colonies was observed (Table 2). The higher number of CFU-GEMM was accompanied by a striking increase in their size. Interestingly, after the males and females spontaneously developed SLE associated with proteinuria and an increase in Abz RAs (groups 7 and 8), a significant increase in BFU-E and a decrease in GFU-GM and CFU-GEMM colonies was observed (Table 2). In contrast to the pre-diseased mice, the CFU-GEMM colonies of the diseased animals were of normal size. Thus, all mice demonstrating detectable level of Abzs activities without a significant increase in proteinuria and anti-DNA Abs can be considered as pre-diseased mice, which in comparison with conditionally healthy young mice are characterized by a specific profile of HSC differentiation, an increased level of lymphocyte proliferation in thymus and spleen, and increased apoptosis of spleen cells (Tables 2–4). The pre-diseased mice differ from the diseased mice (groups 7 and 8) in the profile of HSC differentiation, the size of CFU-GEMM colonies, the level of proteinuria, and Abz RAs, but there is no significant difference in apoptosis and lymphocyte proliferation indexes.
Interestingly, the profile of HSC differentiation in immunized mice (group 9) is quite different from the pre-diseased (group 5) and spontaneously diseased mice (group 7), but comparable with that for young males and females (groups 3 and 4). In addition, bone marrow cell of the immunized males are characterized by low mitogen responsiveness (a 1.3-fold increase), while cells from the lymph nodes, thymus, and spleen are very sensitive to concanavalin A (a 7.4–12-fold increase, Table 3). These data and the similarity of the HSC differentiation profiles for the immunized and young control mice suggest that immunization with DNA does not remarkably affect bone marrow stem cells, and the significant increase in anti-DNA Abs and DNase or ATPase RAs is provided mostly by a significant change in proliferation and differentiation of lymphocyte progenitors in other mouse organs. The maximal increase in lymphocyte proliferation in the immunized males occurs in spleen (Table 3). Thus, the increased level of anti-DNA Abs and Abzs in the immunized mice may be mainly provided by an activation of lymphocyte differentiation and proliferation in different organs and first of all in the spleen, with a parallel significant decrease in cell apoptosis. The very high urine protein concentration and visible markers of SLE demonstrated by the immunized males (Table 1) can be a result of kidney and spleen dysfunction.
As mentioned above, pre-disease is accompanied with a significant change in the profile of HSC differentiation in spleen. These changes in mouse immune system are most likely only temporary, since transition from the pre-diseased (groups 5 and 6) to diseased mice (groups 7 and 8) is associated not only with an increase in the level of anti-DNA Abs and different Abzs, but also with a significant change in the profile of HSC differentiation (Table 2). A change in the profile of HSC differentiation in the diseased mice seems to be the most important factor in the irreversible switching of mouse immune system to an AI mode, since the changes in cell proliferation and apoptosis in different organs occur mainly on transition from healthy to pre-diseased mice and the observed difference in these indexes between pre-diseased and diseased mice is insignificant (Tables 2–4).
Pregnant and lactating females are very interesting groups. Transition from control (group 4) to pregnant females (group 10) leads to: (a) an appearance of Abs catalytic activities (Table 1), (b) a remarkable suppression of HSC proliferation (especially the 5-fold decrease in CFU-GEMM colonies) accompanied with small changes in the number of BFU-E and CFU-GM colonies (Table 2), (c) a significant decrease in apoptosis of cells from all organs and especially the spleen (Table 4), and (d) a change in lymphocyte proliferation in all organs analysed and especially an increase in thymus cell proliferation (Table 3). These findings are in agreement with the literature data on specific “‘immuno-memory’”: switching of the mammalian immune system during pregnancy for collecting all information about possible environmental antigens harmful for newborns ([2–4, 21, 25, 46, 47] and refs therein). As a result, parenteral or oral administration of various antigens to animals late in pregnancy leads to the production of the corresponding Abs, which are accumulated at high levels in the serum, but most of all in milk [46–47].There may be also some degree of autoimmunization during pregnancy similar to that in AI patients, and an increased level of DNA in the sera of normal women during the first trimester, similar to that observed for AI diseases, has been reported [48]. Additional autoimmunization in pregnant women could be a result of the presence of the embryo's antigens in low concentrations in the mother's blood [49]. Specific stimulation of production of various Abs, including DNase Abzs, by the immune system of mothers after viral and bacterial infection and an increase in milk Abzs RAs after allergic diseases during pregnancy were observed [24]. The beginning of lactation may be considered an important period associated with switching the “‘immuno-memory’” of pregnant women into a new mode associated with the beginning of production of different Abs and Abzs mostly in milk [5]. This period is sometimes very dangerous for females since it can lead to a very significant increase in immune reactions and to an ‘AI shock’ phenomenon well known in obstetrics. Many different postnatal AI pathologies including SLE, Hashimoto's thyroiditis, phospholipids syndrome, polymyositis, AI myocarditis, etc. can be stimulated by pregnancy in initially healthy women [29, 50]. One of the most frequent postnatal AI pathologies is Hashimoto's thyroiditis found in 1.9–16.7% of all pregnancies [29–30]. These AI diseases may develop without a strong increase in immune reactions at the beginning of lactation, but more often the AI shock is a specific marker of a future development of typical progressive or chronic AI diseases. AI reactions during pregnancy and after delivery are observed much more often than postnatal AI pathologies of parous women since AI reactions usually cease with the end of lactation. In contrast to pregnant and lactating women, AI reactions in AI patients are usually permanent and the pathologies have chronic character with temporary remissions and exacerbations. Taking these observations together, one can suppose that there may be a significant difference in the mechanisms of AI reactions in lactating females and in patients with AI pathologies. At the same time, one cannot exclude that the molecular mechanisms of immune system activation leading to the production of autoreactive Abs and/or auto-Abs are, to some extent, similar or overlapping in both AI patients and human mothers. Therefore, a comparison of pre-diseased, diseased, pregnant or lactating mice is of a special interest.
During pregnancy (group 10), a very specific reorganization of the profile of HSC differentiation as compared with other mouse groups occurred (Table 2). While the reorganization of HSC differentiation of pre-diseased in comparison with healthy males led to a ∼2–18-fold increase in the number of all colonies (CFU-GEMM, BFU-E, and CFU-GM), the group of pregnant females demonstrated 1.4-fold decrease in CFU-GM and a statistically significant 5-fold decrease in CFU-GEMM colonies. This indicates not only a significant change in the bone marrow HSC differentiation profile, but also suppression of HSC proliferation in pregnant females. Bone marrow HSC proliferation in the pregnant mice was more significantly suppressed in comparison with the spontaneously pre-diseased and diseased mice and, for example, the number of CFU-GEMM colonies in pregnant females was 24–92-fold lower than in the mentioned groups (Table 2).
Interestingly, in the pregnant females (group 10) as compared with the pre-diseased mice (group 5), a significant decrease in average values characterizing lymphocyte proliferation in lymph nodes and especially spleen was observed, while this average index for the thymus was higher (Table 3). In comparison with the pre-diseased and spontaneously diseased mice, the pregnant females are characterized by a very low level of cell apoptosis in different organs, which is comparable with that in the immunized males (Table 4).Thus, the immune system characteristics of pregnant females differ not only from those of healthy, but also from the pre-diseased and diseased males. The most significant differences are observed in the formation of CFU-GEMM colonies and the suppression of lymphocyte proliferation in different organs (Tables 2 and 4).
After the beginning of lactation (4 days after delivery), the profile of HSC differentiation and the level of proliferation of different bone marrow cells were sharply changed: the number of CFU-GEMM and especially CFU-GM colonies increased significantly, while the number of BFU-E colonies did not change remarkably (Table 2). In parallel, we have found a decrease in the average proliferation levels of lymphocytes from lymph nodes and thymus, but an increase in the level of bone marrow cell proliferation (Table 3). A maximal and statistically significant 6.3-fold increase in the spleen cell proliferation was observed. In addition, the beginning of lactation was associated with a tendency to an increase in cell apoptosis, and the maximal and statistically significant 10-fold increase in spleen cell apoptosis was observed at transition from the pregnancy (group 10) to the beginning of lactation (group 11) (Table 4).
Later, 14 days after delivery, when all Abz RAs decreased 2–2.3-fold, a specific and strong change in HSC differentiation was observed again: a statistically significant 2.4- and 8.4-fold increase in the number of BFU-E and CFU-GEMM colonies, respectively, and a 2-fold decrease in the number of CFU-GM colonies was found (Table 2). Interestingly, late in the lactation (14 days after delivery, group 12) as compared with the beginning of lactation (group 11) there was a statistically significant 2.8-fold decrease only in the proliferation of bone marrow lymphocytes and a tendency of decreasing cell proliferation in spleen (1.6-fold decrease in the average values), while the average proliferation indexes for lymph nodes and thymus cells were comparable (Table 3). There was no difference in the apoptosis of lymphocytes from various organs of lactating females at 4 and 14 days after delivery (Table 4). Thus, the profile of HSC differentiation had significantly changed at the beginning of pregnancy, just after delivery and in the late period of lactation. All these female-specific conditions were associated with the appearance of Abzs in the pregnant females, a very significant 6.1–9.3-fold increase in Abs RAs after the delivery, and a ∼2-fold decrease in Abz RAs 14 days after the delivery. As was shown previously, different Abzs with very low activity can sometimes be detected in the sera of healthy volunteers [2–5]. Moreover, we have found neither DNase nor ATPase Abzs in healthy mice, but the amylase activity in young mice, including the control non-AI mice, was detectable (Table 1). Thus, Abzs may be considered ubiquitous even in healthy individuals. At the same time, the production of different Abzs in healthy mammals is most probably suppressed due to apoptosis of specific AI lymphocytes. During pregnancy, the cell apoptosis significantly increased (Table 4), which may be the main reason of the increase in cell AI clones producing anti-DNA Abs and different Abzs (Table 1). Taken together, our data suggest that during both transition states, pre-disease and pregnancy, significant, but different changes in differentiation and proliferation of bone marrow cell progenitors and in cell apoptosis are observed (Table 2). A number of additional changes in HSC differentiation, cell proliferation or apoptosis following the two transition states (pre-disease and pregnancy) lead to comparable increases in auto-Abs and Abzs production for diseased or lactating mice, but it seems that there may be a significant difference in the specific characteristics of an immune system (e.g., clonal differentiation) in these cases in spite of some formal similarity in the HSC differentiation profiles of the lactating (group 12) and spontaneously diseased females (group 8, Table 2).
It should be mentioned that our data concerning pregnant and lactating mice reveal only some of different processes in their immune systems. Human and animal milk contains IgG, IgM, IgA, and sIgA, of which sIgA is the major component (> 85–90%) [51]. After the beginning of lactation, the bulk of women's Abz activities belong to milk IgGs and especially to sIgAs, while the specific activities of their serum Abzs are significantly lower ([21, 25] and rev. refs). sIgA is selectively produced by plasma cells residing in the interstitial tissue underlying the epithelial surfaces of the lactating breast [52]. These lymphoid cells migrate to the breast from the maternal intestinal lymphoid tissues (Peyer's patches) and from the lymphoid in the bronchial tree [52, 53]. The germinal-centre B cells of Peyer's patches express mainly surface IgA along with some IgM or IgG [53]. Such iso-type skewing is the result of B-cell heavy-chain gene switching in the course of clonal differentiation to the precursors of IgA-producing immunocytes, whose preferential induction is the hallmark of gut-associated lymphoid tissue [54]. Milk IgGs may be synthesized locally by such lymphoid tissue and also originate, at least partially, from the mother's circulation [55]. The serum Abs of AI-patients are produced mainly by B lymphocytes of different organs.
The literature data (see above) and our findings suggest that AI diseases originate from specific changes in differentiation and proliferation of HSC.From our point of view, there clearly may be a significant difference in the mechanisms of Abzs production and the biological roles of Abzs in lactating females and in patients with AI pathologies. After the beginning of lactation, the “‘immuno-memory’” of females can be specifically switched to activate the immunocytes in Peyer's patches and lymphoid tissue producing milk Abs, and the increase in Abzs activities in the serum associated with activated lymphocyte pro-liferation mainly in the thymus and the spleen (Table 3) may be to some extent a side effect, since the change in HSC differentiation in pregnant and lactating females could mainly be aimed to modify the activity of Peyer's patches. As mentioned above, in contrast to AI diseases, all auto-Abs and Abzs usually disappear after the end of lactation. It means that the changes in differentiation and proliferation of bone marrow HSC and cells in other tissues and organs occurring in AI patients cannot be easy normalized, while the easy silencing of the ‘immuno-memory’ in lactating women is a programmed phenomenon. Therefore, we infer that the formally similar profiles of HSC differentiation in lactating females 14 days after delivery and in spontaneously diseased females obscure a real existing difference in their immune status. In spite of the formal similarity of the immune system indexes for the diseased and lactating mice, AI reactions in lactating females are not associated with kidney dysfunction and proteinuria, and these groups can differ significantly in a specific cell composition of BFU-E, CFU-GEMM, and CFU-GM colonies and clonal differentiation of the specific lymphocyte precursors in different mouse organs. Nevertheless, our data support the hypothesis that the mechanisms of auto-Abs and Abzs production in ill and in pregnant or lactating females overlap to some extent. The suppression of auto-AI processes in lactating females after the end of lactation implies a possibility of existence of special mechanisms, absent in AI patients, that switch the immune system back to normal.
Acknowledgments
This research was made possible in part by grants from the Presidium of the Russian Academy of Sciences (Molecular and Cellular Biology Program, 10.5, Fundamental Sciences to Medicine Program, 11.8), Russian Foundation for Basic Research (04-04-48211), from RFBR-BFBR (04-04-81017) and funds from the Siberian Division of the Russian Academy of Sciences.
References
- 1.Keinan EE, editor. Catalytic antibodies. Weinheim: Wiley-VCH Verlag GmbH and Co. KgaA; 2005. pp. 1–586. [Google Scholar]
- 2.Nevinsky GA, Buneva VN. Human catalytic RNA-and DNA-hydrolysing antibodies. J Immunol Methods. 2002;269:235–45. doi: 10.1016/s0022-1759(02)00234-x. [DOI] [PubMed] [Google Scholar]
- 3.Nevinsky GA, Favorova OO, Buneva VN. Natural Catalytic Antibodies – New Characters in the Protein Repertoire. In: Golemis E, editor. Protein-protein interactions; a molecular cloning manual. New York: Cold Spring Harbor: Cold Spring Harbor Lab. Press; 2002. pp. 523–34. [Google Scholar]
- 4.Nevinsky GA, Buneva VN. Catalytic antibodies in healthy humans and patients with autoimmune and viral pathologies. J Cell Mol Med. 2003;7:265–76. doi: 10.1111/j.1582-4934.2003.tb00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nevinsky GA, Buneva VN. Natural catalytic antibodies-abzymes. In: Keinan E, editor. Catalytic antibodies. Weinheim: Wiley-VCH Verlag GmbH and Co. KgaA; 2005. pp. 503–67. [Google Scholar]
- 6.Paul S, Volle DJ, Beach CM, Johnson DR, Powell MJ, Massey RJ. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989;244:1158– 62. doi: 10.1126/science.2727702. [DOI] [PubMed] [Google Scholar]
- 7.Kalaga R, Li L, O'Dell JR, Paul S. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheuma-toid arthritis. J Immunol. 1995;155:2695–702. [PubMed] [Google Scholar]
- 8.Lacroix-Desmazes S, Bayry J, Kaveri SV, Hayon-Sonsino D, Thorenoor N, Charpentier J, Luyt CE, Mira JP, Nagaraja V, Kazatchkine MD, Dhainaut JF, Mallet VO. High levels of catalytic antibodies correlate with favourable outcome in sepsis. Proc Natl Acad Sci USA. 2005;102:4109–13. doi: 10.1073/pnas.0500586102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savel'ev AN, Kanyshkova TG, Kulminskaya AA, Buneva VN, Eneyskaya EV, Filatov MV, Nevinsky GA, Neustroev KN. Amylolytic activity of IgG and sIgA immunoglobulins from human milk. Clin Chim Acta. 2001;314:141–52. doi: 10.1016/s0009-8981(01)00691-x. [DOI] [PubMed] [Google Scholar]
- 10.Saveliev AN, Ivanen DR, Kulminskaya AA, Ershova NA, Kanyshkova TG, Buneva VN, Mogelnitskii AS, Doronin BM, Favorova OO, Nevinsky GA, Neustroev K. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol Lett. 2003;86:291–7. doi: 10.1016/s0165-2478(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 11.Saveliev AN, Ivanen DR, Kulminskaya AA, Ershova NA, Kanyshkova TG, Buneva VN, Mogelnitskii AS, Doronin BM, Favorova OO, Nevinsky GA, Neustroev K. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol Lett. 2003;86:291–7. doi: 10.1016/s0165-2478(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 12.Andrievskaya OA, Buneva VN, Naumov VA, Nevinsky GA. Catalytic heterogeneity of polyclonal RNA-hydrolyzing IgM from sera of patients with lupus erythematosus. Med Sci Monit. 2000;6:460–70. [PubMed] [Google Scholar]
- 13.Baranovsky AG, Matushin VG, Vlassov AV, Zabara VG, Naumov VA, Buneva VN, Nevinsky GA. DNA-and RNA-hydrolyzing antibodies from the blood of patients with various forms of viral hepatitis. Biochemistry. 1997;62:1358–66. [PubMed] [Google Scholar]
- 14.Baranovskii AG, Kanyshkova TG, Mogelnitskii AS, Naumov VA, Buneva VN, Gusev EI, Boiko AN, Zargarova TA, Favorova OO, Nevinsky GA. Polyclonal antibodies from blood and cerebrospinal fluid of patients with multiple sclerosis effectively hydrolyze DNA and RNA. Biochemistry. 1998;63:1239–48. [PubMed] [Google Scholar]
- 15.Vlassov A, Florentz C, Helm M, Naumov V, Buneva V, Nevinsky G, Giege R. Characterization and selectivity of catalytic antibodies from human serum with RNase activity. Nucl Acid Research. 1998;26:5243–50. doi: 10.1093/nar/26.23.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odintsova ES, Buneva VN, Nevinsky GA. Casein-hydrolyzing activity of sIgA antibodies from human milk. J Mol Recognit. 2005;18:413–21. doi: 10.1002/jmr.743. [DOI] [PubMed] [Google Scholar]
- 17.Paul S. Mechanism and functional role of antibody catalysis. Appl Biochem Biotechnol. 1998;75:13–23. doi: 10.1007/BF02787705. [DOI] [PubMed] [Google Scholar]
- 18.Kozyr AV, Kolesnikov A, Aleksandrova ES, Sashchenko LP, Gnuchev N, Favorov PV, Kotelnikov MA, Iakhnina EI, Astsaturov IA, Prokaeva TB, Alekberova ZS, Suchkov SV, Gabibov AG. Novel functional activities of anti-DNA auto-antibodies from sera of patients with lymphopro-liferative and autoimmune diseases. Appl Biochem Biotechnol. 1998;75:45–61. doi: 10.1007/BF02787708. [DOI] [PubMed] [Google Scholar]
- 19.Sinohara H, Matsuura K. Does catalytic activity of Bens-Jones proteins contribute to the pathogenesis of multiple myeloma. Appl Biochem Biotechnol. 2000;83:85–94. doi: 10.1385/abab:83:1-3:85. [DOI] [PubMed] [Google Scholar]
- 20.Polosukhina DI, Buneva VN, Doronin BM, Tyshkevich OB, Boiko AN, Gusev EI, Favorova OO, Nevinsky GA. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol Lett. 2006;103:75–81. doi: 10.1016/j.imlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Buneva VN, Kudryavtseva AN, Gal'vita AV, Dubrovskaya VV, Khokhlova OV, Kalinina IA, Galenok VA, Nevinsky GA. Dynamics of antibody nuclease activity in blood of women during pregnan-cy and lactation. Biochemistry. 2003;68:890–900. doi: 10.1023/a:1025703132523. [DOI] [PubMed] [Google Scholar]
- 22.Kanyshkova TG, Semenov DV, Khlimankov D, Buneva VN, Nevinsky GA. DNA-hydrolyzing activity of the light chain of IgG antibodies from milk of healthy human mothers. FEBS Lett. 1997;416:23–6. doi: 10.1016/s0014-5793(97)01163-0. [DOI] [PubMed] [Google Scholar]
- 23.Nevinsky GA, Kanyshkova TG, Semenov DV, Vlassov AV, Gal'vita AV, Buneva VN. Secretory immunoglobulin A from healthy human mothers' milk catalyses nucleic acid hydrolysis. Appl Biochem Biotechnol. 2000;83:115–29. doi: 10.1385/abab:83:1-3:115. [DOI] [PubMed] [Google Scholar]
- 24.Buneva VN, Kanyshkova TG, Vlassov AV, Semenov DV, Khlimankov D, Breusova LR, Nevinsky GA. Catalytic DNA- and RNA-hydrolysing antibodies from milk of healthy human mothers. Appl Biochem Biotechnol. 1998;75:63–76. doi: 10.1007/BF02787709. [DOI] [PubMed] [Google Scholar]
- 25.Semenov DV, Kanyshkova TG, Karotaeva NA, Krasnorutskii MA, Kuznetsova IA, Buneva VN, Nevinsky GA. Catalytic nucleotide-hydrolysing anti-bodies in milk and serum of clinically healthy human mothers. Med Sci Monit. 2004;10:BR23–33. [PubMed] [Google Scholar]
- 26.Nevinsky GA, Kit Y, Semenov DV, Khlimankov D, Buneva VN. Secretory immunoglobulin A from human milk catalyses milk protein phosphorylation. Appl Biochem Biotechnol. 1998;75:77–91. doi: 10.1007/BF02787710. [DOI] [PubMed] [Google Scholar]
- 27.Gorbunov DV, Karataeva NA, Buneva VN, Nevinsky GA. Lipid kinase activity of antibodies from milk of clinically healthy human mothers. Biochim Biophys Acta. 2005;1735:153–66. doi: 10.1016/j.bbalip.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Karataeva NA, Gorbunov D, Prokudin IV, Buneva VN, Kulminskaya AA, Neustroev KN, Nevinsky GA. Human milk antibodies with polysaccharide kinase activity. Immunol Lett. 2006;103:58–67. doi: 10.1016/j.imlet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Amino N, Mori H, Iwatani Y, Tanizawa O, Kawashima M, Tsuge I, Ibaragi K, Kumahara Y, Miyai K. High prevalence of transient post-partum thyrotoxicosis and hypothyroidism. N Engl J Med. 1982;306:849–52. doi: 10.1056/NEJM198204083061405. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka A, Lindor K, Ansari A, Gershwin ME. Fetal microchimerisms in the mother: immunologic implications. Liver Transpl. 2000;6:138–43. doi: 10.1002/lt.500060225. [DOI] [PubMed] [Google Scholar]
- 31.Redhead K, Hill T, Mulloy B. Antimicrobial effect of human milk on Bordetella pertussis. FEMS Microbiol Let. 1990;58:269–73. doi: 10.1111/j.1574-6968.1990.tb13987.x. [DOI] [PubMed] [Google Scholar]
- 32.Gillin FD, Reiner DS, Wang CS. Human milk kills parasitic intestinal protozoa. Science. 1983;221:1290–2. doi: 10.1126/science.6310751. [DOI] [PubMed] [Google Scholar]
- 33.Founel S, Muller S. Antinucleosome antibodies and T cell response in systemic lupus erythematosus. Ann Med Interne. 2002;153:513–9. [PubMed] [Google Scholar]
- 34.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe-Fukunada R, Brannan CI, Copeland NG. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 36.Nishi Y. Evolution of catalytic antibody repertoire in autoimmune mice. J Immunol Methods. 2002;269:213–33. doi: 10.1016/s0022-1759(02)00233-8. [DOI] [PubMed] [Google Scholar]
- 37.Tawfik DS, Chap R, Green BS, Sela M, Eshhar Z. Unexpectedly high occurrence of catalytic antibodies in MRL/lpr and SJL mice immunized with a transition-state analog: is there a linkage to autoimmunity? Proc Natl Acad Sci USA. 2002;92:2145–9. doi: 10.1073/pnas.92.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponomarenko NA, Durova OM, Vorobiev II, Telegin GV, Chamborant OG, Sidorik LL, Suchkov SV, Alekbarova ZS, Gnuchev NV, Gabibov AG. Catalytic antibodies in clinical and experimental pathology: human and mouse models. J Immunol Methods. 2002;269:197–221. doi: 10.1016/s0022-1759(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 39.Mouratou B, Rouyre S, Guedson JL. A method for the detection and screening of catalytic anti-DNA antibodies. J Immunol Methods. 2002;269:147–55. doi: 10.1016/s0022-1759(02)00231-4. [DOI] [PubMed] [Google Scholar]
- 40.Kim JS, Lee SY, Lee WR, Sohn JN, Chung YC, Shim HK, Lee SC, Kwon MH, Kim YS. Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity. J Biol Chem. 2006;281:15287–95. doi: 10.1074/jbc.M600937200. [DOI] [PubMed] [Google Scholar]
- 41.Dubrovskaya VV, Andryushkova AS, Kuznetsova IA, Toporkova LB, Buneva VN, Orlovskaya IA, Nevinsky GA. DNA-hydrolyzing antibodies from sera of autoimmune-prone MRL/MpJ-lpr mice. Biochemistry. 2003;68:1081–8. doi: 10.1023/a:1026350426842. [DOI] [PubMed] [Google Scholar]
- 42.Andryushkova AA, Kuznetsova IA, Orlovskaya IA, Buneva VN, Nevinsky GA. Antibodies with amylase activity from the sera of autoimmune-prone MRL/MpJ-lpr mice. FEBS Lett. 2006;580:5089–95. doi: 10.1016/j.febslet.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 43.Ikehara S, Kawamura M, Takao F. Organ-specific and systemic autoimmune diseases originate from defects in haematopoietic stem cells. Proc Natl Acad Sci USA. 1990;87:8341–4. doi: 10.1073/pnas.87.21.8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toporkova LB, Dubrovskaya VV, Sakhno LV, Tikhonova M, Chernykh ER, Buneva VN, Nevinsky GA, Kozlov VA, Orlovskaya IA. Hematopoetic progenitors colony formation in immunopathogenesis of MRL/MpJ-lpr mice. Russ J Immunol. 2002;7:245–50. [PubMed] [Google Scholar]
- 45.Nicoletti G, Migliorati MC, Pagliacci K. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–5. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 46.Fey H, Butler R, Marti F. The production in the pregnant cow of anti-human immunoglobulin to be used for the antiglobulin test. Vox Sang. 1973;25:245–53. doi: 10.1111/j.1423-0410.1973.tb04369.x. [DOI] [PubMed] [Google Scholar]
- 47.Mestecky J, McGhee JR. Immunoglobulin A. IgA: molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 48.Kazakov VI, Bozhkov VM, Linde VA, Repina MA, Mikhailov VM. Extracellular DNA in the blood of pregnant women. Tsitologiia. 1995;37:232–6. [PubMed] [Google Scholar]
- 49.Newman J. How breast milk protects newborns. Sci Am. 1995;273:76–9. doi: 10.1038/scientificamerican1295-76. [DOI] [PubMed] [Google Scholar]
- 50.Freeman R, Rosen H, Thysen B. Incidence of thyroid dysfunction in an unselected postpartum population. Arch Intern Med. 1986;146:1361–4. [PubMed] [Google Scholar]
- 51.Hanson LA, Carlsson B, Cruz JR. Colostrum-derived immunity and maternal–neonatal interaction. In: Ogra PL, Dayton DH, editors. Immunology of Breast milk. New York: Raven Press; 1979. pp. 145–57. [Google Scholar]
- 52.Roux ME, McWilliams M, Phillips-Quagliata JM, Weisz-Carrington P, Lamm ME. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977;146:1311–22. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butcher EC, Rouse RV, Coffman RL, Nottenburg CN, Hardy RR, Weissman IL. Surface phenotype of Peyer's patch germinal centre cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982;129:2698–707. [PubMed] [Google Scholar]
- 54.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim K, Keller MA, Heiner DC. Immunoglobulin G subclasses in human colostrum, milk and saliva. Acta Paediatr. 1992;81:113–8. doi: 10.1111/j.1651-2227.1992.tb12185.x. [DOI] [PubMed] [Google Scholar]


