Abstract
The tight junctions (TJs) are key players in the control of blood-brain barrier (BBB) properties, the most complex TJs in the vascular system being found in the endothelial cells of brain capillaries. One of the main TJs proteins is occludin, which anchors plasma membranes of neighbour cells and is present in large amounts in the brain endothelia. Previous studies demonstrated that disruption of BBB in various pathological situations associates with changes in occludin expression, and this change could be responsible for malfunction of BBB. Therefore in this study, applying an immunohistochemical approach, we decided to explore the occludin expression in frontal cortex (FC) and basal ganglia in ageing control, Alzheimer's disease (AD), and vascular dementia (VD) brains, as far as all these pathologies associate microangiopathy and disruption of BBB. Strikingly, we found selected neurons, astrocytes and oligodendrocytes expressing occludin, in all cases studied. To estimate the number of occludin-expressing neurons, we applied a stereological approach with random systematic sampling and the unbiased optical fractionator method. We report here a significant increase in ratio of occludin-expressing neurons in FC and basal ganglia regions in both AD and VD as compared to ageing controls. Within the cerebral cortex, occludin was selectively expressed by pyramidal neurons, which are the ones responsible for cognitive processes and affected by AD pathology. Our findings could be important in unravelling new pathogenic pathways in dementia disorders and new functions of occludin and TJs.
Keywords: occludin, blood brain barrier, intercellular tight junctions, Alzheimer, vascular dementia, cerebral angiopathy, CADASIL quantification/stereology
Introduction
The blood brain barrier (BBB) is a physical and metabolic barrier, a specialized system of capillary endothelial cells essential for the maintenance and regulation of the neuronal microenvironment, anatomically formed by components of the ‘neurovascular unit’[1, 2, 3]. The permeability of the BBB is determined by the interendothelial junctions. In mammalian cells, the intercellular junctions are categorized in: tight junctions (TJs), adherent junctions and gap junctions [4].
One of the most important characteristics of the microvasculature of the central nervous system (CNS) is the cell-to-cell contact between two adjacent endothelial cells of the cerebral capillaries through the TJs [5]. In the brain of vertebrates there are two types of cells that bear TJs or TJs-like structures: vascular endothelial cells and oligodendrocytes [6]. The TJs of endothelial cells consists of narrow belt-like structures in the apical region of the lateral plasma membrane circumferentially wrapping each cell and connecting the neighbours [7]. The function of TJs is not only to separate the apical from the basolateral plasma membrane (‘fence function’), but also to restrict the flow through the paracellular pathway (‘gate/barrier function’) [8].
At the BBB level, the endothelial cells of brain capillaries possess the most complex TJs in the vascular system [9], junctions that are thought to be one of the main factors responsible for the tightness of the BBB [10]. The molecular components of TJs are important factors controlling the intramembrane diffusion and maintaining the structural and functional polarity of the endothelial cells [11, 12]. Stabilization of TJs involves a complex network of transmembrane and peripheral proteins, such as occludin, claudin family members, junctional adhesion molecules (JAMs) 1–3, cingulin, spectrin and zonula occludens (ZO)-family members, in which these proteins bind to each other and to the actin cytoskeleton [13].
Occludin is a 65 kD transmembrane protein localized to TJs which anchors ZO-1 and ZO-2 proteins and the plasma membranes of adjacent cells [4]. Occludin is present in large amounts in brain endothelial cells, being undetectable in non-neuronal endothelia [14]. Since it was suggested that the occludin molecules tightly obliterate the interendothelial space, occludin is used as the marker of choice in most pathological studies of BBB [15, 16]. It has also been demonstrated that disruption of BBB in various pathological situations associates with changes in occludin expression [17–20]. A detectable occludin expression was also identified in primary and secondary cultures of neurons and astrocytes from adult mouse [21], being suggested that astrocytes and neurons are capable of retaining or regaining their neuroepithelial characteristics in vitro.
Aging in human beings is associated with significant structural and functional alterations in the BBB and dysregulation of specific TJs proteins was suggested [22, 23]. Age has a strong impact on dementia incidence, an exponential rise to the age of 90 being reported in epidemiological studies [24]. Disruption of BBB is also thought to play an important role in the pathogenesis of cerebral microangiopathy [25] and patients with microangiopathy present white matter changes that ultimately lead to impairment of neuronal flow and cognitive decline in both vascular dementia (VD) and Alzheimer's disease (AD) [26]. Moreover, in addition to cerebral amyloid angiopathy AD patients show profound changes in cerebral microvessels often independent of amyloid deposition [27]. Conversely, it was suggested that amyloid beta (Aβ) effects on TJs protein complexes might be responsible for alteration of BBB integrity and neuropathological findings in AD [28].
Hypertension is one of the main risk factors for VD and a disturbed fence function of the TJs was demonstrated in stroke hypertensive rats at the level of blood–brain barrier endothelial cells [29]. Furthermore oxidative stress, a common pathogenic feature of both dementia types [30], induces a down regulation of the occludin expression that leads to BBB breakdown [31].
The role of TJs proteins is poorly understood in dementia disorders. It is also uncertain whether changes in BBB are a cause or an effect of disease progression [32]. The aim of this study was to comparatively analyse the expression of occludin, one of the main TJs proteins, in human brains of control cases and of several dementia conditions: AD, VD, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and congophilic amyloid angiopathy (CAA).
Materials and methods
The brain material was obtained from the Huddinge Brain Bank, Stockholm, Sweden, in accordance with Swedish law and with the permission of the Karolinska University Hospital Ethical Committee Two brain regions of interest were analysed, with specific anatomical and haemodynamic conditions: cerebral cortex (frontal, Brodmann's area 46/9) and the head of the nucleus caudatus.
The study was based on evaluation of five aging control, 4 AD, 6 VD, 2 CADASIL and 2 CAA postmortem human brains. The control group included the brains of traffic-accident patients or of patients who had no history of long-term illness or neuropsychiatric disorders. The AD group included brains from patients with clinically and pathologically confirmed AD. Clinical diagnosis was based on combined Diagnostic and Statistical Manual of Mental Disorders (DSM)-III-R [33] and National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [34]. The definite neuropathological diagnosis of AD was determined by using Consortium to Establish a Registry for Alzheimer's Disease (CERAD) and National Institute on Aging (NIA)-Reagan Institute Criteria [35, 36]. The VD group included brains from patients with clinically and pathologically diagnosis of VD. The VD group consisted of two cases of single strategic stroke, three cases with dementia multi-infarct and one case with diffuse white matter changes.We also analysed qualitatively two cases of CADASIL and two cases of CAA. Age and sex of all cases are presented in Table 1.
1.
Age and sex of cases analyzed in each study group
| Group | Age | Sex | ||
|---|---|---|---|---|
| Control | 90 | F | ||
| 88 | F | |||
| 86 | F | |||
| 56 | M | |||
| 70 | M | |||
| AD | 87 | F | ||
| 86 | M | |||
| 91 | F | |||
| 72 | F | |||
| VD | 87 | F | ||
| 86 | F | |||
| 65 | M | |||
| 72 | M | |||
| 89 | F | |||
| 77 | F | |||
| CADASIL | 33 | M | ||
| 63 | F | |||
| ICA | 79 | M | ||
| 78 | M | |||
Sectioning and histology
Immunostaining with antibodies raised against occludin was performed on buffered formaldehyde-fixed embedded sections (long-fixation time sections were used). A series of thin sections (7 μm) were cut exhaustively in a randomly chosen saggital plane on the microtome. After the deparaffinization and rehydration procedures the sections were rinsed in distilled water for 5 min. and than heated at 80°C for 20 min in 0.1 M citrate buffer of pH 9. After cooling, the sections were incubated with the primary antibody solution overnight (approximately 16 hrs, dilution 1:100) at 4°C. The primary antibody was a rabbit polyclonal antiserum raised against the C-terminal 150 amino acids of human occludin (Zymed Laboratories, Inc., South San Francisco, CA, USA). Thereafter, the sections were treated with a biotiny-lated secondary antibody (1:300, Vector Laboratories, Burlingame, CA, USA) and with the avidin–biotin-peroxi-dase complex kit (Vector, Burlinggame, CA, USA) with 3,3'-diaminobenzidine-4HCl/H2O2 (DAB, Sigma, St.Louis, MO, USA) as a substrate. Next, the sections were counter-stained with haematoxylin–eosin stain for background. Two types of negative controls slides were run for all the sections (for both frontal and striatal regions). The negative controls were run in an identical manner except the incubation with the primary antibody. For the first set of negative controls we omitted the primary antibody, whereas for the second set we added instead of the primary antibody an unspecific immunoglobulin serum at the same concentration. The sections were then mounted and examined under a Nikon microscope.
Quantification of neurons
To estimate the number of occludin-expressing neurons, we applied a stereological approach on 2-dimension quantification with random systematic sampling and the unbiased optical fractionator method [37], similarly to a previous study [38]. It is known that with an optical dissector probe it is possible to sample isolated particles, in our case neurons, with a uniform probability in the bidimensional space regardless the size, shape or orientation of the tissue [37]. In brief, the area of interest was delineated in low magnification (x 2.5) using the cursor. Due to the clear anatomical borders, we were able to distinguish the grey matter from the white matter. A meander sampling function of the GRID v2.0 program (Olympus, Denmark) was used for stepping through the delineated region with a chosen counting frame. Then, a 100-x oil-immersion objective with a numerical aperture of 1.40 was moved into place and the appropriate counting frame superimposed on the screen. The desired horizontal and vertical step lengths, assisted by a highly precise servo-controlled motorized microscopy stage, were dimensioned for the appropriate distance [231.22 μm (x-step) × 231.22 μm (y-step)] in between the counting frames (1603.9 μm2). The cells in the space were counted by an optical dissector probe (z-axis). The optical dissector uses the simple rule that a neuron is counted if its body cell is in the counter-frame. This procedure ensured the selection of a systematic random sample of sections. The number of neurons was express as a number per squared millimetre.
The neurons could be easily distinguished from the other types of cells due to the presence of the round nucleus with visible cytoplasm and a single large nucleolus, no heterochromatin [39].
Statistical analysis
Statistical analyses of the results were performed using JMP 4 software (SAS Institute, Cary, NC, USA). The neuronal ratio was defined as (number of occludin-expressing neurons/total number of neurons) × 100. To analyse the global variation of the neuronal ratio one-way ANOVA was used. To compare the neuronal ratio between controls, AD and VD (two by two) the Tukey–Kramer honestly significant difference (HSD) test has been used. The CADASIL and CAA cases were not used for statistical analysis, due to the small sample size, but we used them instead for a qualitative description. To compare the neuronal ratio between the frontal and striatal regions, we used the paired Student's t-test. Precision of estimates was evaluated by calculating the coefficient of error (CE = SEM/mean, where SEM represents the standard error of mean).
Results
Occludin expression in neuronal cells
Our first observation was that expression of occludin was increased in neurons from AD and VD brains as compared to control brains, in both frontal and striatum (STR) regions (Table 2 and Fig. 1). The occludin-expressing neurons were in all cases predominantly pyramidal. The neuronal fibres were also stained more intensely in AD and VD as compared to control (Fig. 1). In CADASIL and CAA, we did not notice any difference in neuronal occludin expression as compared to control cases (Fig. 1).
2.
Quantification of total number of neurons and occludin-expressing neurons (positive neurons) in frontal cortex (FC) and striatum (STR) regions in control (5 cases), Alzheimer's disease (AD, 4 cases) and vascular dementia (VD, 6 cases) groups. All values were truncated to two decimals
| Group | Region | Total number of neurons | Number of positive neurons | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | SD | SEM | CE | mean | SD | SEM | CE | ||||||||||||||||||||||||||||||||||||||||||||||
| CONTROL | FC | 23.17 | 3.14 | 1.57 | 6.79 | 5.58 | 1.52 | 0.76 | 13.65 | ||||||||||||||||||||||||||||||||||||||||||||
| STR | 31.58 | 4.32 | 2.16 | 6.84 | 1.04 | 0.08 | 0.04 | 4 | |||||||||||||||||||||||||||||||||||||||||||||
| AD | FC | 20.67 | 4.55 | 2.27 | 11 | 11.25 | 3.13 | 1.57 | 13.92 | ||||||||||||||||||||||||||||||||||||||||||||
| STR | 34.33 | 4.22 | 2.11 | 6.14 | 18.88 | 2.76 | 1.38 | 7.31 | |||||||||||||||||||||||||||||||||||||||||||||
| VD | FC | 22 | 5.56 | 2.78 | 12.65 | 11.25 | 2.71 | 1.36 | 12.06 | ||||||||||||||||||||||||||||||||||||||||||||
| STR | 24.8 | 10.57 | 4.32 | 17.78 | 9.28 | 8.57 | 3.5 | 37.69 | |||||||||||||||||||||||||||||||||||||||||||||
SD = standard deviation, SEM = standard error of the mean, CE = coefficient of error (multiplied by 100).
1.
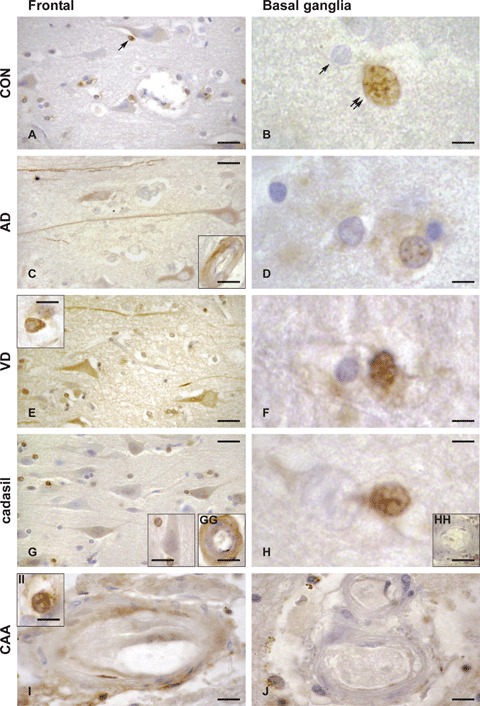
Expression of occludin in control (CON, A, B), Alzheimer's disease (AD, C, D), vascular dementia (VD, E, F), CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, G, GG, H, HH) and CAA (congophilic amyloid angiopathy, I, II, J) brains, in the frontal medial gyrus Brodmann's area 9 (left panels, A, C, E, G, GG, I, II), and basal ganglia, nucleus caudatus (right panels, B, D, F, H, HH, J). Occludin-expressing cells are brownish, as a result of the immunohistochemical staining method with anti-occludin serum detected with avidin–biotin-peroxidase complex kit and DAB substrate. As compared to control brain, in AD and VD brains, occludin expression was increased in neurons and neuronal fibres in the frontal cortex (left panels, A, C, E, G), and in the glial cells of the basal ganglia (right panels, B, D, F, H). Inserts show occludin expression within the brain microvasculature (transversal sections) which is seen only in selected endothelial cells in AD (C) and CAA (I) and in the entire vessel wall in CADASIL (GG). The occludin expression pattern in neurons, astrocytes and oligodendrocytes was similar to control for both CADASIL and CAA cases. Conversely, as compared to control, a more intense occludin expression was noted in the endothelium of CADASIL and CAA cases, mainly in the frontal cortex region. Occludin expression was noticed only in the pyramidal neurons (layer III in the figure) in AD and VD, but not in controls, CADASIL or CAA. Some selected oligodendroglial cells were occludin-positive in the frontal cortex region (arrow in A) in all cases analysed. Occludin-positive astrocytes were identified in VD (E, insert) and in CAA (II). Occludin expression was detected in the microvasculature of frontal cortex in AD (C, insert), CADASIL (GG) and sparsely in CAA (I), but not in control or VD. In the basal ganglia region, intranuclear staining was found almost only in astrocytes in control (B, double arrows, absence in oligodendrocytes denoted by single arrow), AD (D), VD (F) and CADASIL (H) brains. Occludin expression was undetectable in arteries of basal ganglia (nucleus caudatus) in all control and diseased brains (CADASIL, HH and CAA, J). Bars: 5 μm in B, D, F, H and II, 10 μm in E insert, G insert, GG, HH, I and J, 15 μm in A, C, E and G and 20 μm in C, insert.
Subsequently, we used a quantitative approach in order to estimate the number of occludin-expressing neurons in control, AD and VD groups, in FC and STR regions. Table 2 presents comparatively the quantitative data of neurons/mm2 and occludin-positive neurons/mm2 in FC and STR of control, AD and VD groups. The number of neurons did not significantly differ between the groups analysed. Conversely, the statistical analysis showed that there was a significant difference (P < 0.01) for the occludin-positive neurons concerning the global variation both in frontal and striatal region. The area chosen for counting was similar in all cases (with around 100 counting frames/area) and there was no significant difference in the density (number of neurons/area) between the cases analysed. Qualitatively there were no analysed areas where heterogeneity in distribution of occludin was observed.
In the control group, we found a significantly higher ratio of occludin-positive neurons in the FC region (23.97 ± 4.52%) as compared to the striatal region (3.32 ± 0.21%), as shown in Fig. 3 (P < 0.01). In contrast, in AD and VD groups we found no significant difference in ratio of occludin-positive neurons between the frontal and striatal regions (Fig. 3).
3.

Comparative ratios between number of occludin-expressing neurons and total number of neurons in frontal cortex and striatum regions in control (five cases), Alzheimer's disease (AD, four cases) and vascular dementia (VD, six cases). The ratio was calculated with the formula: Ratio = (number of occludin-positive neurons/total number of neurons) × 100. Error bars indicate standard deviation (SD). SD in VD is higher due to the differences in vascular subtype pattern among VD cases. Statistical significance is marked by the following symbols: ‘§’ for comparison between frontal cortex and striatum regions in the control group (p < 0.01), ‘#’ for comparison between frontal region of each AD and VD groups and frontal region in control group (p < 0.05) and ‘*’ for comparison between striatal region of each AD and VD groups and striatal region in control group (p < 0.05).
The ratio of occludin-expressing neurons was significantly higher in the FC region and in the striatal region in the AD group (54.25 ± 6.04, P < 0.05 and 54.49 ± 5.22, P < 0.05, respectively) and in the VD group (51.71 ± 6.13, P < 0.05 and 34.86 ± 27.66, P < 0.05, respectively) as compared to the control group (23.97 ± 4.52 and 3.32 ± 0.21, respectively), as shown in Fig. 3. We did not find any significant differences in neuronal ratios between AD and VD groups in FC and in striatal regions.
We also noticed that within VD group, the highest ratio of occludin-expressing neurons in the STR was obtained for the dementia multi-infarct cases (multiple basal ganglia lesions), as compared with the single strategic infarct and diffuse white matter cases, but due to the limited number of cases per each subgroup the statistical analysis was not performed separately.
OccludinF expression in glial cells
Occludin expression in glial cells was evaluated qualitatively only. In the grey matter of the FC, selective occludin staining of the oligodendroglial cells was found in all diseased and control brains. In the basal ganglia, more astrocytes were positive for occludin than oligodendrocytes. In this region, a positive occludin expression was obvious preferentially in the nucleus (Fig. 1). In the frontal white matter, we were able to identify more occludin-positive oligodendrocytes and astrocytes in AD and VD as compared to the control group (Fig. 2).
2.
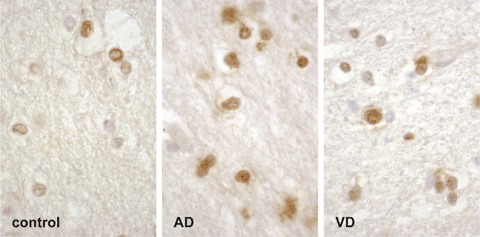
Expression of occludin in control, Alzheimer's disease (AD) and vascular dementia (VD) brains, in the frontal white matter. As compared to control brain, in AD and VD brains, occludin expression is increased in oligodendrocytes and astrocytes (brownish, immunohis-tochemical staining method with anti-occludin serum detected with avidin–biotin-peroxidase complex kit and DAB substrate). Quantification was not performed in this study.
Occludin expression in vessels
At the brain microvasculature level, in the FC of AD and control brains, we observed that occludin expression localized selectively in some of the endothelial cells or in the media layer (Fig. 1, C insert). Conversely, in VD and CADASIL brains, occludin expression localized in most of the vessel wall (Fig. 1, E insert and GG). No occludin staining was noted within the striatal arteries wall.
Occludin in CADASIL and CAA
Observation of the CADASIL and CAA cases showed that the expression of occludin in neurons, oligodendrocytes and astrocytes was similar to control in both regions analysed. However, as compared to control, a more intense occludin staining was obvious throughout the entire arterial wall in CADASIL FC, but not in CADASIL basal ganglia (Fig. 1, GG and H). In CAA, we found a patchy-like occludin staining in the endothelial region of cortical arteries, but a similar to control staining pattern in the basal ganglia. (Fig. 1, I and J).
Discussion
Several lines of evidence suggest that occludin is a protein located selectively in the cerebral endothelium at the level of TJs [29, 40], where it seems to regulate BBB functions [41, 42]. However, occludin expression was also identified in primary and secondary cultures of neurons and astrocytes from adult mouse [21] and it has been demonstrated that neurons and astrocytes regulate synthesis and distribution of occludin in brain capillary endothelial cells in co-cultures [43, 44]. However, other functions were suggested for occludin as well, like involvement in glucose-transport [45], recovery of the blood–nerve barrier [46] and developmental remodelling of the neuroepithelium [47].
To our knowledge, in this study we show for the first time that occludin is expressed by neurons in human brain. In AD brains, we found an increased occludin expression in neurons in both FC and basal ganglia regions in an area- and cell-specific manner. It is particularly remarkable that in the aged control brains almost 1/4th of neuronal cells (mainly pyramidal neurons) in the FC express occludin. In contrast, only 3% of the neurons in basal ganglia are occludin-positive. In dementia conditions, such as AD and VD, expression of occludin in neurons doubled or increased for at least 15 times in cortex and basal ganglia, respectively. This many-fold differences could actually prove a region-specific difference in neural plasticity potential.
The distinct ratio of occludin-expressing neurons in FC and basal ganglia regions in controls could be explained by distinct microvasculature properties and a dissimilar regulation of the neurovascular unit. It is well known, for instance, that corpus STR is more sensitive to ischaemic injury than the cerebral cortex [48] and that striatal arteries are of end-type, with a poor collateral network [49]. Therefore, it is perfectly possible that occludin expression in neurons is regulated also by factors depending on neurovascular coupling, which is different in the striatal and cortical areas. Furthermore, our study shows a rise in the ratio of occludin-expressing neurons in the basal ganglia in AD and VD as compared to control. It is now largely accepted that vascular lesions in the territory of lenticulostriate arteries are a common pathological finding not only in VD, but also in AD [50]. It is also worthy to note that occludin expression in basal ganglia differs among the sub-types of VD. Despite the clinical diagnose of VD, it is to believe that the patho-morphological base of each VD subtype is distinct and therefore it could associate a different occludin expression pattern.
Many studies showed that all mitogen-activated protein kinase (MAPK) pathways are activated in vul-nerable neurons in AD brains [51] and a recent report identified p38 MAPK and extracellular signal-regulated protein kinase (ERK)1/ERK2 as being responsible for regulation of occludin expression [52]. Under these circumstances, it is probable that MAPK-pathways upregulation is responsible for the two-fold-increase in neuronal occludin expression in AD. Moreover, the level of basic fibroblast growth factor (FGF-2) is markedly elevated in AD brains [53] and, on the other hand, in FGF-2-/-/FGF-5-/- double mutant mice, a decrease of occludin expression and a defect in BBB functioning were reported [54].Therefore, it is tempting to speculate that in AD, the high FGF levels trigger an increase in occludin expression, with a possible protective role.
The similarities of high occludin expression and distribution found in both AD and VD could be explained by signalling factors that are equally up regulated in these diseases. One example is the vascular endothelial growth factor (VEGF), which is itself a hypoxia-inducible angiogenic factor that was found elevated in cerebrospinal fluid (CSF) of AD and VD patients as compared to controls [55]. Moreover, it was suggested that polymorphisms within the promoter region of the VEGF gene confer greater risk for AD [56] and neuronal availability of VEGF seems to be reduced by deposition within the senile plaques [57]. A recent report demonstrated that VEGF increases the endothelial permeability by protein kinase C (PKC)-dependent phosphorylation of occludin [58]. Therefore, it is possible that a high VEGF production dysregulates the occludin to a higher and non-functional phosphorylation state in both AD and VD. In turn, an increased syn-thesis could occur to compensate the TJs function.
Moreover, we found a high expression of occludin not only in neurons, but also in astrocytes and oligo-dendrocytes, which means that in different types of CNS cells the mechanisms regulating occludin expression are similar, as far as they respond to the same pathogenic or compensatory factors. In contrast, regulation of occludin in the cerebral microvas-culature did not parallel its regulation in neurons, as far as we found no marked difference in the vessel-staining pattern between control and AD brains. Instead, we showed qualitatively an increase of vascular occludin expression in VD, CADASIL and CAA. These distinct pathways of regulation of the same protein in different brain structures is not new to CNS biology, being seen for instance with the endothelial and neuronal isoforms of nitric oxide synthase [59]. Furthermore, it was recently shown that treatment with the fibrillogenic form of β-amyloid peptide decreases endothelial expression of occludin [28].We may speculate that this mechanism could be involved in the regional and cellular differences seen in our study between occludin expression in microvasculature in AD and VD. However, it was recently reported that in cultured cerebral endothelial cells, experimentally-induced oxidative stress down-regulates occludin expression [31], result that is in apparent conflict to the increased microvascular occludin expression found in VD, CADASIL and CAA brains in our study. Even though oxidative stress could occur in endothelial cells in virtually all vascular brain pathologies, the temporal evolution and intensity of exposure to oxidative stress is completely different in these diseases as compared to an experimental paradigm observed for hours. For instance, it could be the case that oxidative stress down-regulates occludin by an early effect on transcription which results as well in BBB permeability alteration, followed by an adaptive increase in occludin expression at a later stage.
We have also to consider that besides the canonical, 504 amino acids occludin form, other alternative transcripts have been reported, as at least a shorter variant, lacking the fourth transmembrane domain (TM4−) [60] and a longer variant, with a N-terminal sequence of 56 amino acids (1B) [61]. Due to the fact that the antiserum used in our study recognizes the C-terminal 150 amino acids of occludin, it is very probable not to distinguish between the canonical form of occludin and the variant transcripts. Therefore, based on the results of this study, we cannot conclude on the type of occludin transcript which is found in human brain cells or which is responsible for the increase of expression in AD and VD.
In the end, we would like to comment on two other aspects linked to occludin localization showed in our study. First, the intranuclear presence of occludin in astrocytes from basal ganglia suggests that occludin can be transported from cytoplasm to nucleus, a phenomenon seen with factors regulating transcription. Second, the identification of occludin expression selectively in pyramidal neurons, the ones bearing neurofibrillary tangles and being responsible for high cognitive processing, might suggest a role for occludin in cellular responses to neurodegeneration.
In conclusion we show, to our knowledge for the first time, occludin-expressing neurons, astrocytes and oligodendrocytes in human brains. In AD and VD, neurons in FC and basal ganglia overexpress occludin as compared to controls. At the level of the cerebral microvasculature, there is an increase of occludin expression in the predominantly ‘vascular-based’ types of neurodegeneration: VD, CADASIL and CAA, and least in AD. Our findings could be important to elucidate new pathogenic pathways in dementia disorders and open a new horizon for occludin functions in the CNS.
Acknowledgments
This study has been supported by Marie Curie – Eurogendis – fellowship program, Swedish Brain Power Program and Alzheimer Foundation, Sweden. We thank Inga Volkmann for excellent technical support, Stefan Spulber for statistical help and Anca Catrina for providing us the unspecific immunoglobulin serum for the negative controls. Our gratefulness is towards the families that donated the brain material for scientific research.
References
- 1.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins BT, Davis TP. The blood brain barrier/neu-rovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 3.Verkhratsky A, Toescu EC. Neuronal–glial networks as substrate for CNS integration. J Cell Mol Med. 2006;10:826–36. doi: 10.1111/j.1582-4934.2006.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 4.Schneeberger EE, Lynch RD. The tight junction: a multi-functional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 5.Kniesel U, Wolburg H. Tight junctions of the blood brain barier. Cell Mol. Neurobiol. 2000;20:57–76. doi: 10.1023/a:1006995910836. [DOI] [PubMed] [Google Scholar]
- 6.Rubin LL. The blood–brain barrier in and out of cell culture. Curr Opin Neurobiol. 1991;1:360–3. doi: 10.1016/0959-4388(91)90053-a. [DOI] [PubMed] [Google Scholar]
- 7.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771–81. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 8.Gumbiner B. Structure, biochemistry and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–58. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- 9.Nagy Z, Peters H, Hüttner I. Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab Invest. 1984;50:313–22. [PubMed] [Google Scholar]
- 10.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of interendothelial junctions in human blood-brain barrier microvessels. Folia Histochem Cytobiol. 2004;42:67–75. [PubMed] [Google Scholar]
- 11.Bazzoni G, Martinez Estrada O, Dejana E. Molecular structure and functional role of vascular tight junctions. Trends Cardiovasc Med. 1999;9:147–52. doi: 10.1016/s1050-1738(99)00022-5. [DOI] [PubMed] [Google Scholar]
- 12.Cereijido M, Valdes J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161–77. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- 13.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at the tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603–13. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg G. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metallopro-teinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 16.Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood–brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45:325–37. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
- 17.Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–27. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies DC. Blood–brain barrier breakdown in septic encephalopathy and brain tumors. J Anat. 2002;200:639–46. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–94. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plumb J, McQuaid S, Mirakhur M, Kirk J. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2002;12:154–69. doi: 10.1111/j.1750-3639.2002.tb00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer H, Stelzhammer W, Fuchs R, Weiger TM, Danninger C, Probst G, Krizbai IA. Astrocytes and neurons express the tight junction-specific protein occludin in vitro. Exp Cell Res. 1999;250:434–8. doi: 10.1006/excr.1999.4558. [DOI] [PubMed] [Google Scholar]
- 22.Mooradian AD. Potential mechanisms of the age-related changes in the blood–brain barrier. Neurobiol Aging. 1994;15:751–5. doi: 10.1016/0197-4580(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 23.Mooradian AD. Effect of aging on the blood–brain barrier. Neurobiol. Aging. 1988;9:31–9. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- 24.Jorm AF, Jolley D. The incidence of dementia – a meta-analysis. Neurology. 1998;51:728–33. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 25.Nag S. Pathophysiology of blood–brain barrier break-down. Methods Mol Med. 2003;89:97–119. doi: 10.1385/1-59259-419-0:97. [DOI] [PubMed] [Google Scholar]
- 26.Kalaria RN. Vascular factors in Alzheimer's disease. Int Psychogeriatr. 2003;15:47–52. doi: 10.1017/S1041610203008950. [DOI] [PubMed] [Google Scholar]
- 27.Kalaria RN. Cerebral vessels in ageing and Alzheimer's disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 28.Marco S, Skaper SD. Amyloid beta-peptide 1–42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci Lett. 2006;401:219–24. doi: 10.1016/j.neulet.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Lippoldt A, Kniesel U, Liebner S, Kalbacher H, Kirsch T, Wolburg H, Haller H. Structural alterations of tight junctions are associated with loss of polarity in stroke-prone spontaneously hypertensive rat blood-brain barrier endothelial cells. Brain Res. 2000;885:251–61. doi: 10.1016/s0006-8993(00)02954-1. [DOI] [PubMed] [Google Scholar]
- 30.Forero DA, Casadesus G, Perry G, Arboleda H. Synaptic dysfunction and oxidative stress in Alzheimerz's disease: emerging mechanisms. J Cell Mol Med. 2006;10:796–805. doi: 10.1111/j.1582-4934.2006.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmári E, Traweger A, Wejksza K, Bauer HC. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol. 2005;25:129–39. doi: 10.1007/s10571-004-1378-7. [DOI] [PubMed] [Google Scholar]
- 32.Nagy Z. The last neuronal division: a unifying hypothesis for the pathogenesis of Alzheimer's disease. J Cell Mol Med. 2005;9:531–41. doi: 10.1111/j.1582-4934.2005.tb00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM) 4. Washington DC: 1994. Committee on Nomenclature and Statistics. [Google Scholar]
- 34.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 35.Bogdanovic N, Morris JH. Diagnostic criteria for Alzheimer's disease in multi-centre brain banking. In: Cruz-Sanches FF, Ravid R, Cuzner ML, editors. Neuropathological diagnostic criteria for brain banking, Amsterdam. IOS press; 1995. pp. 20–9. [Google Scholar]
- 36.National Institute on Aging and Reagan Institute Working Group on diagnostic criteria for the neu-ropathological assessment o Alzheimer disease. Consensus recommendations for the post-mortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 37.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 38.Lasn H, Winblad B, Bogdanovic N. Neuroglia in the inferior olivary nucleus during normal aging and Alzheimer's disease. J Cell Mol Med. 2006;10:145–56. doi: 10.1111/j.1582-4934.2006.tb00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson TP, Neal JW, Llewellyn L, Thomas C. Neuropathology techniques. London: Oxford University Press; 2003. [Google Scholar]
- 40.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood–brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 41.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–57. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 42.Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood–brain barrier disruption after stroke. Stroke. 2002;33:1706–11. doi: 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- 43.Schiera G, Bono E, Raffa MP, Gallo A, Pitarresi GL, Di Liegro I, Savettieri G. Synergistic effects of neurons and astrocytes on the differentiation of brain capillary endothelial cells in culture. J Cell Mol Med. 2003;7:165–70. doi: 10.1111/j.1582-4934.2003.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiera G, Sala S, Gallo A, Raffa MP, Pitarresi GL, Savettieri G, Di Liegro I. Permeability properties of a three-cell type in vitro model of blood–brain barrier. J Cell Mol Med. 2005;9:373–9. doi: 10.1111/j.1582-4934.2005.tb00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussar P, Tserentsoodol N, Koyama H, Yokoo-Sugawara M, Matsuzaki T, Takami S, Takata K. The glucose transporter GLUT1 and the tight junction protein occludin in nasal olfactory mucosa. Chem Senses. 2002;27:7–11. doi: 10.1093/chemse/27.1.7. [DOI] [PubMed] [Google Scholar]
- 46.Hirakawa H, Okajima S, Nagaoka T, Takamatsu T, Oyamada M. Loss and recovery of the blood–nerve barrier in the rat sciatic nerve after crush injury are associated with expression of intercellular junctional proteins. Exp Cell Res. 2003;284:196–210. doi: 10.1016/s0014-4827(02)00035-6. [DOI] [PubMed] [Google Scholar]
- 47.Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure-remodelling of the neuroepithelium prior to neurogenesis. Dev Biol. 1996;180:664–79. doi: 10.1006/dbio.1996.0336. [DOI] [PubMed] [Google Scholar]
- 48.Garcia JH, Liu K-F, Ho K-L. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudatoputamen and cortex. Stroke. 1995;26:636–43. doi: 10.1161/01.str.26.4.636. [DOI] [PubMed] [Google Scholar]
- 49.Feekes JA, Hsu SW, Chaloupka JC, Cassel MD. Tertiary microvascular territories define lacunar infarcts in the basal ganglia. Ann Neurol. 2005;58:18–30. doi: 10.1002/ana.20505. [DOI] [PubMed] [Google Scholar]
- 50.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62:1287–301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer's disease. Neurosignals. 2002;11:270–81. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- 52.Campbell M, Collery R, McEvoy A, Gardiner TA, Stitt AW, Brankin B. Involvement of MAPKs in endo-statin-mediated regulation of blood-retinal barrier function. Curr Eye Res. 2006;31:1033–45. doi: 10.1080/02713680601013025. [DOI] [PubMed] [Google Scholar]
- 53.Stopa EG, Gonzalez AM, Chorsky R, Corona RJ, Alvarez J, Bird ED, Baird A. Basic fibroblast growth factor in Alzheimer's disease. Biochem Biophys Res Commun. 1990;171:690–6. doi: 10.1016/0006-291x(90)91201-3. [DOI] [PubMed] [Google Scholar]
- 54.Reuss B, Dono R, Unsicker K. Functions of fibrob-last growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood–brain barrier permeability: evidence from mouse mutants. J Neurosci. 2003;23:6404–12. doi: 10.1523/JNEUROSCI.23-16-06404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2002;23:237–43. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 56.Del Bo R, Scarlato M, Ghezzi S, Martinelli Boneschi F, Fenoglio C, Galbiati S, Virgilio R, Galimberti D, Galimberti G, Crimi M, Ferrarese C, Scarpini E, Bresolin N, Comi GP. Vascular endothelial growth factor gene variability is associated with increased risk for AD. Ann Neurol. 2005;57:373–80. doi: 10.1002/ana.20390. [DOI] [PubMed] [Google Scholar]
- 57.Yang SP, Bae DG, Kang HJ, Gwag BJ, Gho YS, Chae CB. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer's disease. Neurobiol Aging. 2004;25:283–90. doi: 10.1016/S0197-4580(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 58.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–15. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 59.Mungrue IN, Bredt DS. nNOS at a glance: implications for brain and brawn. J Cell Sci. 2004;117:2627–29. doi: 10.1242/jcs.01187. [DOI] [PubMed] [Google Scholar]
- 60.Ghassemifar MR, Sheth B, Papenbrock T, Leese HJ, Houghton FD, Fleming TP. Occludin TM4-: an isoforms of the tight junction protein present in primates lacking the fourth transmembrane domain. J Cell Sci. 2002;115:3171–80. doi: 10.1242/jcs.115.15.3171. [DOI] [PubMed] [Google Scholar]
- 61.Muresan Z, Paul DL, Goodenough DA. Occludin 1B, a variant of the tight junction protein occludin. Mol Biol Cell. 2000;11:627–34. doi: 10.1091/mbc.11.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]


