Abstract
We report the clinical case of a genital outbreak with both Herpes Simplex Type 1 (HSV-1) and Herpes Simplex Type 2 (HSV-2) during pregnancy. Herpes was presumptively identified by clinical presentation of lesion and Tzanck smear while serotypes were identified by cell culture and polymerase chain reaction (PCR). This case report highlights the need for increased surveillance of both serotypes in genital infection of pregnant women for effective disease management and reduced risk of transmission. Increasing rates of genital infection with HSV-1, the possibility of genital co-infection with HSV-1 and HSV-2 and the non-specificity and lack of sensitivity of traditional viral isolation methods may lead to under-diagnosis of genital HSV-1 infections unless molecular diagnostic methods, such as polymerase chain reaction (PCR) are routinely deployed in the clinical setting.
Keywords: HSV, Herpes, PCR, co-infection, pregnancy
Case report
A 23-year-old woman presents for her initial obstetric visit at 9 weeks gestation with chief concern of 1-week-old painful blisters in her perineum. She reported no previous history of similar rashes in other regions of her body and no significant past medical problems, surgeries or hospitalizations. She denied any history of sexually transmitted infections and was unaware of any past exposures. She was otherwise healthy without any recent fevers, chills, oral lesions, myalgias or arthralgias. She denied any similar rash on her husband. On physical exam, there were multiple coalesced 2–3 mm vesicles and crusted papules circumscribing the skin immediately around the external genitalia. No lesions were appreciated on speculum exam of the internal mucosa. Patient was negative for HIV, Chlamydia trachomatis/Neisseria gonorrhea (as determined by ELISA and PACE2C assays, respectively).
Materials and methods
Clinical Specimens. Using Dacron sterile swabs, vesicle fluid and cells were collected by vigorously scraping the base of several lesions. Swabs were placed in Micro TestÔ M4 transporter (Remel, Lenexa, KS) for use in molecular diagnostic testing and traditional viral isolation methods.
Viral Culture and Direct Immunofluorescent Analysis (DFA). Shell vial cell cultures, each containing a 12-mm diameter round cover slip with a monolayer of BHK cells were inoculated with patient specimen as previously described [1]. After centrifugation for 40 min at 700 x g and incubation at 36°C for 24 hr and 48 hr, respectively, cover slips from four shell vials were stained with type-specific anti-glycoprotein G (gG) antibodies (Remel PathoDx Herpes Typing Kit).
Molecular Analysis. The same patient sample swabs used for viral isolation were subsequently returned to the remaining transport media and transported to the Molecular Diagnostics laboratory. Swabs were expressed by compression against the side of the transport tube. DNA was extracted using the MagnaPure Compact system (Roche Diagnostics) and real-time polymerase chain reaction (PCR) was performed on the LightCycler using Light Cycler FastStart DNA Master PLUS Hybridization probes and primers targeting the UL30 gene of Herpes Simplex Virus (HSV) polymerase followed by a second test for subtyping using primers targeting the HSV-1 gD and the HSV-2 gG, as previously described [2, 3]. Each run included positive and negative controls. The Green Fluorescent Protein (GFP) is used as a sample extraction control and detected with specific primers (a known amount of GFP was added to the sample prior to the nucleic acid extraction).
Results
Herpes simplex-characteristic cytopathic effect (prominent plaques, rounded cells) was observed in BHK cells inoculated with patient specimen at 24 hrs after infection confirming the previous results obtained with the Tzanck smear which showed herpes virus family-characteristic multi-nucleated syncytial giant cells (data not shown). DFA with serotype specific antibodies (Remel PathoDx Herpes Typing Kit) at 24 hrs post-inoculation identified the presence of HSV-2 (Fig. 1A) but not HSV-1. However, at 48 hrs post-inoculation, both HSV-2 and HSV-1 (Fig. 1B) serotypes were present and detected by DFA.
1.
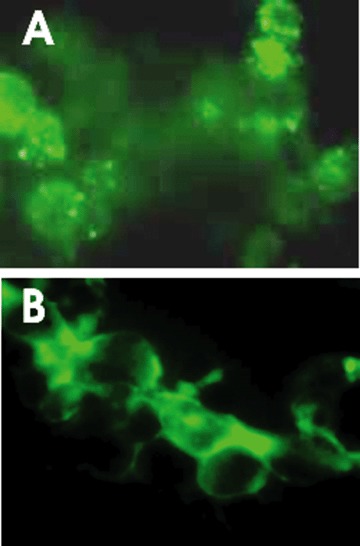
Positive DFA staining with HSV-2 gG (A) and HSV-1 gG (B) antibodies at 24 hrs and 48 hrs postafter- infection in cell culture, respectively.
A screening PCR assay targeting the HSV polymerase was performed (Fig. 2A) using the GFP as a DNA extraction control using the primers listed in Fig. 3. Results show that HSV is present and the specific serotype was further identified by a second PCR test targeting the HSV-1 gD and the HSV-2 gG (Fig. 2B). Both tests were performed in the same day the specimen was received in the laboratory with a turnaround time not exceeding 5 hrs.
2.
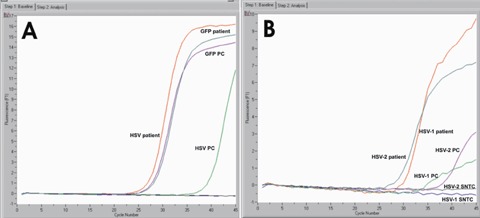
A: real-time fluorescence measurements by PCR using the LightCycler instrument (Roche Diagnostics) targeting the UL30 gene of HSV; GFP was used as a DNA extraction control. SNTC = sample negative template control; GFP PC = Green Fluorescent Protein positive control; GFP patient = clinical specimen spiked with GFP; HSV PC = HSV positive control; HSV patient = patient specimen results. (B) real-time fluorescence measurements by PCR using the LightCycler instrument (Roche Diagnostics) targeting the HSV-1 gD and the HSV-2 gG. HSV-1 patient = HSV-1 detected in patient specimen; HSV-2 patient: HSV-2 detected in patient specimen.
3.

Forward (F), reverse (R), and probes (P) for generic HSV (UL30), type specific (HSV-1-gD, HSV-2-gG), and DNA extraction control (GFP) used in PCR assay.
Discussion
Current prenatal care practices involve screening methods to determine pregnant women who are at risk for transmitting herpes to their newborn. The women most at risk of transmitting herpes to their newborn are those seronegative who acquire a primary infection from her partner during pregnancy. In the US, approximately 800,000 pregnant women acquire HSV each year [4] and incidence in neonates is about 1 in 3,200 live births.
Recent studies showed that compared to 1988–1994, the seroprevalence of HSV-1 decreased between 1999 and 2004, but the incidence of genital herpes caused by HSV-1 may be increasing [5]. Co-infection with HSV-1 and HSV-2 (which has decreased from 14.6% in 1988–1994 to 10.5% in 1999–2004) influences the epidemiology of clinical genital herpes (less frequent recurrences, 5.9%) compared to persons seropositive for HSV-2 only (16.2%) [6]. 13 % of pregnant women showed sero-prevalence for both HSV-1 and HSV-2 [7]. Considering the higher seroprevalence of HSV-1 compared to HSV-2 in pregnant women (63% versus 22%) and the population trend of increasing HSV-1 genital infections, determining the herpes serotype is a useful issue not only from an epi-demiological standpoint, but also in assessing prognosis and management of potential disseminated infection in neonates and/or disease management of the mother-to-be. (which may include central nervous system (CNS) involvement). Moreover, HSV-2 is less sensitive than HSV-1 to acyclovir [8] and it was reported that CSF remains positive in HSV-2 infection after completion of acy-clovir therapy [9].
Neonatal HSV infection, which is caused by both HSV-1 and HSV-2 [10,11], is a severe disease with high mortality and morbidity. While neonate infection with either HSV-1 or HSV-2 is associated with poor prognosis in terms of neurological outcome, in adults
HSV-2 may cause a self-limiting aseptic meningitis while HSV-1 accounts for nearly all cases of herpes encephalitis (and about 10 to 20% of all viral encephalitis cases). The molecular mechanism responsible for the different outcome of adult CNS infection is largely unknown, but a serotype-specific modulation of neuronal apoptosis has been proposed [12].
This case illustrates the importance of laboratory testing and especially molecular testing in aiding clinical diagnosis of genital herpes. Tzanck preparations and cell culture methods are non-specific in that they cannot distinguish between types 1 and 2, and varicella-zoster virus. Immunofluorescent staining offers such distinction but relies on virus growth in culture that often is positive only after 48 hrs post-inoculation and depends on the presence of infectious virus in the lesions. As such, healing lesions and asymptomatic shedding can only be detected in a timely and specific manner by molecular diagnostic methods.
In this particular case, it was impossible to discern based on patient history whether the lesions were the result of a primary or recurrent infection with either HSV-1 or HSV-2. Based on the current literature review, we can only assume that such occurrences (of either a primary infection in conjunction with a recurrent outbreak or two primary infections with distinct serotypes in the same genital area) are rare. Whether the delay of HSV-1 detection in culture is due to a more advanced stage of lesion healing or a reduced virus inoculum is also not known. However, the laboratory capability to perform same day testing with serotype identification significantly reduced the turnaround time and accuracy of results reporting. The patient was counselled on the laboratory findings and was started on a course of Acyclovir 400 mg three times daily per 7 days.
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense of the U.S. Government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
References
- 1.Gleaves CA, Wilson DJ, Wold AD, Smith TF. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 hrs post-inoculation. J. Clin. Microbiol. 1985;21:29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura H, Ito Y, Futamura M, Ando Y, Yabuta Y, Hoshino Y, Nishiyama Y, Morishima T. Quantitation of viral load in neonatal herpes simplex virus infection and comparison between type 1 and type 2. J. Med. Virol. 2002;67:349–353. doi: 10.1002/jmv.10084. [DOI] [PubMed] [Google Scholar]
- 3.Stöcher M, Leb V, Bozic M, Kessler HH, Halwachs-Baumann G, Landt O, Stekel H, Berg J. Parallel detection of five human herpes virus DNAs by a set of real-time polymerase chain reactions in a single run. J. Clin. Virol. 2003;26:85–93. doi: 10.1016/s1386-6532(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control Fact Sheet. 2004. available at: http://www.cdc.gov/std/healthcomm/fact_sheets.htm, accessed on 07 April 2007.
- 5.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seropreva-lence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, Markowitz LE. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J. Infect. Dis. 2002;185:1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- 7.Xu F, Markowitz LE, Gottlieb SL, Berman SM. Seroprevalence of herpes simplex virus types 1 and 2 in pregnant women in the United States. Am. J. Obstet. Gynecol. 2007;196:e1–e6. doi: 10.1016/j.ajog.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Kimberlin DW, Lakeman FD, Arvin AM, Prober CG, Corey L, Powell DA, Burchett SK, Jacobs RF, Starr SE, Whitley RJ. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J. Infect. Dis. 1996;174:1162–1167. doi: 10.1093/infdis/174.6.1162. [DOI] [PubMed] [Google Scholar]
- 9.Whitley R. Neonatal herpes simplex virus infection. Curr. Opin. Infect. Dis. 2004;17:243–6. doi: 10.1097/00001432-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Kimberlin D. Herpes simplex virus, meningitis and encephalitis in neonates. Herpes. 2004;11:65A–676A. [PubMed] [Google Scholar]
- 11.Hirsch MS, Kaplan JC, D'Aquila RT. Antiviral agents. In: Fields BN, Knipe DM, Howly PM, editors. Virology. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 431–466. [Google Scholar]
- 12.Perkins D. Virus signalling and apoptosis in the central nervous system infection. Front Biosci. 2005;10:2804–2819. doi: 10.2741/1737. [DOI] [PubMed] [Google Scholar]


