Abstract
Mast cells (MC) have been implicated in both normal and pathological angiogenesis, such as that in chronic inflammatory diseases and tumors. This assumption is partially supported by the close structural association between MC and blood vessels and the recruitment of these cells during tumor growth. MC release a number of angiogenic factors among which tryptase, a serine protease stored in MC granules, is one of the most active. In this study, we correlate the extent of angiogenesis with the number of tryptase-reactive MC in tissue fragments from pterygium and normal bulbar conjunctiva investigated by immunohistochemistry, using two murine monoclonal antibodies against the endothelial cell marker CD31 and the MC marker tryptase. Angiogenesis, measured as microvessel density, was highly correlated with MC tryptase-positive cell count in pterygium tissues. These results suggest that the characteristic neovascularization observed in pterygium may be sustained, at least in part, by MC angiogenic mediators, in particular tryptase.
Keywords: angiogenesis, mast cell, pterygium, tryptase
Introduction
Pterygium is a surface ocular lesion that begins growing from limbal epithelium that invades the cornea centripetally followed by conjunctival epithelium, exhibiting degenerative and hyperplastic changes as well as proliferative, inflammatory features and a rich vasculature. Seifert and Sekund [1] identified intraepithelial capillaries in the optical half of 42.3% pterygia, providing evidence of neovascularization in pterygium.
Angiogenesis entails the new vessel formation from pre-existing vessels and occurs both in physiological, i.e. cyclically in female reproductive system, and pathological conditions, such as chronic inflammations and tumors [2, 3].
In eye diseases, angiogenesis causes severe loss of vision [4] and several angiogenic cytokines have been implicated in pterygium pathogenesis, such as fibroblast growth factor-2 (FGF-2), tumor necrosis factor alpha (TNF-α), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-) and vascular endothelial growth factor (VEGF) [5, 6]. More recently, Aspiotis et al.[7] demonstrated overexpression of VEGF and the absence of thrombospondin-1 (TSP-1), a well-known angiogenesis inhibitor, from almost half of the pterygium cases examined.
There is an increased number of mast cell (MC) in pterygium containing FGF-2 in their secretory granules [8, 9].The density of MC is highly correlated with the extent of both normal and pathological angiogenesis, such as that in chronic inflammatory diseases and tumors [10].
In this study, we correlate the extent of angiogenesis with the number of tryptase-reactive MC in tissue fragments from pterygium and normal bulbar conjunctiva.
Materials and methods
Patients and study design
The study group included 31 cases of surgical excised pterygium, (11 males and 20 females) whose ages ranged from 21 to 68 years. All patients underwent excision by the bare sclera technique at the Department of Pathology, Cancer Center of Solca, Cuenca, Ecuador. Most of the lesions were located on the nasal side and only the head of primary pterygium was included as pterygium sample. Ten nasal epibulbar conjunctival segments, excised during cataract surgery near the limbus, were used as control tissues. The study protocol was approved by the local Research Ethic Committee, and informed consent was obtained from all patients.
Immunohistochemistry
Tissue samples were fixed by immersion in 10% formalin 0.2 M phosphate buffer, pH 7.3 for 3–4 hrs and processed for paraffin embedding. Two murine monoclonal antibodies (MAbs) against the endothelial cell marker CD31 (MAb 1A10) and tryptase (MAb AA1, Dako, Glostrup, Denmark) were used. Briefly, 4 m thick sections were collected on 3-amino-propyl-triethoxysilane coated slides, deparaffinized by the xylene-ethanol sequence, rehydrated in a graded ethanol scale and in TRIS-buffered saline (TBS, pH 7.6), and incubated overnight at 4°C with MAbs 1A10 (1:25 in TBS) and AA1 (1:1500 in TBS), after prior antigen retrieval by enzymatic digestion with Ficin (Sigma, St Louis, MO, USA) for 30 min at room temperature for tryptase, and in a pressure cooker for 90 sec in EDTA buffer, pH 8 for CD31. The immunoreaction was performed with alkaline phosphatase anti-alkaline phosphatase (APAAP, Dako) and Fast Red as chromogen for tryptase, and with the streptavidin-peroxidase complex (LSAB2, Dako) and 3.3 diaminobenzidine tetrahydrochloride (Dako) 5% as chromogen for CD31, followed by hematoxylin counterstaining. An unrelated monoclonal IgG1 produced by the P3×63/Ag8 mouse secretory myeloma replacing the MAbs served as negative controls.
Microvessel counts
These were simultaneously assessed without knowledge of the final pathological diagnosis by two investigators with a double-headed light microscope (Axioplan II, Zeiss, Oberkochen, Germany). Four to six 200 × fields covering almost the whole of each of four sections per sample were examined with a 144-intersection point square reticulum (0.78 mm2) inserted in the eyepiece. Care was taken to select microvessels, i.e. capillaries and small venules, from all the CD31-stained vessels. They were identified as transversally sectioned tubes with a single layer of endothelial cells, either without or with a thin basement membrane. Each assessment was agreed upon in turn. Microvessels were counted with a planimetric point-count method, according to which only microvessels transversally cut occupying the reticulum points were counted. As the microvessel diameter was smaller than the distance between adjacent points, only one transversally sectioned microvessel could occupy a given point. Microvessels transversally sectioned outside the points and those longitudinally or tangentially sectioned were omitted. Therefore, it was sufficiently certain that a given microvessel was counted only once, even in the presence of several of its section planes. As almost the entire section was analysed per sample, and as transversally sectioned microvessels hit the intersection points randomly, the method allowed objective counts. Means SD (Standard Deviation) and medians were determined for each section, sample and group of samples.
MC counts
MC were highlighted in every second section adjacent to that stained for microvessels with tryptase, counted in 6 to 8 250 × fields, covering almost the whole section, inside a square reticulum (0.25 mm2), and calculated as means ±SD and median for each group of samples.
Statistics
The significance of changes in the counts of microvessels and MC tryptase-reactive MC was assessed with parametric (Fisher's test) and non-parametric (Kruskal-Wallis test) analysis of variance, followed by the Duncan (t), Bonferroni (t) and Wilcoxon tests to compare groups. Correlations between counts were assessed with Pearson's (r) coefficient and simple regression analysis. The chi-square test was split into the linear and the residual component according to Cochran. Data were computed with the Statistical Analysis Software (SAS, SAS Institute, Cary, NC).
Results
Pterygium tissues were more vascularized than normal conjunctiva. Table 1 shows the microvessel and tryptase positive MC counts on adjacent tissue sections selected from patients affected from pterygium and from bulbar conjunctival of control subjects. There are significant differences between these two groups concerning the microvessel density (chi-square = 33.2, df = 3, P < 0.001; F = 35.2, P < 0.001) and MC counts (chi-square = 37.4, df = 3, P < 0.001; F = 38.5, P < 0.001).
1.
Correlation between microvessel counts and mast cell counts in specimens of pterygium and matching normal conjunctiva immunostained with anti-CD31 and anti-tryptase antibodies
P < 0.001 compared with normal conjunctiva.
These differences are also shown in Figure 1, which shows the distinct microscopic patterns of microvessel and MC density between pterygium and bulbar conjunctival. Intense angiogenic activity was observed particularly at the sub epithelial area of pterygium, while MC were generally scattered throughout the interstitial stroma, where they rested near or around the blood capillaries.
1.
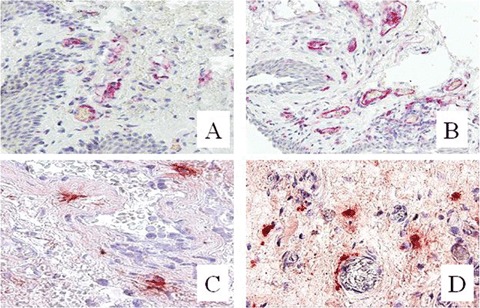
Immunohistochemical staining for CD31 positive microvessels (A, B) and tryptase-positive mast cells (C, D). Note a higher microvessel and mast cell density in human pterygium (B, D), as compared to normal conjunctiva (A, C). Original magnifications: A–D, × 250.
Discussion
The pathogenesis of pterygium is still controversial. Many angiogenic cytokines have been implicated and immunoreactivity for these growth factors has been demonstrated in epithelial cells, endothelial cells, fibroblasts and inflammatory cells [5–7]. Here, we show that angiogenesis in human pterygium, measured as microvessel counts, is highly correlated with MC tryptase-positive counts. This finding is consistent with previous observations indicating that MC are strikingly associated with angiogenesis in tumors [10].
MC contain many angiogenic factors and a variety of cytokines, such as TGF-β, TNF-α, interleukin-8 (IL-8), FGF-2 and VEGF, implicated in normal as well as tumor-associated neoangiogenesis [11]. These cytokines are involved both in normal as well as tumor-associated angiogenesis. The spectrum of cytokines expressed appears to vary depending on the maturity state of the MC and of the tissue of residence. Qu et al.[12] demonstrated that FGF-2 is localized in the cytoplasmic and extruded granules of MC in several human tissues. Grutzkan et al.[13] demonstrated the expression of VEGF in the human MC line HMC-1 and in human skin MC. Nerve growth factor (NGF), also contained in MC secretory granules, induces endothelial cell proliferation in vitro and angiogenesis in vivo in the chick embryo chorioallantoic membrane (CAM) assay [14].
Blair et al.[15] have shown that tryptase released by MC at an angiogenesis site may play an important role in neovascularization. Direct addition of tryptase to microvascular endothelial cells cultured on Matrigel caused a pronounced increase of capillary growth, which was suppressed by specific tryptase inhibitors. Moreover, tryptase directly induced endothelial cell proliferation in a dose-dependent fashion. Inside MC granules, tryptase is stored at high concentration in a macromolecular complex with heparin proteoglycan. The interaction with heparin is known to be essential for maintaining enzymatic activity [16]. As shown, tryptase exerts a direct angiogenic activity on endothelial cell precursors, stimulating their differentiation and assembly into mature vascular tubes. Being tryptase involved in tissue remodeling, it is supposed to also act indirectly on tissue neovascularization by releasing latent angiogenic factors bound to the extracellular matrix. As a result, tryptase is the major MC protease and one of the most powerful angiogenic mediators released by human MC. We have previously demonstrated that in multiple myeloma, B-cell non-Hodgkin's lymphoma, myelodysplastic syndromes, B-cell chronic lymphocytic leukemia, melanoma and endometrial cancer, there is a striking association between MC and microvessel counts and both increase in function of malignancy [10, 17].
In line with these reports showing a close relationship between increased number of tryptase-positive MC and tumor progression, our data suggest that tryptase-positive MC may contribute, at least partly to pterygium-associated angiogenesis, together with the angiogenic cytokines secreted by epithelial and endothelial cells, fibroblasts and inflammatory cells.
Suggestions for antiangiogenic therapy for pterygium gain support from a number of studies: TNP-470, a potent fungus-derived inhibitor of angiogenesis, inhibited the proliferation of fibroblasts isolated from active pterygium in vitro[18]. In this contest, tryptase antagonization could be used as antiangiogenic approach in the treatment of human pterygium.
Acknowledgments
Supported in part by Associazione Italiana per la Ricerca sul Cancro AIRC (National and Regional Funds) Milan, Fondazione Italiana per la Lotta al Neuroblastoma; Genoa and MIUR (FIRB 2001, PRIN 2005 and Project CARSO Consortium 72/2), Rome, Italy.
References
- 1.Seifert P, Sekundo W. Capillaries in the epithelium of pterygium. Br J Ophthalmol. 1998;82:77–81. doi: 10.1136/bjo.82.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis. Ann Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsanou E, Ioachim E, Stefaniotou M, Gorezis S, Charalabopoulos K, Bagli H, Peschos D, Psilas K, Agnantis NJ. Immunohistochemical study of angio-genesis and proliferative activity in epiretinal membranes. Int J Clin Pract. 2005;59:1157–61. doi: 10.1111/j.1368-5031.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 5.Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996;98:195–201. doi: 10.1016/s0065-1281(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 6.Marcovich AL, Morad Y, Sandbank J, Huszar M, Rosner M, Pollack A, Herbert M, Bar-Dayan Y. Angiogenesis in pterygium: Morphometric and immuno-histochemical study. Curr Eye Res. 2002;25:17–22. doi: 10.1076/ceyr.25.1.17.9959. [DOI] [PubMed] [Google Scholar]
- 7.Aspiotis M, Tsanou E, Gorezis S, Joachim E, Skyrlas A, Stefaniotou M, Malomou-Mitsi V. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1 Eye. 2006. Advance online publication, 2006, June 23. [DOI] [PubMed]
- 8.Powers MR, Qu Z, O'Brien B, Wilson DJ, Thompson JE, Rosenbaum JT. Immunolocalization of bFGF in pterygia: association with mast cells. Cornea. 1997;16:545–9. [PubMed] [Google Scholar]
- 9.Nakagami T, Murakami A, Okisaka S, Ebihara N. Mast cells in pterygium: number and phenotype. Jpn J Ophthalmol. 1999;43:75–9. doi: 10.1016/s0021-5155(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 10.Ribatti D, Crivellato E, Roccaro AM, Ria R, Vacca A. Mast cell contribution to angiogenesis related to tumour progression. Clin Exp Allergy. 2004;34:1660–4. doi: 10.1111/j.1365-2222.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- 11.Crivellato E, Beltrami CA, Mallardi F, Ribatti D. The mast cell: an active participant or an innocent bystander. Histol Histopathol. 2004;19:259–70. doi: 10.14670/HH-19.259. [DOI] [PubMed] [Google Scholar]
- 12.Qu Z, Kayton RJ, Ahmadi P, Liebler JM, Powers MR, Planck SR, Rosembaum JT. Ultrastructural immunolocalization of basic fibroblast growth factor in mast cell secretory granules: morphological evidence for bFGF release through degranulation. J. Histochem. Cytochem. 1998;46:1119–28. doi: 10.1177/002215549804601004. [DOI] [PubMed] [Google Scholar]
- 13.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF 206. Mol Biol Cell. 1998;9:875–84. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappalà G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interactions lead to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–9. doi: 10.1096/fj.01-1000fje. [DOI] [PubMed] [Google Scholar]
- 15.Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691–700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallgren J, Estrada S, Karlson U, Alving K, Pejler G. Heparin antagonists are potent inhibitors of mast cell tryptase. Biochemistry. 2001;40:7342–9. doi: 10.1021/bi001988c. [DOI] [PubMed] [Google Scholar]
- 17.Ribatti D, Finato N, Crivellato E, Marzullo A, Mangieri D, Nico B, Vacca A, Beltrami CA. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 2005;193:1961–5. doi: 10.1016/j.ajog.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 18.Kria L, Ohira A, Amemiya T. TNP-470 (a fungus-derived inhibitor of angiogenesis) reduces proliferation of cultured fibroblasts isolated from primary pterygia: A possible drug therapy for pterygia. Curr Eye Res. 1998;17:986–93. doi: 10.1076/ceyr.17.10.986.5245. [DOI] [PubMed] [Google Scholar]


