Abstract
The anti-resorptive agent zoledronic acid inhibits key enzymes in the mevalonate pathway, disrupting post-translational modification and thereby correct protein localization and function. Inhibition of prenylation may also be responsible for the reported anti-tumour effects of zoledronic acid, but the specific molecular targets have not been identified. Cenp-F/mitosin, a kinetochore-associated protein involved in the correct separation of chromosomes during mitosis, has been shown to undergo post-translational prenylation and may therefore be a novel target contributing to the anti-tumour effects of zoledronic acid. We investigated whether zoledronic acid causes loss of Cenp-F from the kinetochore in breast cancer cells, to determine if the reported anti-tumour effects may be mediated by impairing correct chromosome separation. MDA-MB-436, MDA-MB-231 and MCF-7 breast cancer cells and MCF-10A non-malignant breast epithelial cells were treated with zoledronic acid in vitro, and the effect on Cenp-F localization was analysed by immunoflourescence microscopy. Zoledronic acid caused loss of Cenp-F from the kinetochore, accompanied by an increase in the number of cells in pro-, /prometa- and metaphase in all of the cancer cell lines. There was also a significant increase in the number of lagging chromosomes in mitotic cells. The effects of zoledronic acid could be reversed by inclusion of an intermediary of the mevalonate pathway, showing that the loss of Cenp-F from the kinetochore was caused by the inhibition of farnesylation. In contrast, no effect was seen on Cenp-F in non-malignant MCF-10A cells. This is the first report showing a specific effect of zoledronic acid on a protein involved in the regulation of chromosome segregation, identifying Cenp-F as a potential new molecular target for NBPs in tumour cells.
Keywords: zoledronic acid, Cenp-F, mitosin, breast cancer, prenylation
Introduction
Zoledronic acid, a nitrogen-containing bisphosphonate (NBP), is a potent inhibitor of bone resorption commonly used to treat cancer-induced bone disease from solid tumours and multiple myeloma [1]. Zoledronic acid induces apoptosis of osteoclasts through inhibition of key enzymes of the mevalonate pathway, which is responsible for post-translational modification of a wide range of proteins including signalling GTPases [2]. The molecular target for NBPs has been identified as farnesyl diphosphate (FPP) synthase, and binding of NBP to FPP synthase prevents the formation of FPP and its downstream metabolite geranyl geranyl diphosphate (GGPP) [3].
The mevalonate pathway constitutes an important part of the metabolic process resulting in cholesterol synthesis, which is ubiquitous to all nucleated cells, and zoledronic acid may therefore also induce apoptosis of other cell types, including tumour cells. In support of this, there is increasing evidence from both in vitro and in vivo model systems for a role of NBPs as potential anti-tumour agents, reviewed in Ref. [4]. The majority of the reported effects can be overcome by replenishing the tumour cells with farnesol or genanylgeraniol, providing substrates for isoprenylation. Small GTPases of the Rab, Ras and Rho families have been the most studied targets for NBPs, as they play important roles in maintaining normal osteoclast function. However, with approximately 300 peptides with prenylation motifs identified in the human proteome, there are a large number of additional potential targets for drugs that interfere with this form of post-translational modification [5]. An interesting protein in this context is the centromeric protein Cenp-F (mitosin) that has been shown to undergo farnesylation, and together with other proteins such as Cenp-E, cytoplasmic dynein, MAD1, MAD2, Bub1 and BubR1 form the protein complex responsible for kinetochore assembly, microtubule attachment, microtubule dynamics and spindle checkpoint signalling during mitosis [6]. The mature kinetochore plays a critical role in chromosome segregation, mediating the establishment and maintenance of kinetochore-microtubule attachments and the regulation of the spindle assembly checkpoint (SAC). Cenp-F is a 367 kD protein expressed in a cell cycle dependent manner. Only low levels of Cenp-F are present in S-phase, which accumulates during the cell cycle resulting in a peak at G2/M-phase. In S/G2-phase, Cenp-F is mainly distributed in the nucleus but is excluded from the nucleoli [7, 8]. There is a dynamic spatial and temporal distribution of Cenp-F during mitosis progression. In late G2, a sub-pool of protein is localized at the nuclear envelope with the bulk of Cenp-F distributed within the nuclear matrix. It is first detectable at the kinetochores in late G2 to early prophase, and is reported to be one of the first proteins to associate with the kinetochores, suggesting a role for Cenp-F in its initial assembly [6]. Cenp-F exhibits several motifs that have been proposed to contribute to its function and localization, including farnesylation sites. Between late S-phase and prophase the protein is modified by farnesylation at a CAAX box motif at the C-terminus [9, 10]. Hussein and Taylor showed that the localization of Cenp-F to the nuclear envelope in G2/M and the kinetochores in early mitosis, as well as Cenp-F degradation, is dependent on its farnesylation [9]. Studies with farnesyltransferase inhibitors (FTIs) suggested that farnesylation of Cenp-F is also necessary for its function at the G2/M transition [11]. Taken together, there is emerging evidence that inhibition of Cenp-F farnesylation results in disruption of correct kinetochore assembly, subsequently leading to delayed cell cycle progression and inhibition of cell proliferation. As NBPs have been shown to affect protein prenylation, as well as inhibit tumour cell proliferation [12, 13], we have investigated whether zoledronic acid causes delocalization of Cenp-F from the kinetochore in breast cancer cells. We found that cancer cells treated with zoledronic acid displayed loss of Cenp-F from the kinetochore, accompanied by an accumulation of cells in early stages of mitosis and disrupted chromosome alignment, whereas this does not happen in non-malignant MCF-10A cells. The effects could be counteracted by addition of farnesol (FOH), showing that they were mediated through inhibition of the mevalonate pathway. Our data are the first to show an effect of zoledronic acid on kinetochore assembly in breast cancer cells, and we propose that Cenp-F may be a novel target protein contributing to the anti-tumour effects of zoledronic acid.
Materials and methods
Cell lines and tissue culture
The hormone-independent human breast cancer cell lines MDA-MB-436 and MDA-MB-231 and the hormone-dependent human breast cancer cell line MCF-7 were obtained from the European collection of cell cultures (Salisbury, UK). MDA-MB-436, MDA-MB-231 and MCF-7 cells were cultured in RPMI-1640 medium supplemented with 10% foetal calf serum (both from Gibco, Invitrogen, Paisley, UK) and harvested with trypsin (0.05%)–EDTA (0.02%) (PAA Laboratories GmbH, Pasching, Germany). MCF-10-A cells were cultured in DMEM:HAMS nutritient mix F12 + Glutamax medium (Gibco, Invitrogen, Paisley, UK) supplemented with 5% horse serum (Invitrogen, Paisley, UK) 10 μg/ml insulin, 20 ng/ml epidermal growth factor and 0.1 μM hydrochortisol (all from SIGMA-Aldrich, Steinheim, Germany).
Drugs and chemicals
Zoledronic acid ([2-(imidazol-1-yl)-hydroxy-ethylidene-1,1-bisphosphonic acid, disodium salt, 4.5 hydrate]) supplied as the hydrated di-sodium salt by Novartis Pharma AG, Basel, Switzerland. A 10 mM stock solution was prepared in PBS and stored at −20°C. Farnesol (trans-trans farnesol, (E,E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol trans,trans-3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol) was stored at room temperature and geranylgeraniol (trans-3,7,11–15-Tetramethyl-2,6,10,14-hexadecatetraen-1-ol), stored at −20°C, were both obtained from SIGMA-Aldrich. A 50 mM stock solution of Clodronate (dichloromethylene bisphosphonate disodium tetrahydrate) in dH2O was prepared and stored at 4°C. Clodronate was from Leiras Oy, Turku, Finland.
Cell treatment
After harvesting, cells were seeded (MDA-MB-231 and MDA-MB-436 at 2 × 104 cells/ml, MCF-7 at 4 × 104 cells/ml and MCF 10-A at 4 × 104 cells/ml, 0.5 ml per chamber) into chamber slides (Lab-Tek, Nalge Nunc International, NY, USA). Cells were incubated for 24 hrs at 37°C and 5% CO2 prior to treatment. Subsequently, medium was discarded and fresh medium supplemented with the drug and/or chemical of interest was added at the concentration and exposure time indicated in the result section.
Immunofluorescence staining
Cells were fixed in 3.7% formalin for 10 min., washed three times in PBS and permeabilized in 0.1% Triton X-100 for 5 min. After three wash steps, non-specific binding sites were blocked with 2% BSA and 5% normal horse serum in PBS. Cells were incubated over night at 4°C with anti-Cenp-F (610768, BD Transduction Laboratories, Erembodegen, Belgium, 1:400), washed three times, then incubated with a biotinylated anti-mouse (BA-2000, Vector Laboratories Inc., Burlingame, CA, USA, 1:100) for 1 hr at ambient temperature, washed three times and then incubated for 1 hr in Fluorescein-Avidin D at ambient temperature (Fluorescein A-2001, Vector Laboratories Inc. 1:250). Cells were mounted with Prolong Gold anti-fade reagent with DAPI (Molecular Probes, Invitrogen, OR, USA).
Microscopy and scoring
Immunofluorescence microscopy was performed with a fluorescence and phase contrast inverted microscope (Leica DMI 4000B, Germany) with a 40.0×:0.60 DRY objective. Images were acquired with LAS AF software (Leica Microsystems CMS GmbH) and a DFC 300F× R2 Camera (Version:1.8.0).
Forty cells in pro-, prometa- and metaphase were scored for Cenp-F localization at or not at the kinetochore. Only cells with a clear punctuate pattern of Cenp-F localized within the DAPI stained DNA were scored as ‘cells with Cenp-F localized at the kinetochore’. Mitotic cells of each mitotic stage (pro-, prometa-, meta-, ana-, telophase) were counted for mitosis progression experiments. The degree of chromosome condensation (DAPI) was used as criteria for mitotic stages. For quantification of mitotic cells with lagging chromosomes, 100 mitotic cells were counted per drug concentration.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software (Version 5.01). Experiments with various drug exposure times and concentrations were analysed by two-way ANOVA and Bonferroni post test. For differences between means in experiments with only one exposure time, one-way ANOVA and Dunnett post test was performed. In all cases, a P-value of <0.05 was considered significant.
Results
Zoledronic acid inhibits Cenp-F/mitosin localization at the kinetochore in breast cancer cells
The farnesylated protein Cenp-F, involved in regulation of mitosis, represents a potential novel molecular target for zoledronic acid. Displacement and/or dysfunction of mitotic proteins may be involved in mediating the reported anti-tumour effects of NBPs (Fig. 1). We therefore investigated the effects of zoledronic acid on Cenp-F kinetochore localization in MDA-MB-436, MDA-MB-231 and MCF-7 human breast cancer cells. Cells were exposed to increasing doses of zoledronic acid for either 24 or 48 hrs, fixed and Cenp-F localization in mitotic cells was assessed using fluorescence microscopy. Effects of the used treatment schedules on cell viability were ruled out by initial experiments in which no effect on MDA-MB-436 cell apoptosis was observed after treatment with 25 μM zoledronic acid for 72 hrs or on MCF-7 cells after 48 hrs at 25 μM (data not shown).
Fig 1.
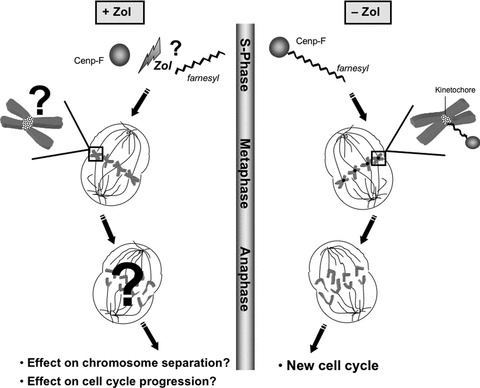
Schematic representation of the potential effects on Cenp-F following zoledronic acid treatment. Cenp-F farnesylation, which is crucial for Cenp-F localization to the kinetochores, takes place during late S-phase. This enables the protein to take an active part in kinetochore assembly, chromosome alignment/segregation and G2/M transition. The potential effects of inhibition of Cenp-F farnesylation by zoledronic acid and the resulting consequences for the cell are depicted in the left panel.
Cenp-F staining of untreated MDA-MB-436 cells at different stages of mitosis showed localization of the protein as reported in previous studies [7, 14], with clear kinetochore staining in pro-, prometa- and metaphase. Following treatment with zoledronic acid, there was a marked redistribution of Cenp-F from the kinetochore, resulting in a dispersed staining pattern (Fig. 2). The punctuate pattern of Cenp-F staining was evident in control cells (Fig. 2A and C) whereas the distinct pattern was completely abolished in zoledronic acid treated cells (Fig. 2B and D) which exhibited diffuse staining for Cenp-F.
Fig 2.
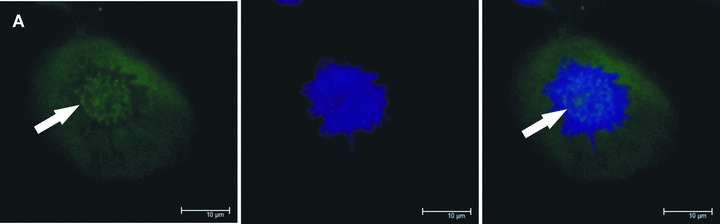
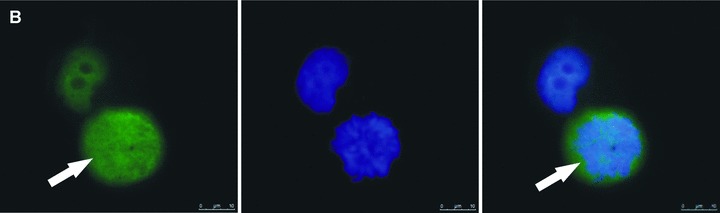
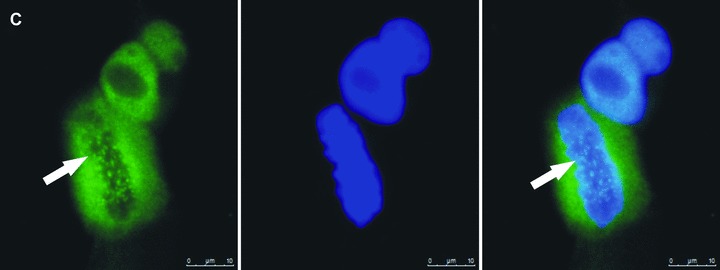
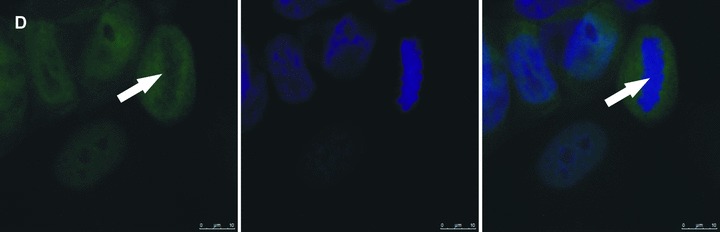
Zoledronic acid induces delocalization of Cenp-F from the kinetochore. In untreated MDA-MB-436 cells, Cenp-F is localized at the kinetochore (A, C). (B) and (D) show images of MDA-MB-436 cells treated with zoledronic acid (10 μM for 24 hrs + 24 hrs incubation in drug-free medium) at the respective mitotic stages to (A) and (C), but lacking Cenp-F associated with the kinetochore. Cells were stained using a specific antibody to Cenp-F (green) and DNA visualized by DAPI (blue). White arrows indicate cells of interest.
Quantitation showed that compared to untreated control, all of the cancer cell lines displayed a significant decrease in the number of mitotic cells in either prophase, prometaphase or metaphase with Cenp-F localized at the kinetochore following 24 hrs treatment with 10 μM and 25 μM zoledronic acid (Fig. 3). The reduction was 50.0% and 33.3% vs. 91.7%, both P < 0.001, MDA-MB-436 (Fig. 3A); 58.3% and 58.8% vs. 94.2%, P < 0.01 and P < 0.01, MDA-MB-231 (Fig. 3B); and 46.7% and 36.7% vs. 81.7%, P < 0.01 and P < 0.001, MCF-7 (Fig. 3C). Following 24 hrs exposure, there was no significant difference in the effect caused by the two drug concentrations, a dose of 10 μM was sufficient to cause significant levels of Cenp-F delocalization. This effect was further enhanced following 48 hrs continuous exposure to the drug. A total of 10 and 25 μM zoledronic acid caused a decrease in the percentage of MDA-MB-436 cells with Cenp-F localized at the kinetochore to 25.8% and 10.8%, respectively, (both P < 0.001 vs. control 90.0%), 49.2% and 20.0% in MDA-MB-231 cells (both P < 0.001 vs. control 96.7%) and 24.2% and 20.0% in MCF-7 cells (both P < 0.001 vs. control 80.8%).
Fig 3.
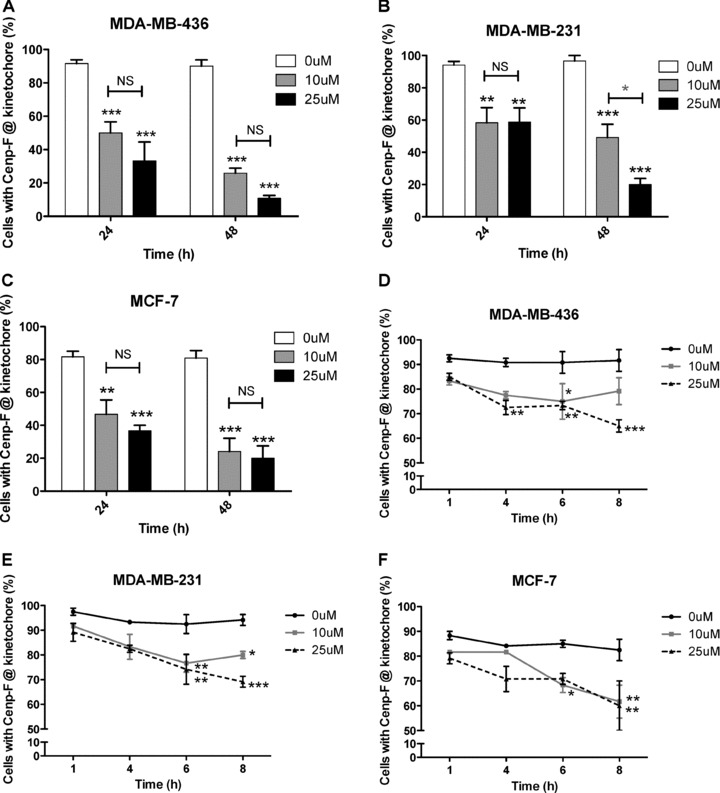
Zoledronic acid significantly reduces the percentage of cells with Cenp-F localized at the kinetochore. MDA-MB-436, MDA-MB-231 and MCF-7 cells were treated with 0, 10 or 25 μM zoledronic acid for 1, 4, 6, 8, 24 (followed by incubation in medium for up to 48 hrs) or cells were continuously exposed to zoledronic acid for 48 hrs. The percentage of cells in pro-, prometa- and metaphase with Cenp-F localized at the kinetochore was determined. Results are the mean ± SEM of three experiments. *P < 0.05, **P < 0.01, ***P < 0.001, for differences between each treatment group compared to corresponding control. *P < 0.05, for differences between corresponding treatment groups.
These data show that 24 hrs exposure to 10 μM zoledronic acid is sufficient to significantly reduce the number of mitotic breast cancer cells with Cenp-F localized at the kinetochore and this effect was increased with higher concentrations and prolonged treatment times. Because MDA-MB-436 cells appear to have the greatest sensitivity to zoledronic acid treatment, the cell line was chosen for a range of experiments. Furthermore a GFP transfected version of this cell line has been used in previous in vivo studies exploring the potential anti-tumour effects of zoledronic acid [15].
To confirm that the observed effect on Cenp-F delocalization is due to zoledronic acid’s ability to inhibit protein prenylation, experiments with the non-NBP clodronate were performed. Because clodronate is known to be less potent at inhibiting bone resorption than zoledronic acid [1] a dose of 500 μM was used to treat MDA-MB-436 cells for 48 hrs. Clodronate treatment did not cause any change in Cenp-F localization at the kinetochore, supporting that this effect is due to inhibition of FPP synthase by zoledronic acid (data not shown).
Zoledronic acid is rapidly cleared from the circulation to bone following in vivo infusion, and peripheral tissues, including tumours, will therefore only be exposed to this drug for a limited period of time. To establish the minimum exposure time required to affect Cenp-F localization, we determined the percentage of mitotic breast cancer cells with Cenp-F at the kinetochore following exposure to 10 or 25 μM zoledronic acid for 1, 4, 6 and 8 hrs. As shown in Fig. 3, treating MDA-MB-436 cells with 25 μM zoledronic acid for 4 hrs significantly decreased the proportion of cells with Cenp-F at the kinetochore compared to control (72.5% vs. 90.8%, P < 0.01, Fig. 3D). Similar decreases were observed after 6 hrs treatment of MDA-MB-231 cells with 10 and 25 μM zoledronic acid (76.7% and 74.2% vs. 92.5%, P < 0.01 and P < 0.01, Fig. 3E) and in MCF-7 cells treated with 10 μM (68.3% vs. 85.0%, P < 0.05, Fig. 3F). Increasing the zoledronic acid exposure time to 8 hrs caused significant reductions in kinetochore-associated Cenp-F-levels in all cell lines, with the highest effect found in MDA-MB-436 cells (65% vs. 91.7% in the control P < 0.001, Fig. 3D). These data show that treatment of breast cancer cells with zoledronic acid for as short as 6−8 hrs induced a significant decrease of cells with Cenp-F localized at the kinetochore. Although prolonged exposure (24–48 hrs) resulted in a more pronounced effect, shorter exposure times (6–8 hrs) may be sufficient to affect tumour cells by this mechanism.
Doses of zoledronic acid used in some in vitro experiments (10–25 μM for up to 48 hrs) are not clinically achievable as plasma levels of the drug, following 4 mg i.v. infusion, only reach up to 1–2 μM. Therefore, we investigated whether lower concentrations of zoledronic acid could affect the localization of Cenp-F. MDA-MB-436 cells were treated with 0, 4, 6, 10 or 25 μM zoledronic acid for 48 hrs continuously and were analysed for Cenp-F localization. As shown in Fig. 4, treatment with 6 μM zoledronic acid for 48 hrs significantly decreased the percentage of cells with Cenp-F at the kinetochore when compared to untreated control (59.2% vs 90%, P < 0.05), and a dose-dependent increase in the number of cells without Cenp-F at the kinetochore was observed (33.3% and 21.2% vs control 90%, P < 0.001 and P < 0.001).
Fig 4.
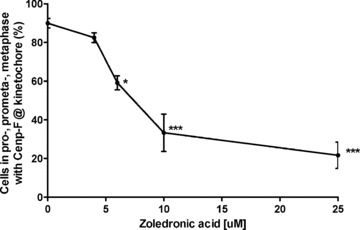
Low zoledronic acid doses affect Cenp-F localization at the kinetochore. MDA-MB-436 cells were treated with 0, 4, 6, 10 and 25 μM zoledronic acid for 48 hrs prior to DNA and Cenp-F staining. Cells were then analysed by microscopy for Cenp-F localization. Results are the mean ± SEM of three experiments. *P < 0.05, ***P < 0.001 for differences between each treatment group compared to control.
These data show that a concentration of zoledronic acid as low as 6 μM is sufficient to induce loss of Cenp-F at the kinetochore, suggesting that high drug concentrations are not required to affect fast growing cancer cells.
Effects of zoledronic acid on mitosis progression in breast cancer cells
Localization of Cenp-F to the kinetochore is dependent on farnesylation of the CAAX motif at the C-terminus of the protein, and studies using siRNA have found that Cenp-F-depleted cells show a delay in mitosis progression [9, 16]. Inhibition of Cenp-F farnesylation caused by FTI treatment is reported to induce a similar inhibition in cell cycle progression to that seen in Cenp-F-depleted cells [17]. We therefore investigated whether zoledronic acid induced depletion of Cenp-F from the kinetochore had an effect on mitosis progression in breast cancer cells. This was assessed following treatment of MDA-MB-231, MDA-MB-436 and MCF-7 cells with increasing doses of zoledronic acid for up to 48 hrs, and determination of the number of cells in each mitotic stage (prophase, prometaphase, metaphase, anaphase and telophase) compared to untreated control. A significant increase of MDA-MB-231 cells in pro-, prometa- and metaphase was observed after exposure to 10 μM zoledronic acid for 24 hrs (83.3% vs. 65.8%, P < 0.05, Fig. 5B) and in MDA-MB-436 cells after 48 hrs treatment with 25 μM zoledronic acid (91.6% vs. 76.6%, P < 0.05, Fig. 5A). MCF-7 cells in prophase, prometaphase and metaphase increased significantly after 24 hrs treatment with 10 μM zoledronic acid (84.2% vs. 65%, P < 0.05, Fig. 5C) but not at 48 hrs to either drug concentration. These data suggest that 25 μM zoledronic acid for 48 hrs has little effect on mitosis progression in human breast cancer cells when determining the effect regardless to Cenp-F localization of the cells.
Fig 5.
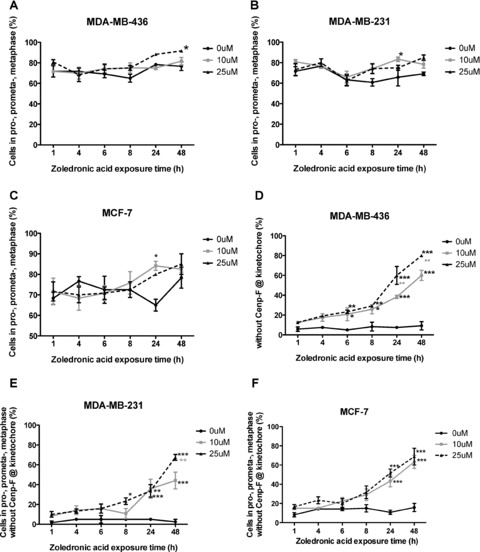
Zoledronic acid causes an increase of breast cancer cells in pro-, prometa- and metaphase lacking Cenp-F at the kinetochore. MDA-MB-436, MDA-MB-231 and MCF-7 cells were treated with 0, 10 or 25 μM zoledronic acid for 1, 4, 6, 8, and 24 hrs (followed by incubation for up to 48 hrs in medium), or cells were continuously exposed to zoledronic acid for 48 hrs. The percentage of cells in pro-, prometa-, meta-, ana- and telophase was determined. Percentages of cells in pro-, prometaphase and metaphase regardless of Cenp-F localization are depicted in (A–C). In addition, the percentage of cells in pro-, prometa- and metaphase without Cenp-F localized at the kinetochore was determined (D–F). Results are the mean ± SEM of three experiments. *P < 0.05, **P < 0.01, ***P < 0.001, for differences between each treatment group compared to corresponding control. **P < 0.01 for differences between treatment groups.
We next investigated whether progression of cells in early stages of mitosis without Cenp-F at the kinetochore was affected to a greater extend compared to cells with correct Cenp-F localization. Breast cancer cells were treated with zoledronic acid for up to 48 hrs, stained for Cenp-F, and the number of cells in pro-, prometa- and metaphase lacking Cenp-F at the kinetochore was determined. The number of cells in ana- and telophase was also assessed. In MDA-MB-436 cells, 10 and 25 μM zoledronic acid induced a significant increase in the percentage of pro-, prometa- and metaphase cells without Cenp-F at the kinetochore after 6 hrs, compared to control (20.8% and 23.3% vs. 5%, P < 0.05 and P < 0.01, Fig. 5D). This was further enhanced with increasing treatment times (60% and 80% vs. 9.2% at 48 hrs, P < 0.001 and P < 0.001). Although an effect of treatment was seen in all cell lines, it was less pronounced in MDA-MB-231 and MCF7, compared to MDA-MB-436 cells. A significant increase in MDA-MB-231 cells in pro-, prometa and metaphase without Cenp-F at the kinetochore could be seen following 8 hrs treatment with 25 μM zoledronic acid compared to control (23.3% vs. 5%, P < 0.05, Fig. 5E). This increased to 44.2% and 67.5% (both P < 0.001 compared to control of 2.5%), following 48 hrs treatment with 10 and 25 μM. In MCF-7 cells, 24 hrs exposure to 10 and 25 μM zoledronic acid increased the cells in pro-, prometa- and metaphase to 43.3% and 51.7% vs. control levels of 10.8%, both P < 0.001 (Fig. 5F), reaching maximum after 48 hrs continuous exposure to 10 and 25 μM (63.3% and 68.75% vs. 15.8%, P < 0.001 and P < 0.001). These data show that exposing breast cancer cells to 10 μM zoledronic acid for a period of 6–8 hrs is sufficient to induce a significant increase in the number of MDA-MB-231 and MDA-MB-436 cells lacking Cenp-F at the kinetochore with a shift towards prophase, prometaphase and metaphase. In comparison, the estrogen-dependent cell line MCF-7 seems to be less sensitive to the drugs effect on the cell.
In order to confirm that the effect of zoledronic acid on Cenp-F is specific to fast proliferating cell lines, such as cancer cells, the same experiment was performed with the non-malignant breast cancer cell line MCF-10A. In these cells no change in Cenp-F localization or mitosis progression was observed after zoledronic acid treatment (25 μM for 8 or 24 hrs followed by incubation in drug-free medium up to a total of 48 hrs, or 48 hrs continuous exposure), suggesting that rapidly proliferating cancer cells are more sensitive to the effects of zoledronic acid treatment (data not shown).
The mevalonate pathway intermediary FOH reverses zoledronic acid induced Cenp-F delocalization from the kinetochore
Zoledronic acid causes inhibition of FPP synthase, a key enzyme of the mevalonate pathway, resulting in reduced levels of prenylated proteins [3]. In order to determine whether the zoledronic acid induced reduction of Cenp-F associated with the kinetochore was mediated via inhibition of the mevalonate pathway, we investigated the effects of combining zoledronic acid with the mevalonate pathway intermediates GGOH and FOH.
MDA-MB-436 cells were treated with 25 μM zoledronic acid ± 50 μM /100 μM FOH or 50 μM /100 μM GGOH for 48 hrs, and the number of cells with Cenp-F localized at the kinetochore was determined. As expected, zoledronic acid caused a reduction in the percentage of cells with Cenp-F at the kinetochore, but there was no significant effect of either FOH or GGOH alone compared to control (Fig. 6). Treatment with 25 μM zoledronic acid combined with either 50 μM or 100 μM FOH showed a significant increase in cells with Cenp-F localized at the kinetochore, compared to cells treated with zoledronic acid alone (49.2% and 60.0% vs. 16.7%, P < 0.01 and P < 0.01, Fig. 6A). In contrast, inclusion of GGOH did not significantly affect the ability of zoledronic acid to cause loss of Cenp-F (Fig. 6B) when used at identical concentrations to FOH. When using higher GGOH doses (150 μM) together with 25 μM zoledronic acid, the effect of the drug on Cenp-F localization at the kinetochore could be reversed (data not shown).
Fig 6.
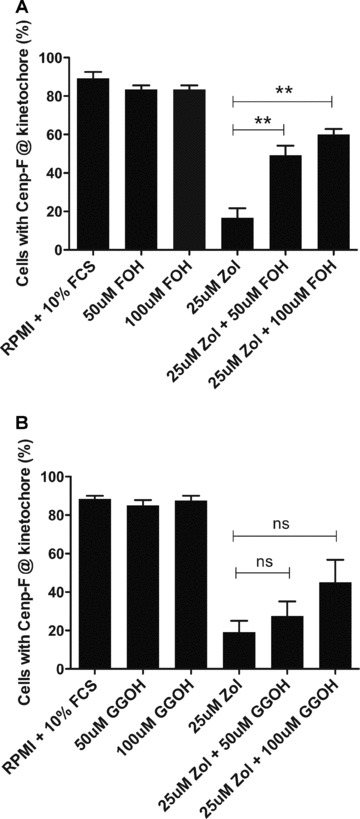
The delocalization of Cenp-F from the kinetochore is reversed by low concentrations of FOH but not GGOH. MDA-MB-436 cells were treated with 25 μM zoledronic acid in the presence or absence of 50 μM/100 μM FOH (A) or 50 μM/100 μM GGOH (B) for 48 hrs. Cells in pro-, prometa- and metaphase with Cenp-F localized at the kinetochore were determined. Results are the mean ± SEM of three experiments. **P < 0.01 for differences between each combined treatment group compared to zoledronic acid alone.
These data show that lower doses of FOH are superior to GGOH in reversing the effect on Cenp-F induced by zoledronic acid, which is in agreement with the proposed importance of farnesylation for Cenp-F localization and function.
Zoledronic acid disrupts chromosome alignment and spindle morphology in breast cancer cells
Several siRNA studies have reported that depletion of Cenp-F results in chromosome misalignment as well as in increased numbers of cells with multipolar spindles [8, 16, 18, 19]. We therefore investigated whether the zoledronic acid induced reduction of kinetochore-associated Cenp-F was associated with changes in chromosome alignment and/or spindle morphology. MDA-MB-436 cells were exposed to 4, 6, 10 and 25 μM zoledronic acid for 48 hrs and stained for Cenp-F and DNA. The cells that scored positive for misalignment had chromosomes distributed broadly around the prometaphase-, and metaphase plates and/or chromosomes remained far outside of these structures. We found that the number of mitotic cells without Cenp-F at the kinetochore and misaligned chromosomes was significantly increased following treatment with 10 and 25 μM zoledronic acid when compared to control (4.7% and 7.3% vs. 0.3%, P < 0.05 and P < 0.001, Fig. 7). In addition to the drug-induced disruption of chromosome alignment (Fig. 7A) we also observed cells containing tripolar spindles (Fig. 7B), but the increase was not statistically significant for any of the drug concentrations tested. The same experiment was undertaken using the non-malignant breast cell line MCF-10A but no effect on either chromosome alignment or spindle morphology was observed (data not shown). In agreement with other published data, our results show that depletion of Cenp-F from the kinetochore may cause failure in chromosome alignment and abnormalities in spindle morphology.
Fig 7.
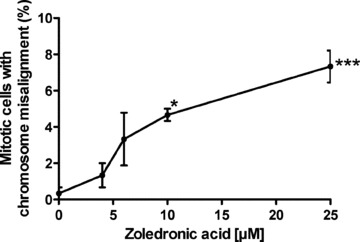
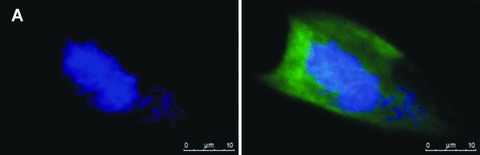
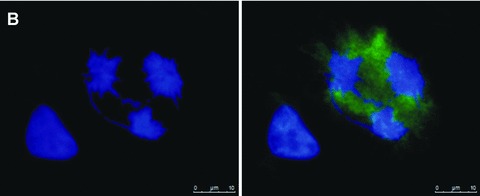
Zoledronic acid induces a significant increase of breast cancer cells with chromosomes lagging outside of the metaphase plate. MDA-MB-436 cells were treated with 0, 4, 6, 10 and 25 μM zoledronic acid for 48 hrs. Cells were stained for Cenp-F and DNA and 100 mitotic cells were counted in each treatment group. Cells were scored for mitotic structures with lagging chromosomes and without Cenp-F localized at the kinetochore. Results are the mean ± SEM of three experiments. *P < 0.05, ***P < 0.001 for differences between each treatment group compared to corresponding control. Example images of MDA-MB-436 cells treated with zoledronic acid for 48 hrs showing the induced chromosome misalignment (A) and formation of tripolar spindles (B). Cells were stained using a specific antibody to Cenp-F (green) and DNA visualized by DAPI (blue).
Discussion
The direct anti-tumour effects of NBPs, in particular zoledronic acid, are still controversial, and the specific molecular mechanisms responsible remain to be identified. There is, however, an agreement that in a range of tumour models, zoledronic acid does inhibit cell growth as well as induce apoptosis, and these effects can be reversed by incorporation of intermediaries of the mevalonate pathway [20, 21, 22]. Regardless of the cell type, NBPs inhibit FPP synthase, resulting in a depletion of the pool of prenylated forms of proteins required for a variety of cellular functions. The effects of zoledronic acid on prenylated proteins such as the small GTPases, including Ras, Rab and Rho, have been studied in some detail. These GTPases are involved in the regulation of vesicular trafficking, cytoskeletal arrangement and cell survival, and are therefore key targets for NBPs in osteoclasts, recently reviewed by Coxon et al. [23]. So far no studies of the effects of zoledronic acid on other types of prenylated proteins have been reported, despite more than 300 peptides with prenylation motifs having been identified in the human proteome [17], together with 60 farnesylated eukaryotic proteins [11]. Studies aiming to identify the molecular effects of zoledronic acid are important to increase our understanding of its proposed anti-tumour action. One example is the study by Marra et al. [24] who found a zoledronic acid induced decrease in Cyr61, an angiogenic inducer, in prostate cancer cells. We have investigated the effects of zoledronic acid on Cenp-F, a farnesylated protein implicated in the assembly of the kinetochore structures required for correct chromosome alignment and separation during mitosis (Table 1). We found that in both hormone-dependent and -independent breast cancer cells, zoledronic acid does cause loss of Cenp-F from the kinetochore. Our findings are in agreement with the results reported by Schafer-Hales et al., who investigated the effect of the FTI lonafarnib on 15 human cancer cell lines, including the breast cancer cell lines MCF-7, MDA-MB-231 and MDA-MB-436 [11]. They reported a reduction in the percentage of Cenp-F at the kinetochore after drug treatment, suggesting that farnesylation is required for the correct localization of Cenp-F. In contrast, exposure of zoledronic acid to the non-malignant breast cell line MCF-10-A did not affect Cenp-F localization and the conflicting results may be influenced by several factors including a difference in drug uptake, different effects on FPPs or the effect could be tumour cell specific possibly associated with the increased proliferation rate found in malignant cell types.
Table 1.
Summary of findings
| Research question | Cell line | Cenp-F localization at the kinetochore | ||||||
|---|---|---|---|---|---|---|---|---|
| Reduced | Not reduced | |||||||
| Effect of zol on Cenp-F localization at the kinetochore | MDA-MB-436 | X | ||||||
| MDA-MB-231 | X | |||||||
| MCF-7 | X | |||||||
| Effect of Clodronate on Cenp-F localization at the kinetochore | MDA-MB-436 | X | ||||||
| Effect of low zol concentrations on Cenp-F localization at the kinetochore | MDA-MB-436 | X (from 6 μM) | X (below 4uM) | |||||
| Effect of FOH/GGOH on zol-induced Cenp-F delocalization | MDA-MB-436 | X zol + GGOH | X zol + FOH | |||||
| Research question | Cell line | Cells in pro-, prometa-, metaphase (%) | ||||||
| Increased | No effect | |||||||
| Effect of zol on mitosis progression | MDA-MB-436 | X (only 25 μM, 48 hrs) | X | |||||
| MDA-MB-231 | X (only 10 μM, 24 hrs) | X | ||||||
| MCF-7 | X (only 10 μM, 24 hrs) | X | ||||||
| Effect of zol on mitosis progression without Cenp-F localization at the kinetochore | MDA-MB-231 | X | ||||||
| MDA-MB-436 | X | |||||||
| MCF-7 | X | |||||||
| MCF-10A | X | |||||||
| Research question | Cell line | Chromosome misalignment | ||||||
| Yes | No | |||||||
| Effect of zol-induced Cenp-F delocalization on chromosome alignment | MDA-MB-436 | X | ||||||
| MCF-10A | X | |||||||
We could show that the zoledronic acid induced delocalization of Cenp-F in cancer cells was caused by inhibition of the mevalonate pathway, because the presence of FOH, but not GGOH, effects of zoledronic acid on Cenp-F localization were significantly reversed. The requirement for farnesylation of Cenp-F for kinetochore localization has also been reported by Hussein and Taylor [9]. Post-translational prenylation is mainly involved in ensuring correct membrane association of proteins [25], and it is not clear why lack of farnesylation should cause Cenp-F redistribution, as the kinetochore does not include any membrane structures. It has been suggested that farnesylation is also involved in protein–protein interactions, and that accumulation of unprenylated forms of proteins may compete for effectors [23]. In this context, it may be of importance that two proteins closely associated with both Cenp-F and the kinetochore, Cenp-E and lamin B, are also farnesylated and therefore likely to be affected by zoledronic acid treatment [17, 26, 27]. Hence it is possible that in addition to Cenp-F, several other proteins involved in ensuring correct chromosome segregation will be affected by zoledronic acid, but this remains to be established.
The standard dosing regimen of zoledronic acid used in treatment of cancer-induced bone disease is a 4 mg infusion every 3–4 weeks, and the high affinity of NBPs for bone and their rapid clearance from the general circulation means that peripheral tumours will be exposed to these drugs for no more than a few hours [20]. This has been one of the main arguments against the ability of zoledronic acid to exert direct anti-tumour effects during standard clinical use. The majority of in vitro and in vivo studies reporting anti-tumour effects of NBPs have involved the use of very high or frequent repeated dosing. Although there was some variation between the cell lines, 6-hr treatment with zoledronic acid did cause a significant increase in the number of mitotic cells lacking Cenp-F at the kinetochore, indicating that prolonged exposure to the drug is not required in order to generate this effect.
Protein farnesylation is increasingly investigated as a potential target in cancer care and FTIs are currently the subject of investigation in a number of clinical trials. However results are contrary with some studies reporting no beneficial effects (Schering-Plough, Lonafranib in Non-small-cell lung cancer) whereas others could report anti-tumour effects when giving the FTI R115777 in combination with Capecitabine (Eastern Cooperative Oncology Group). The efficacy of FTIs in cancer treatment therefore remains to be established and the contrary results may implicate that a greater anti-cancer benefit could be achieved when targeting both farnesylated and geranylgeranylated proteins as done by NBPs. However, farnesylated proteins do play a major role in cancer proliferation and progression and should not be underestimated as potential anti-cancer targets.
In studies using FTIs, it has been shown that inhibition of Cenp-F farnesylation does result in a delay in cell cycle progression rather than a complete block, and there was an increase in the proportion of lung cancer cells accumulating in G2/M in the presence of FTIs [28, 29]. Delayed cell cycle progression is likely to affect fast growing tumour cells, and we show that three different breast cancer cell lines show a similar response to zoledronic acid in relation to Cenp-F, suggesting that this is not a phenomenon associated with a single cell line. In contrast, non-dividing osteoclasts will be unaffected by disruption of chromosome separation. Delocalization of Cenp-F is therefore unlikely to be involved in the inhibition of bone resorption caused by zoledronic acid, where the correct membrane localization of small GTPases are of greater importance due to their established roles in osteoclast function [2, 23]. The lack of the effect of zoledronic acid on Cenp-F localization seen in MCF-10A cells suggests that the drug does not exhibit a general activity in all cell types.
Several studies have reported NBP-induced reduction of tumour cell proliferation, including neuroblastoma [30], lung [31], prostate [32] and breast [33]. Several studies reported an effect of FTIs on cancer cell progression through G2/M phase, suggesting that this is caused by inhibition of protein farnesylation. Ashar et al. found that the FTI SCH 66336 induced accumulation of human lung cancer cells in G2/M-phase, but did not find any association with Cenp-F depletion from the kinetochore [17, 34]. Crespo et al. reported that treatment of lung cancer cells with the FTI-2153 caused a significant increase in prometaphase cells, again without any effect of the drug on Cenp-F localization at the kinetochore [29]. In contrast, Hussein and Taylor have shown that Cenp-F farnesylation is required for progression through G2 to M-phase, and that Cenp-F farnesylation is essential for localization to the kinetochore and the nuclear membrane, suggesting a possible connection between the farnesylation status and intracellular protein localization [9].
We further determined whether the drug affected the ability of the cells to enter mitosis, or if progression through M-phase was delayed at a particular stage. Therefore, we treated breast cancer cells with zoledronic acid and assessed the percentage of cells in each mitotic phase, that is pro-, prometa-, meta-, ana- and telophase, by microscopy. Although first experiments showed little effect of zoledronic acid on mitosis progression, it was determined whether cells lacking Cenp-F at the kinetochore were more prone to show changes in mitosis progression. There was a significant increase of cells in pro-, prometa- and metaphase lacking Cenp-F at the kinetochore in all breast cancer cell lines tested when compared to control. These findings suggest that the zoledronic acid induced lack of Cenp-F at the kinetochore plays a role in the accumulation of cells in the early stages of mitosis and that impairment of the Cenp-F and kinetochore association causes disruption of M-phase progression. The observed accumulation of cells in early stages of M-phase following treatment with zoledronic acid suggests a cell cycle delay at either the progression from G2 to M-phase, or a delay of progression from prophase to metaphase. Published studies of the effects of zoledronic acid on the cell cycle of different cancer cell lines have shown an accumulation of cells in S-phase [12, 13]. Our results reflect the findings of Merrell et al. who reported a decrease of cells in S and a concomitant increase in G1 phase in MDA-MB-231 cells treated with 10 μM zoledronic acid for 72 hrs [35].
The impaired chromosome alignment observed in zoledronic acid treated cells could be due to insufficient microtubule-chromosome attachment as it has been shown that Cenp-F depletion can cause failure/loss of Cenp-E, cytoplasmic dynein and dynactin accumulation at the kinetochore [8]. Cenp-E is a plus end motor protein and is therefore important for correct microtubule attachment [36], and Cenp-E is also a farnesylated protein. Similar effects were reported on cell cycle progression and chromosome alignment after protein silencing of Cenp-E compared to Cenp-F [37]. The effect of the drug on chromosome alignment and M-phase progression may be a combined effect of faulty Cenp-F, Cenp-E (and probably other prenylated kinetochore proteins) localization and maintenance. However, because Cenp-E recruitment to the kinetochore has been shown to be partly dependent on Cenp-F [6], it is most likely that loss of Cenp-F from the kinetochore is the ‘master switch’ for the observed effects.
Once we had established that zoledronic acid caused disruption of M-phase progression, we investigated the consequences of Cenp-F delocalization on chromosome alignment and separation, as this may potentially explain the disruption of mitosis progression. Following zoledronic acid treatment there was a significant increase in the number of cells lacking Cenp-F at the kinetochore that had chromosomes distributed far outside of the normal metaphase plate. This phenotype reflects the findings of other studies of the effects of Cenp-F depletion on chromosome separation. Yang et al. showed that Cenp-F depletion by RNAi caused severe defects in chromosome alignment, accompanied by reduced sister kinetochore tension and premature chromosome decondensation, finally resulting in apoptosis [8]. Holt et al. reported that siRNA depletion of Cenp-F resulted in disrupted chromosome alignment and prolonged mitosis, possibly mediated by the activation of the SAC through the checkpoint kinase BubR1 [16]. Impaired chromosome alignment, due to faulty kinetochore-microtubule attachment, could activate the SAC and thus stop cells from progressing through mitosis, resulting in the observed accumulation of cells in pro- and prometaphase [8, 16]. It has also been shown that Cenp-F is a microtubule binding protein that may be part of the microtubule-chromosome attachment machinery [18, 28]. In addition to the impaired chromosome alignment, Cenp-F-depleted cells show abnormalities in spindle morphology [16]. Yang et al. found that approximately 16% of HeLa cells lacking Cenp-F exhibited multipolar spindles, and that Cenp-F-depleted cells in late pro- or metaphase had abnormal bipolar spindles [8]. In a comprehensive study of the effects of Cenp-F depletion, Bomont found that this would result in gross chromosome missegregation in only a subset of cells, whereas in the majority of cells the effect was mitotic delay [28]. In agreement with these results, we observed the presence of multipolar spindles in zoledronic acid-treated cells lacking Cenp-F at the kinetochore.
Our data show that in rapidly proliferating breast cancer cells, zoledronic acid causes disruption of Cenp-F localization at the kinetochore. The effects were caused by inhibition of the mevalonate pathway, most likely by reducing the levels of post-translational farnesylation of Cenp-F. This is the first report showing that zoledronic acid may exert part of its anti-tumour effect by disrupting kinetochore assembly and subsequently reducing tumour cell proliferation.
Acknowledgments
PDO is funded by Breast Cancer Campaign UK. Zoledronic acid was a kind gift from Novartis Pharma. We thank Dr. Peter Grabowski for his help with the fluorescence microscopy studies and Dr. Karen Sisley for her help with the evaluation of mitotic stages.
References
- 1.Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–7. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 2.Coxon FP, Helfrich MH, Van’t Hof R, et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–76. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 3.Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 4.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34:453–75. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer cell. 2005;7:297–300. doi: 10.1016/j.ccr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Johnson VL, Scott MI, Holt SV, et al. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci. 2004;117:1577–89. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- 7.Liao H, Winkfein RJ, Mack G, et al. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol. 1995;130:507–8. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Guo J, Chen Q, et al. Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol Cell Biol. 2005;25:4062–74. doi: 10.1128/MCB.25.10.4062-4074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussein D, Taylor SS. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci. 2002;115:3403–14. doi: 10.1242/jcs.115.17.3403. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Chang KH, He D, et al. The C terminus of mitosin is essential for its nuclear localization, centromere/kinetochore targeting, and dimerization. J Biol Chem. 1995;270:19545–50. doi: 10.1074/jbc.270.33.19545. [DOI] [PubMed] [Google Scholar]
- 11.Schafer-Hales K, Iaconelli J, Snyder JP, et al. Farnesyl transferase inhibitors impair chromosomal maintenance in cell lines and human tumors by compromising CENP-E and CENP-F function. Mol Cancer Ther. 2007;6:1317–28. doi: 10.1158/1535-7163.MCT-06-0703. [DOI] [PubMed] [Google Scholar]
- 12.Dumon JC, Journé F, Kheddoumi N, et al. Cytostatic and apoptotic effects of bisphosphonates on prostate cancer cells. Eur Urol. 2004;45:521–8. doi: 10.1016/j.eururo.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Ory B, Blanchard F, Battaglia S, et al. Zoledronic acid activates the DNA S-phase checkpoint and induces osteosarcoma cell death characterized by apoptosis-inducing factor and endonuclease-G translocation independently of p53 and retinoblastoma status. Mol Pharmacol. 2007;71:333–43. doi: 10.1124/mol.106.028837. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Mancini MA, Chang KH, et al. Characterization of a novel 350-kiloDalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol Cell Biol. 1995;15:5017–29. doi: 10.1128/mcb.15.9.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottewell PD, Monkkonen H, Jones M, et al. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100:1167–78. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 16.Holt SV, Vergnolle MA, Hussein D, et al. Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci. 2005;118:4889–900. doi: 10.1242/jcs.02614. [DOI] [PubMed] [Google Scholar]
- 17.Ashar HR, James L, Gray K, et al. Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem. 2000;275:30451–57. doi: 10.1074/jbc.M003469200. [DOI] [PubMed] [Google Scholar]
- 18.Feng J, Huang H, Yen TJ. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma. 2006;115:320–9. doi: 10.1007/s00412-006-0049-5. [DOI] [PubMed] [Google Scholar]
- 19.Laoukili J, Kooistra MR, Bras A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 20.Neville-Webbe HL, Rostami-Hodjegan A, Evans CA, et al. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer. 2005;113:364–71. doi: 10.1002/ijc.20602. [DOI] [PubMed] [Google Scholar]
- 21.Coxon JP, Oades GM, Kirby RS, et al. Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU Int. 2004;94:164–70. doi: 10.1111/j.1464-4096.2004.04831.x. [DOI] [PubMed] [Google Scholar]
- 22.Virtanen SS, Väänänen HK, Härkönen PL, et al. Alendronate inhibits invasion of PC-3 prostate cancer cells by affecting the mevalonate pathway. Cancer Res. 2002;62:2708–14. [PubMed] [Google Scholar]
- 23.Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:307–12. doi: 10.1016/j.coph.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Marra M, Santini D, Meo G, et al. Cyr61 downmodulation potentiates the anticancer effects of zoledronic acid in androgen-independent prostate cancer cells. Int J Cancer. 2009;125:2004–1. doi: 10.1002/ijc.24648. [DOI] [PubMed] [Google Scholar]
- 25.McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–67. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusiñol AE, Sinensky MS. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J Cell Sci. 2006;119:3265–72. doi: 10.1242/jcs.03156. [DOI] [PubMed] [Google Scholar]
- 27.Tamanoi F, Kato-Stankiewicz J, Jiang C, et al. Farnesylated proteins and cell cycle progression. J Cell Biochem. 2001;37:64–70. doi: 10.1002/jcb.10067. [DOI] [PubMed] [Google Scholar]
- 28.Bomont P, Maddox P, Shah JV, et al. Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 2005;24:3927–39. doi: 10.1038/sj.emboj.7600848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crespo NC, Ohkanda J, Yen TJ, et al. The farnesyltransferase inhibitor, FTI-2153, blocks bipolar spindle formation and chromosome alignment and causes prometaphase accumulation during mitosis of human lung cancer cells. J Biol Chem. 2001;276:16161–7. doi: 10.1074/jbc.M006213200. [DOI] [PubMed] [Google Scholar]
- 30.Peng H, Sohara Y, Moats RA, et al. The activity of zoledronic Acid on neuroblastoma bone metastasis involves inhibition of osteoclasts and tumor cell survival and proliferation. Cancer Res. 2007;67:9346–55. doi: 10.1158/0008-5472.CAN-06-4508. [DOI] [PubMed] [Google Scholar]
- 31.Li YY, Chang JW, Chou WC, et al. Zoledronic acid is unable to induce apoptosis, but slows tumor growth and prolongs survival for non-small-cell lung cancers. Lung Cancer. 2008;59:180–91. doi: 10.1016/j.lungcan.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Caraglia M, Marra M, Leonetti C, et al. R115777 (Zarnestra)/Zoledronic acid (Zometa) cooperation on inhibition of prostate cancer proliferation is paralleled by Erk/Akt inactivation and reduced Bcl-2 and bad phosphorylation. J Cell Physiol. 2007;211:533–43. doi: 10.1002/jcp.20960. [DOI] [PubMed] [Google Scholar]
- 33.Verdijk R, Franke HR, Wolbers F, et al. Differential effects of bisphosphonates on breast cancer cell lines. Cancer Lett. 2007;246:308–12. doi: 10.1016/j.canlet.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Ashar HR, James L, Gray K, et al. The farnesyl transferase inhibitor SCH 66336 induces a G(2) –> M or G(1) pause in sensitive human tumor cell lines. Exp Cell Res. 2001;262:17–27. doi: 10.1006/excr.2000.5076. [DOI] [PubMed] [Google Scholar]
- 35.Merrell MA, Wakchoure S, Lehenkari PP, et al. Inhibition of the mevalonate pathway and activation of p38 MAP kinase are independently regulated by nitrogen-containing bisphosphonates in breast cancer cells. Eur J Pharmacol. 2007;570:27–37. doi: 10.1016/j.ejphar.2007.05.075. [DOI] [PubMed] [Google Scholar]
- 36.Yen TJ, Li G, Schaar BT, et al. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992;359:536–39. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]
- 37.Tanudji M, Shoemaker J, L’Italien L, et al. Gene silencing of CENP-E by small interfering RNA in HeLa cells leads to missegregation of chromosomes after a mitotic delay. Mol Biol Cell. 2004;15:3771–81. doi: 10.1091/mbc.E03-07-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]


