Abstract
Premature birth accounts for approximately 75% of neonatal mortality and morbidity in the developed world. Despite this, methods for identifying and treating women at risk of preterm labour are limited and many women still present in preterm labour requiring tocolytic therapy to suppress uterine contractility. The aim of this study was to assess the utility of Kv7 channel activators as potential uterine smooth muscle (myometrium) relaxants in tissues from pregnant mice and women. Myometrium was obtained from early and late pregnant mice and from lipopolysaccharide (LPS)-injected mice (day 15 of gestation; model of infection in pregnancy). Human myometrium was obtained at the time of Caesarean section from women at term (38–41 weeks). RT-PCR/qRT-PCR detected KCNQ and KCNE expression in mouse and human myometrium. In mice, there was a global suppression of all KCNQ isoforms, except KCNQ3, in early pregnancy (n= 6, P < 0.001 versus late pregnant); expression subsequently increased in late pregnancy (n= 6). KCNE isoforms were also gestationally regulated (P < 0.05). KCNQ and KCNE isoform expression was slightly down-regulated in myometrium from LPS-treated-mice versus controls (P < 0.05, n= 3–4). XE991 (10 μM, Kv7 inhibitor) significantly increased spontaneous myometrial contractions in vitro in both human and mouse myometrial tissues (P < 0.05) and retigabine/flupirtine (20 μM, Kv7 channel activators) caused profound myometrial relaxation (P < 0.05). In summary, Kv7 activators suppressed myometrial contraction and KCNQ gene expression was sustained throughout gestation, particularly at term. Consequently, activation of the encoded channels represents a novel mechanism for treatment of preterm labour.
Keywords: potassium channel, uterus, preterm labour, KCNQ, KCNE
Introduction
Spontaneous preterm labour is a major cause of preterm birth (<37/40 weeks of gestation). Preterm births account for ∼6–7% of all deliveries in developed countries and up to 25% in undeveloped nations. In the developed world, approximately 75% of neonatal deaths and a majority of neonatal intensive care admissions, arise from preterm babies born before 32 weeks of gestation [1]. In addition, the short- and long- term economic consequences of preterm birth are considerable [2, 3].
Clinical management strategies for women at risk of preterm labour are restricted as women cannot currently be identified accurately from the antenatal population. As a result, most women present in threatened preterm labour requiring tocolytic (agents that inhibit uterine contractions) treatment. However, the tocolytics currently employed clinically (oxytocin receptor antagonists, β2 mimetics and nifedipine) provide only limited benefit either in prevention of labour or in regard to neonatal outcome [4]. Development of more effective tocolytic therapies would have major benefits for maternal and neonatal health worldwide and lead to substantive reductions in health care costs.
Improved understanding of the processes responsible for suppressing myometrial (uterine smooth muscle) contractility during normal pregnancy will inevitably inform development of effective therapeutic strategies. Potassium channel expression and function is particularly relevant as these channels are major contributors to the resting membrane potential, thereby influencing cell excitability and repolarization in the smooth muscle of the myometrium. In human myometrial smooth muscle, the resting membrane potential becomes less negative toward the end pregnancy and labour onset [5] and this is likely to be driven in part by down-regulation of (functional) potassium (K+) channels [6, 7]. We have shown that voltage-dependent K+ channels encoded by the ‘ether–a-go-go related’ gene (KCNH1) become functionally redundant at the end of mouse pregnancy due to up-regulation of accessory β subunits [8]. Paradoxically, however, we and others have also demonstrated that voltage-dependent (Kv) channels in general still play a key role in rat and murine myometrium at term [9, 10]. The conclusion, therefore, is that subsets of Kv channels exist to enable repolarization and myometrial relaxation during the rhythmic contractile activity associated with labour [7, 11]. These Kv channel subtypes represent novel targets for tocolysis as expression in myometrium is coincidental with the time of labour.
Recent studies from our laboratory in murine myometrium indicate that expression of KCNQ (α-subunits) and KCNE (β-subunits) genes, which encode Kv7 channels, are modulated during the oestrous cycle [12] where they contribute to non-pregnant mouse myometrial contractility. The aim of the present study was to establish the expression and function of myometrial Kv7 channels during pregnancy in mouse and human myometrium and, with a view to development of new tocolytic strategies, to determine whether Kv7 channel activators act as uterine relaxants at term. To address their potential role in infection associated preterm labour and to establish the influence of infection on KCNQ/KCNE expression, an intraperitoneal model of lipopolysaccharide (LPS)-induced preterm labour was utilized. This model induces a systemic inflammatory reaction and shares many of the characteristics of the inflammatory response associated with infection in human preterm labour [13].
Materials and methods
All reagents were supplied by Sigma-Aldrich (Gillingham, UK) unless otherwise stated in the text.
Tissue collection
Time-mated mice
Female C57/BL6 mice (Charles River Laboratories, Margate, UK) were killed by cervical dislocation, in accordance with UK Home Office guidelines, in early (days 6–7) and late pregnancy (days 17–18). Uterine horns were dissected, opened longitudinally and foetuses/placentae removed. The decidua/endometrium was then gently removed using a cotton bud. Myometrium was dissected and either immediately snap frozen in liquid nitrogen for RNA and protein isolation and stored at −80°C or placed straight into ice cold phosphate-buffered saline (PBS, Sigma-Aldrich) for isometric tension recording. Samples for RNA extraction were later transferred to RNAlaterICE (according to manufacturers recommendations: Ambion, Warrington, UK).
Lipopolysaccharide treated mice
C57/BL6 mice (8+ weeks, n= 6) were mated during 2 hr time periods. Day 0 was determined by visualization of copulatory plug and on the 15th day of pregnancy, mice were injected intraperitoneally with 100 μg LPS from Escherichia coli, serotype 0111:B4 (Sigma, St. Louis, MO, USA) in sterile saline (n= 4) or with vehicle alone (n= 3). Mice injected with LPS delivered approximately 16 hrs after injection if left undisturbed (data not shown, [14]), but for the present study mice were killed by CO2 inhalation 8 hrs after injection for myometrium mRNA analysis. The uterus was dissected and the number of foetuses within the uterus or birth canal recorded. Foetuses and foetal membranes were removed and myometrium snap frozen in liquid nitrogen and stored at −80°C.
Human myometrial tissue
Myometrium was obtained, with informed written consent, from women undergoing Caesarean section at term (>37 weeks; n= 9) not in labour, as approved by the Guy’s and St Thomas’ Ethics Committee (EC00/137). Biopsies were obtained from the upper edge of the lower segment incision and placed into ice-cold PBS. The tissue was immediately dissected in a sterile Petri dish on ice into small segments and then either snap frozen for RNA extraction or longitudinal muscle strips placed in ice-cold PBS for isometric tension recording.
RNA isolation and cDNA synthesis
Myometrium from time-mated animals and human myometrial tissue
Total RNA (tRNA) was isolated from early and late pregnant murine myometrium (n= 6) and term non-labouring (TNL) human myometrial tissue (n= 3). Tissue was homogenized using the TissueLyser (Qiagen, Crawley, UK) in Trizol® (Invitrogen, Paisley, UK) and tRNA extracted as per manufacturers recommendations. Samples were quantified using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Labtech, Ringmer, UK). cDNA was synthesized from 500 ng tRNA, using 0.25 μg random hexanucleotide primers (Promega, Southampton, UK) and 200 IU Superscript III (Invitrogen).
Myometrium from LPS-treated mice
tRNA was extracted using the guanidinium isothiocyanate method (4 M guanidinium isothiocyanate, 25 mM sodium citrate, 0.5% sarkosyl, and 0.1 M β-mercaptoethanol). Caesium chloride (0.4 g/ml homogenate) was added and samples centrifuged (20 min., 9200 ×g). The supernatant was layered onto 1 ml of 5.7 M CsCl cushion (plus 0.1 M ethylenediaminetetraacetic acid [EDTA], 25 mM sodium acetate) and centrifuged overnight at 30,000 rpm (Beckman Coulter Optima LE-80k ultracentrifuge, Brea, CA, USA; SW 55 Ti rotor). The RNA pellet was ethanol-precipitated and resuspended in Tris-EDTA (pH 6.5). cDNA was synthesized using the iScript kit (Bio-Rad, Hercules, CA, USA).
Conventional PCR
PCR was carried out on cDNA using Illustra hot start mastermix (GE Healthcare, Chalfont St Giles, UK) using the PCR primers described in Table 1 (initial denaturation: 95°C for 5 min.; cycling: 94°C for 1 min., 60°C for 30 sec., 72°C for 1 min. for a total of 35 cycles, with a final extension at 72°C for 10 min.). Samples were run on 2% agarose gels stained with SYBR safe (Invitrogen) and visualized using GeneSnap software on a G-BOX system (Syngene, Cambridge, UK).
Table 1.
List of primers used for qRT-PCR
| Gene | Primer sequence | Amplicon length | Gen bank accession number |
|---|---|---|---|
| Mouse | |||
| KCNQ1 | (+) 5′-ATGCTCTGTGGTGGGGGGTG-3′ | 185 | NM_008434 |
| (−) 5′-CTTCTGCCTCTGCTTCTGCTGG-3′ | |||
| KCNQ2 | (+) 5′-TGACCACTTTGACACCTACGCAGA-3′ | 150 | NM_008434 |
| (−) 5′-GGAAGAGCAAAGAACGAGACACCA-3′ | |||
| KCNQ3 | (+) 5′-CTTCCATATCGACCCCTTCCAACA-3′ | 144 | AB000504 |
| (−) 5′-GCGCTTGAAACAGCCAGTGG-3′ | |||
| KCNQ4 | (+) 5′-ATGGGGCGCGTAGTCAAGGT-3′ | 169 | BB022155 |
| (−) 5′-GGGCTGTGGTAGTCCGAGGTG-3′ | |||
| KCNQ5 | (+) 5′-CGCGTTCGTTTTTCTCCTTGTG-3′ | 199 | AF249747 |
| (−) 5′-CCTCAGTCTTCCTTGCCATCCTCT-3′ | |||
| KCNE1 | (+) 5′-TTCCTCCAGCAACTGACTGCC-3′ | 162 | AF263836 |
| (−) 5′-TGGCCAGAAAGGGCAGAACA-3′ | |||
| KCNE2 | (+) 5′-TATGGACAGCTGGAGGAGGAACAC-3′ | 133 | NM_008424 |
| (−) 5′-GGCCACCACGATGAACGAGA-3′ | |||
| KCNE3 | (+) 5′-GTCACTGTGGGCAGTCTCATCC-3′ | 82 | NM_134110 |
| (−) 5′-CGTAGTACACATGATAGGGGTCACTACG-3′ | |||
| KCNE4 | (+) 5′-AGGCTGTGGGGGGAGGCTAT-3′ | 175 | NM_020574 |
| (−) 5′-GGTGCACATCAGGAGAGGAGGAC-3′ | |||
| KCNE5 | (+) 5′-ATGAACTGCAGCGAGAGCCAAC-3′ | 173 | NM_021342 |
| (−) 5′-TCGTTACCCTTGGCGCTGGT-3′ | |||
| GAPDH | (+) 5′-TTGATGGCAACAATCTCCAC-3′ | 110 | NM_001001303 |
| (−) 5′-CGTCCCGTAGACAAAATGGT-3′ | |||
| β2M | (+) 5′-TTCAGTATGTTCGGCTTCCC-3′ | 103 | NM_009735 |
| (−) 5′-TGGTGCTTGTCTCACTGACC-3′ | |||
| ARBP | (+) 5′-GATGCCCAGGGAAGACAG-3′ | 90 | NM_007475.2 |
| (−) 5′-ACAATGAAGCATTTTGGATAATCA-3′ | |||
| β-Actin | (+) 5′-ATGGAGGGGAATACAGCCC-3′ | 149 | NM_007393 |
| (−) 5′-TTCTTTGCAGCTCCTTCGTT-3′ | |||
| B-Gluc | (+) 5′-CTCTGGTGGCCTTACCTGAT-3′ | 73 | NM_010368.1 |
| (−) 5′-CAGTTGTTGTCACCTTCACCTC-3′ | |||
| Human | |||
| KCNQ1 | (+) 5′-CATCACCCACATCTCACAGC-3′ | 124 | NM_000218.2 |
| (−) 5′-GTCCCGCACATCGTAAGG-3′ | |||
| KCNQ2 | (+) 5′-GACAAGGACCGCACCAAG-3′ | 123 | NM_004518.3 |
| (−) 5′-CACCAGGAAGTCCAGCTTCT-3′ | |||
| KCNQ3 | (+) 5′-CGAGTTTGCTTTGAGGATCTG-3′ | 119 | NM_004519.2 |
| (−) 5′-AGGCAATCAGCACAAAGATGT-3′ | |||
| KCNQ4 | (+) 5′-TGCCCCCTCAGAGGAAGT-3′ | 119 | NM_004700.2 |
| (−) 5′-CCACCAGGAACTTGAGAATCC-3′ | |||
| KCNQ5 | (+) 5′-AGTCCCACCAAAGTGCAGA-3′ | 120 | NM_019842.2 |
| (−) 5′-AGTGCCAAGGGCTGTGTC-3′ | |||
Quantitative RT-PCR
Real-time PCR was carried out with the use of SYBR Green chemistry (Bioline, London, UK) on a RotorGene 6000 (Qiagen, Crawley, UK) using the primers as listed in Table 1. A pre-PCR cycle was run for 15 min. at 95°C followed by 45 cycles of 95°C for 20 sec., 60°C for 20 sec. and 72°C for 20 sec. followed by a final extension at 72°C for 15 sec. Melt-curve analysis was performed to confirm the presence of one single product and non-template controls run to assess contamination. Cycle threshold values were used for analysis and abundance data were obtained by the use of quantified cDNA to generate a standard curve. Standards were quantified using absorbance and 10-fold serial dilutions (1010 to 10 copies) run in parallel with the samples. Abundance data for the genes of interest were expressed relative to the two most stable housekeeping genes from a panel of five using GeNorm software [15]. In early pregnant tissues, the housekeepers used for final analysis were β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and GAPDH and β2-microglobulin were used for late pregnant tissues.
Isometric tension recording
For both mouse and human protocols, small longitudinal myometrial strips (5 mm × 2 mm × 2 mm) were mounted and maintained at 37°C, pH 7.4 in organ baths (oxygenated – 95% O2, 5% CO2) physiological salt solution (PSS, pH 7.4 in mM: NaCl 119, KCl 4.7, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, EDTA 0.025, glucose 6 and CaCl2 2.5). Early pregnant strips were subjected to 12.75 mN of tension, late pregnant to 14.72 mN and human myometrial strips to 38.25 mN (approximately 1.5 times original length). After development of spontaneous contractile activity with a stable baseline (mouse 40 min., human 1–2 hrs). A baseline period of contractile activity was recorded, before the addition of either XE991 (Tocris Bioscience, Bristol, UK, 1–10 μM, broad-spectrum Kv7 channel inhibitor), chromanol 293B (Tocris Bioscience, 1–30 M, preferential inhibitor of KCNQ1 containing complexes,) or retigabine/flupirtine (20 μM, Kv7 channel activator). Retigabine (20 μM) and XE991 (10 μM) were also added to oxytocin (OT, 10−9 M) augmented contractions. Time- and gestation-matched control experiments using appropriate vehicles [dimethylsulfoxide (DMSO) for retigabine, flupirtine and chromanol 293B; H2O for XE991; PSS for OT] were carried out in parallel.
Contractility data were obtained using MacLab™ hardware and analysed using Chart v4.2 software (ADI Instruments, Chalgrove, UK). Contractile periods were assessed using mean integral tension (MIT), the sum of the integrals for each contraction divided by the duration of the period assessed. All treatment data were then expressed as a percentage of the preceding baseline periods (MIT as% of baseline period). The frequency of contractions was also assessed.
Statistical analysis
Relative abundance data were analysed (Stata version 9.2 StataCorp, College Station, TX, USA), using generalized estimating equations with semi-robust standard errors for each group: KCNQ or KCNE [16]. Overall tests for differences between early and late gestation were carried out using repeated measures ANOVA (RM ANOVA. LPS data were analysed using Mann–Whitney U-test and summary data presented as medians (min–max). The Student’s t-test was used to assess isometric tension recording. Data were expressed as mean ± standard error. P≤ 0.05 was considered significant. ‘n’ refers to the number of animals or human beings unless otherwise stated.
Results
KCNQ/KCNE expression in myometrium from pregnant mice
The presence of KCNQ isoforms in pregnant mouse myometrium was established by qRT-PCR (Fig. 1A). In early pregnant mouse myometrium, the relative abundance of mRNA expression was KCNQ3 > KCNQ4 > KCNQ5 > KCNQ1 > KCNQ2 (Fig. 1A, P < 0.001). In contrast, in late pregnant mouse myometrium, the relative abundance was KCNQ1 > KCNQ5 > KCNQ4 > KCNQ3 > KCNQ2 (Fig. 1A P < 0.001). Figure 1 also clearly shows that KCNQ1, 2, 4 and 5 are suppressed in early compared to late pregnant tissue (P < 0.001) and non-pregnant tissue (P < 0.01, compared to data from McCallum et al.). KCNQ3 expression was higher in non-pregnant tissues (P < 0.01) and early pregnant tissues compared to late pregnant tissues (Fig. 1A).
Fig 1.
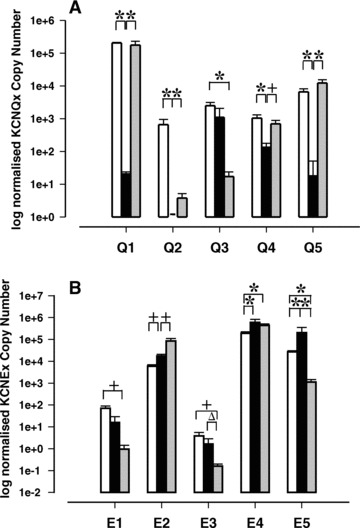
Expression of (A) KCNQ and (B) KCNE genes in non-pregnant, early and late pregnant mouse myometrial tissue. Non-pregnant (oestrous, white bars, n= 6; data taken from McCallum et al., for comparison), early pregnant (black bars, n= 6, gestation days 6–7) and late pregnant mouse myometrium (grey bars, n= 6, gestation days 17–18). Data are expressed as log geometric mean of copy number (± S.E.) normalized to housekeeping genes. (*P < 0.001, +P < 0.01, ΔP < 0.05).
Transcripts for all KCNE genes (Fig. 1B) were detected in myometrium from early and late pregnant mice. The relative abundance of KCNE gene expression in early pregnancy was as follows: KCNE4 > KCNE5 > KCNE2 > KCNE1 > KCNE3 (P < 0.001). In late gestational samples the abundance was: KCNE4 > KCNE2 > KCNE5 > KCNE1 > KCNE3 (P < 0.001). There was clear regulation of KCNE mRNA over gestation. In early pregnancy, KCNE5 was more highly expressed than in late pregnant tissues and non-pregnant tissues (P < 0.001, non-pregnant data from McCallum et al.) and KCNE3 expression was suppressed in late pregnancy (P < 0.05). KCNE1 demonstrated a trend towards a decrease in expression although this was not statistically significant. KCNE2 expression was increased over the course of gestation from non-pregnant to late pregnant (P < 0.01, Fig. 1B). KCNE4 did not appear to be regulated with gestation.
Kv7 channel inhibition with XE991 increases spontaneous myometrial contractions
XE991 (1 μM) enhanced contractility in myometrial tissue from late pregnant animals (MIT increased by 45%; P < 0.01), but there was limited effect on contraction frequency compared to time-matched vehicle controls (data not shown).
The effect of XE991 (10 μM) was more pronounced (Fig. 2A, B). Mean data (Fig. 2C, D) indicated that XE991 promoted a significant increase in MIT of 70% (compared to vehicle control, Fig. 2C, P < 0.05) in early pregnant mouse myometrium. This effect was larger in tissues taken from late pregnant animals (160% greater than gestation matched vehicle controls, P < 0.01, Fig. 2D). XE991 also increased contraction frequency (by 50% in early gestation, P < 0.01, versus vehicle control) and 100% in late gestation (P < 0.01) (data not shown). In contrast, application of C293B (1–30 μM) had no effect on myometrial contractility in tissues from late pregnant animals (n= 8–10, data not shown).
Fig 2.
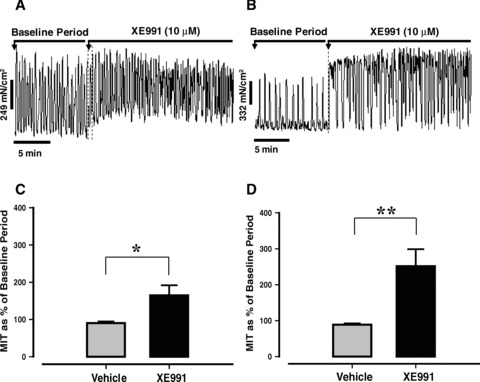
Effect of XE991 (10 μM) on spontaneous myometrial contractions in tissues from mice in early (A) and late pregnancy (B). (C) Mean effect of XE991 (10 μM) (±S.E.) in early pregnancy (n= 10 from 9 animals) versus vehicle control (n= 10 from 9 animals). (D) Mean effect of XE991 (10 μM) (±S.E.) in late pregnancy (n= 14 from 8 animals) compared to vehicle controls (n= 14 from 8 animals). (*P < 0.05, **P < 0.01).
Flupirtine and retigabine suppress myometrial contractility
Application of flupirtine (20 μM) reduced myometrial contractility in both early and late pregnant mouse myometrial tissues, an effect that was reversed by XE991 (10 μM, Fig. 3A, B). The effect of flupirtine was significantly greater in myometrium from late gestation compared to early gestation (P < 0.05). In ∼40% of late pregnant myometrial strips flupirtine completely abolished myometrial contractions. This effect was not observed in any tissues from early pregnant animals.
Fig 3.
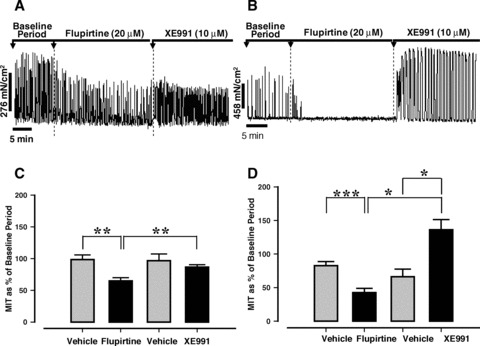
The effect of flupirtine (20 μM) on spontaneous myometrial contractions from mice in early (A) and late pregnancy (B). The impact of flupirtine (20 μM, mean ± S.E.) and reversal by XE991 (10 μM) in myometrium from animals in early (C, n= 6) and late pregnancy (D, n= 10 from 9 animals) compared to time and gestation-matched vehicle controls (C, n= 6; D, n= 9 from 8 animals). (*P < 0.05, **P < 0.01, ***P < 0.001).
Similarly, retigabine (20 μM), a structurally distinct analogue of flupirtine, suppressed late pregnant myometrial contractility (40%, P < 0.01) when compared to vehicle control (Fig. 4A, B) and the effect was reversed by addition of XE991 (10 μM). Retigabine also reduced contraction frequency by 35% (P < 0.01). Retigabine effectively abolished all myometrial contractions in ∼70% of late pregnant tissue strips.
Fig 4.
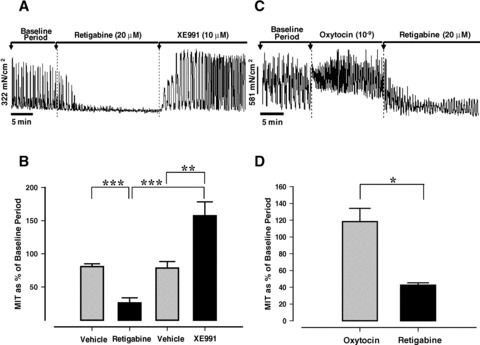
The effect of retigabine (20 μM) on spontaneous (A) and oxytocin induced (10−9 M, C) myometrial contractions in tissue from mice in late pregnancy. (B); mean data (±S.E.) for retigabine (20 μM) and reversal by XE991 (10 μM, n= 10 from 9 animals) compared to vehicle controls (n= 9 from 8 animals). (D); Mean (± S.E.) for retigabine versus oxytocin period (n= 3). (*P < 0.05, **P < 0.01, ***P < 0.001).
The effect of retigabine and XE991 on agonist driven contractions
Application of retigabine (20 μM) resulted in a decrease in the frequency, amplitude and basal tone of oxytocin (10−9 M) driven contractions (Fig. 4C). MIT was suppressed by ∼80% (P < 0.05 compared to OT period, Fig. 4D) and contraction frequency was reduced by 50% (data not shown) although this did not quite reach significance. Inhibition of Kv7 channels with XE991 (10 μM) on oxytocin-driven contractions reduced the basal tone and amplitude and MIT (mean ± S.E.: 171.2 ± 22.9%versus 94.9 ± 22.4%; P < 0.05). There was no effect on contraction frequency (mean ± S.E.: 126.7 ± 11.5%versus 123.7 ± 14.4%).
The impact of LPS treatment on myometrial KCNQ/KCNE expression
KCNQ and KCNE mRNA was detected in myometrium from LPS-treated mice at 8 hrs after injection. There was a generalized suppression of most of the KCNQ/KCNE isoforms compared to myometrium taken from vehicle-treated control mice (Fig. 5A, B, P < 0.05), with the exception of KCNQ4 and KCNE5.
Fig 5.
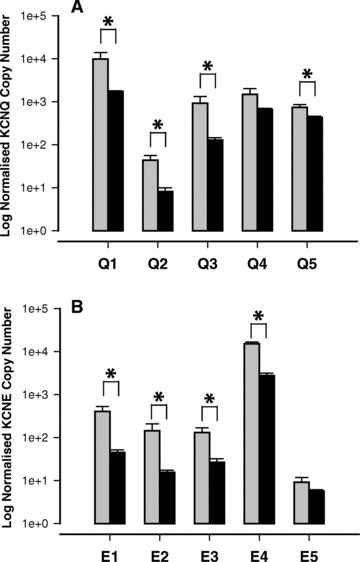
The expression of KCNQ (A) and KCNE (B) mRNA transcripts in myometrial tissue taken from mice 8 hrs after being injected (IP) with LPS (100 ng). Vehicle control (grey bars, n= 3) and LPS (black bars, n= 4). Data are expressed as log mean of copy number (± S.E.) normalized to geometric mean of two housekeeping genes (*P < 0.05).
The expression and function of Kv7 channels in human myometrial smooth muscle
Figure 6(A) and (B) clearly shows that all KCNQ isoforms with the exception of KCNQ5 are expressed in human myometrial smooth muscle tissue. RT-PCR data suggest that KCNQ4 and KCNQ1 are the most abundant α-subunit isoforms. The channels encoded by these genes are functional in late pregnant human myometrial smooth muscle as Kv7 channel modulators have a significant impact on myometrial contractility (Fig. 6C, D). Application of 10 μM XE991 (7C) increased the frequency of spontaneous myometrial contractions by 219% whereas retigabine (n= 3, 20 μM) relaxed human myometrial tissues (n= 3, MIT decreased by 50% compared to vehicle controls, Fig. 6D).
Fig 6.
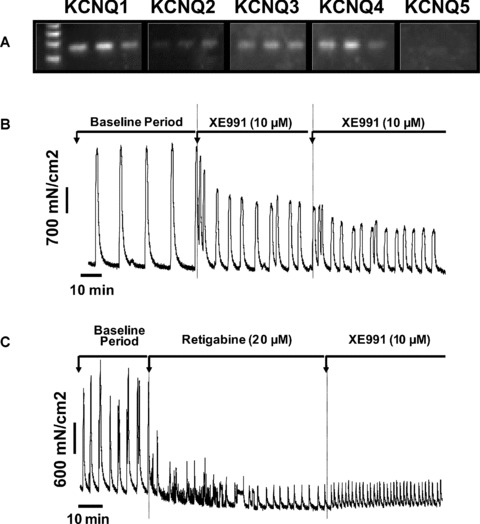
The expression (A) and function (B–C) of KCNQ isoforms in myometrial tissue from pregnant women at term (n= 3). KCNQ1 (124 bp), KCNQ2 (123 bp), KCNQ3 (119 bp), KCNQ4 (119 bp), KCNQ5 (120 bp). The effect of (B) XE991 (10 μM) and (C) retigabine (20 μM) on spontaneous myometrial contractions in vitro (not in labour, representative of n= 3).
Discussion
These data clearly demonstrate, for the first time, a significant functional impact of Kv7 channels in pregnant mouse and human myometrium. The observation that the majority of mouse KCNQ isoforms are most highly expressed in late pregnancy and are not regulated by a generalized inflammatory response in mice suggests that Kv7 channels may ultimately represent novel targets for therapy in relation to pregnancy disorders such as preterm labour. The profound relaxation induced by Kv7 channel activators in vitro in human tissues is particularly important, as the activator retigabine (currently in phase III clinical trials [17]) has been used safely in human beings for the treatment of non-reproductive disorders and flupirtine has been licensed in some countries for use as an analgesic [18].
All KCNQ and KCNE isoforms were expressed in mouse myometrium in early and late gestation, but the majority of KCNQ isoforms were up-regulated in late pregnancy. This contrasts to published data on other K+ channels α-subunits including ROMK1 [19], Kv4.1 and 4.3 [20], KATP, and SK3 [14, 21] which decrease in expression with gestation.
It is apparent from our data that KCNQ1 containing complexes, similar to those found in the heart, are non-functional in mouse myometrium as chromanol (potent inhibitor of these complexes at low concentrations) had no effect on myometrial activity. This could be partially attributed to the co-assembly of KCNQ1 with KCNE4 as this is known to inhibit the K+ current through KCNQ1 encoded channels [22]. Indeed preliminary co-immunoprecipitation data from our lab indicates that these two subunits may interact. The lower expression levels of most KCNQ mRNA in early pregnancy were supported by functional data as the activator flupirtine could only partially suppress myometrial contractions in early pregnant animals. In late pregnant animals both flupirtine and retigabine entirely suppressed contractile activity in 40% and 70% of tissues studied, respectively.
In contrast, to other isoforms, KCNQ3 expression was highest in early pregnancy, potentially indicating a contribution of this isoform to resting cell excitability. In neurones, KCNQ3 is the underlying constituent of the M current when in multimeric combination with KCNQ2 [23]. The M-current is a non-inactivating potassium current involved in subthreshold activity in many neuronal cell types. Due to the low KCNQ2 expression throughout pregnancy in myometrium, formation of this complex is unlikely. However, KCNQ5 and KCNQ3 have also been shown to form a multimer that may contribute to the native M-current [24]. KCNQ3 could also form multimers with other KCNQ proteins or exist as a non-functional homomeric channel [25].
KCNE isoforms show a different pattern of regulation across pregnancy to KCNQ genes. A significant increase is seen in KCNE2 mRNA between early and late pregnant myometrium, mirroring data previously found by our lab in BALBC mice [8]. Conversely, KCNE3 and KCNE5 appear to be down-regulated in late pregnant myometrial tissue. KCNE4, is also highly expressed in all tissues studied throughout gestation, and as discussed earlier may serve to inhibit KCNQ1 function [22]. Interestingly, all KCNE isoforms can also associate with KCNQ4 and interactions with KCNE4 in particular can augment the K+ current [26].
KCNE isoforms can also associate with non-KCNQ encoded α-subunits [27–34] and our data suggest that KCNE2 may play an important functional role in inhibiting the ERG current in late pregnant mouse myometrium, potentially promoting activation of the uterine smooth muscle at term [8]. This functional suppression of ERG currents occurs at the same point in gestation when there is an up-regulation of a majority of Kv7 channel encoding isoforms. This strengthens the hypothesis that KCNE isoforms may function as molecular switches at the end of pregnancy and regulate more than one Kv gene family in parallel.
The application of the broad-spectrum Kv7 inhibitor XE991 to myometrial tissue in vitro also demonstrated the key role Kv7 channels play in modulating contractile activity in both early and late pregnant myometrium, although the effect appeared more marked in tissues from late gestation.
Our data also demonstrate that Kv7 channels impact on oxytocin-induced contractile activity as XE991 led to a fusion of contractile activity. Significantly, retigabine effectively suppressed oxytocin induced contractile activity. This is important as oxytocin may be involved in stimulating contractions during labour.
The use of a generalized model of inflammation in pregnant mice allowed assessment of the impact of an inflammatory milieu on myometrial KCNQ/KCNE gene expression. Most of the KCNQ and KCNE mRNA expression in myometrial tissue, except for KCNQ4 and KCNE5, was suppressed in tissues from LPS-treated tissues compared to vehicle controls. This could indicate that modulation of these channels is associated with the onset of infection-associated preterm labour. However, quantification of gene expression would suggest that mRNA levels are still relatively high and that functional Kv7 channels are likely to be expressed and contribute to control of uterine activity during preterm labour in this mouse model.
In human myometrium, taken from women prior to labour onset at term, all the major KCNQ transcripts are expressed, except for KCNQ5. The functional contribution of these channels is illustrated by the relaxation provoked by retigabine and the impact of XE991 on contractile frequency. These data further strengthen our proposal for Kv7 channels as targets for development of novel tocolytics. However, it is essential that the role of Kv7 channels in other reproductive tissues also be determined to address any potential for adverse influences on maternal and neonatal outcome. The expression profile of KCNQ and KCNE genes is different in the uterus from those in heart and brain, but may have resemblance to vascular and other smooth muscles. The reported side effects of retigabine and flupirtine to-date are mainly central nervous system related and reported to be mild [17, 35]. Nifedipine use as a tocolytic is associated with systemic cardiovascular effects [36], but these side-effects are not been reported with treatment with retigabine/flupirtine use in clinical trials/as an analgesic and would be a distinct benefit.
In summary, the maintained presence of Kv7 channels in the murine myometrium throughout gestation and the demonstrable functional impact of Kv7 activators and inhibitors provide clear evidence for a key role for these channels in regulating rhythmic myometrial activity at term. Furthermore, given that functional Kv7 channels are present in term human myometrial tissue, then Kv7 channel directed drugs currently in development may represent novel tocolytics for use in preterm labour.
Acknowledgments
The authors thank Dr. M Schwake (University of Kiel) for the gift of retigabine, the BSU at St Thomas’ Hospital for their assistance and support with animal handling and all staff on the labour ward of St Thomas’ Hospital for their assistance with the collection of human myometrial samples. This study was funded by Tommy’s the Baby Charity (registered charity no.: 1060508), KCL School of Medicine. The work on human tissue was funded by Action Medical Research (registered charity no.: 208701) and Rosetrees Trust (registered charity no.: 298582). LPS model work supported by the National Institutes of Health (HD-037831 to S.K.E.) and the March of Dimes (21-FY08-566 to S.K.E.).
References
- 1.Steer P. The epidemiology of preterm labour. BJOG. 2005;112:1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 2.Mangham LJ, Petrou S, Doyle LW, et al. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. 2009;123:e312–27. doi: 10.1542/peds.2008-1827. [DOI] [PubMed] [Google Scholar]
- 3.Russell RB, Green NS, Steiner CA, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 4.Gyetvai K, Hannah ME, Hodnett ED, et al. Tocolytics for preterm labor: a systematic review. Obstet Gynecol. 1999;94:869–77. doi: 10.1016/s0029-7844(99)00329-4. [DOI] [PubMed] [Google Scholar]
- 5.Parkington HC, Tonta MA, Brennecke SP, et al. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am J Obstet Gynecol. 1999;181:1445–51. doi: 10.1016/s0002-9378(99)70390-x. [DOI] [PubMed] [Google Scholar]
- 6.Sanborn BM. Ion channels and the control of myometrial electrical activity. Semin Perinatol. 1995;19:31–40. doi: 10.1016/s0146-0005(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 7.Khan RN, Matharoo-Ball B, Arulkumaran S, et al. Potassium channels in the human myometrium. Exp Physiol. 2001;86:255–64. doi: 10.1113/eph8602181. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood IA, Yeung SY, Tribe RM, et al. Loss of functional K+ channels encoded by ether-a-go-go-related genes in mouse myometrium prior to labour onset. J Physiol. 2009;587:2313–26. doi: 10.1113/jphysiol.2009.171272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaronson PI, Sarwar U, Gin S, et al. A role for voltage-gated, but not Ca2+-activated, K+ channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharmacol. 2006;147:815–24. doi: 10.1038/sj.bjp.0706644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith RC, McClure MC, Smith MA, et al. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 2007;5:41. doi: 10.1186/1477-7827-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007;18:332–9. doi: 10.1016/j.semcdb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCallum LA, Greenwood IA, Tribe RM. Expression and function of K(v)7 channels in murine myometrium throughout oestrous cycle. Pflugers Arch. 2009;457:1111–20. doi: 10.1007/s00424-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 13.Koscica KL, Ananth CV, Placido J, et al. The effect of a matrix metalloproteinase inhibitor on inflammation-mediated preterm delivery. Am J Obstet Gynecol. 2007;196(551):e1–3. doi: 10.1016/j.ajog.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Pierce SL, Kresowik JD, Lamping KG, et al. Overexpression of SK3 channels dampens uterine contractility to prevent preterm labor in mice. Biol Reprod. 2008;78:1058–63. doi: 10.1095/biolreprod.107.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Boill. 2002;(3):0034.1–0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang K, Zeiger S. Longitudinal analysis using generalised linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 17.Porter RJ, Nohria V, Rundfeldt C. Retigabine. Neurotherapeutics. 2007;4:149–54. doi: 10.1016/j.nurt.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribkoff VK. The therapeutic potential of neuronal KCNQ channel modulators. Expert Opin Ther Targets. 2003;7:737–48. doi: 10.1517/14728222.7.6.737. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren DW, Moore JJ, Chang SM, et al. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med. 1997;216:57–64. doi: 10.3181/00379727-216-44156. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Takimoto K. Differential expression of Kv4 pore-forming and KChIP auxiliary subunits in rat uterus during pregnancy. Am J Physiol Endocrinol Metab. 2005;288:E335–41. doi: 10.1152/ajpendo.00250.2004. [DOI] [PubMed] [Google Scholar]
- 21.Curley M, Cairns MT, Friel AM, et al. Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol Hum Reprod. 2002;8:941–5. doi: 10.1093/molehr/8.10.941. [DOI] [PubMed] [Google Scholar]
- 22.Grunnet M, Jespersen T, Rasmussen HB, et al. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542:119–30. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HS, Pan Z, Shi W, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–3. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 24.Wickenden AD, Zou A, Wagoner PK, et al. Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br J Pharmacol. 2001;132:381–4. doi: 10.1038/sj.bjp.0703861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bal M, Zhang J, Zaika O, et al. Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis. J Biol Chem. 2008;283:30668–76. doi: 10.1074/jbc.M805216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strutz-Seebohm N, Seebohm G, Fedorenko O, et al. Functional coassembly of KCNQ4 with KCNE-beta- subunits in Xenopus oocytes. Cell Physiol Biochem. 2006;18:57–66. doi: 10.1159/000095158. [DOI] [PubMed] [Google Scholar]
- 27.Cui J, Kagan A, Qin D, et al. Analysis of the cyclic nucleotide binding domain of the HERG potassium channel and interactions with KCNE2. J Biol Chem. 2001;276:17244–51. doi: 10.1074/jbc.M010904200. [DOI] [PubMed] [Google Scholar]
- 28.Um SY, McDonald TV. Differential association between HERG and KCNE1 or KCNE2. PLoS ONE. 2007;2:e933. doi: 10.1371/journal.pone.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis A, McCrossan ZA, Abbott GW. MinK, MiRP1, and MiRP2 diversify Kv3.1 and Kv3.2 potassium channel gating. J Biol Chem. 2004;279:7884–92. doi: 10.1074/jbc.M310501200. [DOI] [PubMed] [Google Scholar]
- 30.Choi E, Abbott GW. The MiRP2-Kv3.4 potassium channel: muscling in on Alzheimer’s disease. Mol Pharmacol. 2007;72:499–501. doi: 10.1124/mol.107.039206. [DOI] [PubMed] [Google Scholar]
- 31.Abbott GW, Butler MH, Bendahhou S, et al. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell. 2001;104:217–31. doi: 10.1016/s0092-8674(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 32.Gordon E, Roepke TK, Abbott GW. Endogenous KCNE subunits govern Kv2.1 K+ channel activation kinetics in Xenopus oocyte studies. Biophys J. 2006;90:1223–31. doi: 10.1529/biophysj.105.072504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Decher N, Bundis F, Vajna R, et al. KCNE2 modulates current amplitudes and activation kinetics of HCN4: influence of KCNE family members on HCN4 currents. Pflugers Arch. 2003;446:633–40. doi: 10.1007/s00424-003-1127-7. [DOI] [PubMed] [Google Scholar]
- 35.Porter RJ, Partiot A, Sachdeo R, et al. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- 36.van Geijn HP, Lenglet JE, Bolte AC. Nifedipine trials: effectiveness and safety aspects. BJOG. 2005;112:79–83. doi: 10.1111/j.1471-0528.2005.00591.x. [DOI] [PubMed] [Google Scholar]


