Abstract
MafA is a pancreatic transcriptional factor that controls β-cell-specific transcription of the insulin gene. However, the role of MafA in the regulation of pancreatic transdifferentiation and reprogramming in human stem cells is still unclear. In this study, we investigate the role of MafA in placenta-derived multipotent stem cells (PDMSCs) that constitutively expressed Oct-4 and Nanog. PDMSCs were isolated and transfected with MafA using a lentivector. Our results showed that overexpression of MafA in PDMSCs significantly up-regulated the expression of pancreatic development-related genes (Sox17, Foxa2, Pdx1 and Ngn3). Microarray analysis suggested that the gene expression profile of MafA-overexpressing PDMSCs was similar to that of pancreas and islet tissues. MafA increased the expression levels of the mRNAs of NKx2.2, Glut2, insulin, glucagons and somatostatin, and further facilitated the differentiation of PDMSCs into insulin+ cells. The glucose-stimulated responses to insulin and c-peptide production in MafA-overexpressing PDMSCs were significantly higher than in PDMSCs with vector control. Our results indicated that MafA-overexpressing PDMSCs were more resistant to oxidative damage and oxidative damage-induced apoptosis than PDMSCs carrying the vector control were. Importantly, the expression of MafA in PDMSCs xenotransplanted into immunocompromised mice improved the restoration of blood insulin levels to control values and greatly prolonged the survival of graft cells in immunocompromised mice with STZ-induced diabetes. In summary, these data suggest that MafA plays a novel role in the reprogramming of stem cells into pancreatic β-progenitors, promotes the islet-like characteristics of PDMSCs, as well as functionally enhances insulin production to restore the regulation of blood glucose levels in transplanted grafts.
Keywords: MafA, reprogramming, placenta-derived multipotent stem cells, insulin+ cells
Introduction
Diabetes mellitus has been recognized as the most prevalent and serious metabolic disease as the number of diabetic patients is increasing worldwide [1]. Type I diabetes, which is due to a loss of insulin generating capacity, is caused by progressive degeneration of β cell functional capacity in the islets of Langerhans. Type II diabetes results from the destruction of insulin receptors and/or the insulin transport system. Transplantation of islets of Langerhans is a promising therapy for the treatment of type I diabetes [1]. However, numerous obstacles remain, such as the limited number of available donor cells, the difficult process of islet isolation and the cost of the procedure, which have largely discouraged its use in the treatment of diabetic patients to date [2].
MafA is a member of the musculoaponeurotic fibrosarcoma oncogene family, a subgroup of the basic leucine zipper (bZip) transcription factors with homology to the v-Maf oncoprotein [3]. MafA is one of the pancreatic transcription factors regulating the expression of the β-cell-specific insulin gene [3]. During pancreatic development, MafA expression is first detected during the initial stage of insulin-producing cell production [4]. Recently, Zhang et al. reported that mice deficient in MafA developed diabetes due to impaired insulin secretion [5]. This study also revealed that MafA knock-out mice displayed reduced levels of Insulin1, Insulin2, Pdx-1, NeuroD1 and Glut-2 expression, and exhibited age-dependent pancreatic islet abnormalities. More recently, the elegant study of Zhou et al. identified a specific combination of three transcription factors (Ngn3, Pdx1 and MafA) that reprograms differentiated pancreatic exocrine cells in adult mice into cells that closely resemble β-cells in vivo[6]. Therefore, MafA is the principal factor required for β-cell development, reprogramming, differentiation and maturation as well as the maintenance of insulin-secreting function [6]. However, whether MafA plays a role in the reprogramming of adult human tissues and/or stem cells into pancreatic progenitor cells is still an open question.
Human term placenta is usually regarded as medical waste in the delivery room. Recently, placenta-derived multipotent stem cells (PDMSCs) from human term placenta were isolated and demonstrated to possess multi-lineage differentiating capacity [7]. PDMSCs, which are fibroblast-like cells, can be cultured in vitro and induced to differentiate into cells of various mesenchymal tissues, including adipocytes, osteoblasts and chondrocytes [7]. In contrast to other human stem cells, PDMSCs can be considered as ‘very young’ progenitor cells and they entail less immunological problems than adult stem cells during allogenic transplantation [8]. Furthermore, PDMSCs have shown the potential to differentiate into a neurological lineage [9], and have been suggested as an alternative resource for the generation of hepatic progenitor cells [10]. Our previous data demonstrated that PDMSCs constitutively expressing Oct-4 and Nanog have the ability to generate insulin+ cells in vitro and in vivo[11]. In the present study, we investigate whether MafA is capable of promoting the reprogramming potential and insulin production for pancreatic lineage and islet-like characteristics from PDMSCs. First, we overexpressed MafA from a cDNA plasmid delivered by lentivector in PDMSCs. By using gene expression microarrays and transcriptome distance analysis, we found that MafA promotes pancreatic islet-related transcriptome reprogramming in PDMSCs, with up-regulation of pancreatic development-associated genes. The results of Western blotting further demonstrated that MafA significantly up-regulates the expression of pancreatic β-progenitor-related proteins in the reprogramming of PDMSCs. Next, we investigated the potential of MafA-overexpressing PDMSCs for pancreatic-lineage differentiation in modified serum-free medium for pancreatic induction. Our data demonstrated that MafA can effectively promote PDMSC differentiation into insulin- and glucagon+ cells in vitro and in vivo. Our data also showed that MafA plays a crucial role in promoting the islet-like characteristics of PDMSC and further reprograms PDMSC into pancreatic β-progenitors. Therefore, this approach could be a novel therapeutic target for diabetes.
Materials and methods
Isolation of placenta-derived multipotent stem cells
This research follows the tenets of the Declaration of Helsinki and informed consent was obtained from the donor patients. The human term placenta tissue was dissected and digested with collagenase P (Roche, Indianapolis, IN, USA) in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered saline for 7 hrs at 37°C. The isolation procedure for PDMSC isolation has been described previously [11]. The dissociated cells obtained from human placenta were negative for CD45 and glycophorin-A after depletion of CD45+ and glycophorin-A+ cells using micromagnetic beads. These cells were then plated in human fibronectin coated (5 ng/ml, Sigma-Aldrich, St. Louis, MO, USA) 96-well plates. Expansion medium consisted of Dulbecco’s modified Eagle’s medium with 1 g/l of glucose [low-glucose Dulbecco’s modified Eagle’s medium (DMEM-L), Gibco®; Invitrogen, Grand Island, NY, USA] and 10% foetal bovine serum (Gibco®) supplemented with 10 ng/ml basic fibroblast growth factor (bFGF), 10 ng/ml epidermal growth factor (EGF), 10 ng/ml platelet-derived growth factor (PDGF)-BB (R&D Systems, Minneapolis, MN, USA), 100 units/ml penicillin, 1000 μg/ml streptomycin and 2 mM L-glutamine (Gibco®).
The Preparation of MafA-lentiviral production and infection (step 1)
The gene coding for MafA was cloned by the PCR using human genomic DNA as the template by two primers, a N-primer (5′-GGATCCATGGCCGC GGAGCTGGC-3′) and a C primer (5′-GGATCCCTACAGGAAGAAGTCGGCCGTGC-3′). The BamHI restriction sites are underlined. The PCR fragment (1059 bps) was ligated into pGEM(T) to make the TA cloning vector pGEM(T) (Promega, Madison, WI, USA)/MafA plasmid. After verification of the sequence, the pGEM(T)/MafA plasmid was digested by restriction enzyme BamH1. The BamH1 site flanked MafA gene fragments was purified and subcloned into lentivirus-based expression vector PLV-EF1a-GFP (green fluorescent protein) or lentivirus-based expression vector (PLV) (BiOSETTIA, San Diego, CA, USA)-EF1a-puro vector. These lentivirus based vector will allow efficient and stable transduction of a wide variety of cells. 293T cells were plated on a 10 cm dish and transfected with lentiviral vectors by lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to manufactures’ instructions and the previous protocol [12]. Supernatants were collected 48 hrs after transfection and then were filtered; the viral titres were then determined by FACS at 48 hrs after transduction. Subconfluent cells were infected with lentivirus with GFP at a multiplicity of infection of 5 in the presence of 8 μg/ml polybrene (Sigma-Aldrich). Seven days after transduction (step 1, Fig. 1), the MafA-overexpressing PDMSC-GFP cells were sorted by FACSAria™ Cell Sorter (BD Biosciences, San Jose, CA, USA) (Fig. S1).
Fig 1.
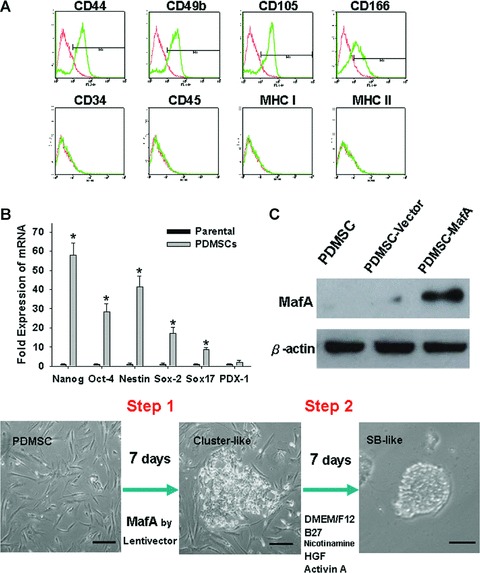
Characterization of PDMSCs and two-step induction protocol. (A) The phenotypes of PDMSCs analyzed by flow cytometry (Positive: red line; blue colour: control). (B) Q-PCR analysis showing that PDMSCs express the mRNAs of Nanog, Nestin, Oct-4, Sox2, Sox17 and PDX1. (C) PDMSCs were transduced with a lentivirus carrying the MafA gene. The protein expression levels of MafA in vector control PDMSCs and MafA-overexpressing PDMSCs were verified by Western blotting. (D) Outline of the differentiation two-step protocol and stage-specific cell morphology. Essential factor manipulations at each stage are also shown. Data shown here are the mean ± S.D. of three independent experiments. *P < 0.001. Bar = 30 μm.
Selection of insulin+ cells with serum-free pancreatic induction medium (step 2)
PDMSCs (105 cells) were then washed twice with HBS solution, then placed on 10 cm and cultured with DMEM (25 mM glucose)/F12 serum-free medium (composed: 1:1 of DMEM/F12, 0.6% glucose, 25 μg/ml insulin, 100 μg/ml transferrin, 20 nM progesterone, 60 μM putrescine, 30 nM selenium chloride, 2 mM glutamine, 3 mM sodium bicarbonate, 5 mM HEPES buffer, 2 μg/ml heparin, 20 ng/ml EGF, 20 ng/ml b-FGF and 20 ng/ml HGF (PeproTech Asia, Rehovot, Israel), 10 mM nicotinamide (Gibco) and activin A 100 ng/ml) for 7 days culture (step 2, Fig. 1). The induction medium was changed twice and sub-cultured once with the ratio of 1:3 a week.
Microarray analysis and bioinformatics
Total RNA was extracted from cells using Trizol reagent (Life Technologies, Bethesda, MD, USA) and the Qiagen RNAeasy (Qiagen, Valencia, CA, USA) column for purification. Affymetrix HG U133 Plus 2.0 microarrays containing 54,675 probe sets for >47,000 transcripts and variants, including 38,500 human genes. A typical probeset contains eleven 25-mer oligo nucleotide pairs (a perfect match and a mismatch control). For microarray analysis, sample labelling, hybridization and staining were carried out by Affymetrix standard protocol with affyQCReport. Probeset was normalized with loess method of all microarrays. The average-linkage distance was used to assess the similarity between two groups of gene expression profiles as described below [13]. The difference in distance between two groups of sample expression profiles to a third was assessed by comparing the corresponding average linkage distances (the mean of all pair-wise distances (linkages) between members of the two groups concerned). The error of such a comparison was estimated by combining the standard errors (the standard deviation of pair-wise linkages divided by the square root of the number of linkages) of the average-linkage distances involved [14]. Classical multidimensional scaling was performed with the standard function of the R program to provide a visual impression of how the various sample groups are related.
Cell survival analysis by MTT assay and detection of apoptotic cells with annexin V staining
For evaluation of cell survival, cells were seeded on 24-well plates at a density of 2 × 104 cells/well, followed by the addition of methyl thiazol tetrazolium (MTT; Sigma) at the end of cell culture. The amount of MTT formazan product was determined using a microplate reader following absorbance at 560 nm (SpectraMax 250, Molecular Devices, Sunnyvale, CA, USA). Annexin V staining was used to determine the percentage of apoptotic cells. Cells were harvested and stained with fluorescein isothiocyanate-labelled annexin V (BD Biosciences, San Diego, CA, USA) and propidium iodide (PI; Sigma) in the dark at room temperature for 30 min. The percentage of annexin V+ and PI– cells was determined by flow cytometry.
Measurement of insulin and C-peptide secretion
After 7 days of differentiation, cells were washed twice with PBS and incubated for 3 hrs in DMEM-LG (5.6 mM glucose). After 3 hrs, the medium was sorted and stored at –20°C. Cells were then washed twice with PBS and incubated for 3 hrs in DMEM-HG (25 mM glucose). Thereafter, the medium was sorted and stored at –20°C. For the analysis of C-peptide content, culture medium was sorted using the insulin and C-peptide ELISA kit (Mercodia, Uppsala, Sweden) according to the manufacturer’s instructions. Briefly, 25 μl of sample were added to 50 μl of assay buffer and incubated 1 hr at room temperature on a shaker. After washing six times with washing buffer, 100 μl of enzyme conjugate was added and incubated 1 hr on a shaker. TMB substrate (200 μl) was then added and incubated for 15 min. Finally, 50 μl of stop solution was added to the wells and mixed for 5 sec. on a shaker, and the samples were analyzed following absorbance at 450 nm.
Transplantation into Streptozotocin induced-hyperglycemic mice
All procedures involving animals were performed in accordance with the institutional animal welfare guidelines of Taipei Veterans General Hospital. Eight-week-old severe combined immunodeficiency (SCID) mice were treated with Streptozotocin (STZ, 200 mg/kg, Sigma) freshly dissolved in 0.025 M sodium citrate (pH 4.0). PDMSCs and differentiated PDMSCs (2 × 105 total at the end of step 2) were injected into the subcapsule of the left kidney of SCID mice following the previously described protocol [11]. Blood sampling from the retro-orbital plexus was performed every 2 days and measured using a OneTouch® SureStep (LifeScan, Milpitas, CA, USA) [11]. In vivo GFP imaging was performed with an illuminating device (LT-9500 Illumatool TLS equipped with excitation illuminating source [470 nm] and filter plate [515 nm]). The integrated optical density of green fluorescence intensity was captured and analyzed using Image Pro-plus software [13].
Statistical analysis
Statistical analysis was performed with the one-way or two-way ANOVA test followed by Tukey’s test. A value of P < 0.05 was considered statistically significant.
Results
MafA promotes the reprogramming of PDMSCs toward the pancreatic lineage
We previously isolated PDMSCs as a follow-up to the protocol of negative immunoselection (CD45 and glycophorin-A). PDMSCs possessed multilineage potential for differentiation into mesodermal- (osteocyte and adipocyte) and endodermal- (pancreatic-like) lineage cells. Consistent with the previous report [11], flow cytometry analyses revealed that PDMSCs were strongly positive for CD44, CD49b, CD105 and CD166, but negative for CD45, CD34, MHC I and MHC II (Fig. 1A). The levels of Oct-4, Nanog, Nestin, Sox-2 and Sox17 in PDMSCs were elevated compared to the primary culture of fibroblast cells as detected by RT-PCR (P < 0.05; Fig. 1B), with sustained expression of all genes detected through 10 passages in this study. To investigate the role of MafA in PDMSCs (fifth passage), the overexpression of MafA in PDMSCs was induced using a lentivector (Fig. 1C and D: step 1). MafA-overexpressing PDMSCs developed a cluster-like morphology, and cell aggregates were observed at day 7 after infection (Fig. 1D). Under the serum-free pancreatic induction medium (Fig. 1D: step 2), MafA-overexpressing PDMSCs formed 3D spheroid-bodies (3D-SB; Fig. 1D) more easily than the vector control (PDMSCs without overexpression of MafA).
We next examined the expression profile of MafA-overexpressing PDMSCs (step 1: after 7 days post-transfection only; Fig. 1D) using microarray analysis. The profiles of the differentially expressed genes based on their functions in the Gene Ontology database of MafA-overexpressing PDMSCs are displayed in Fig. 2A and Table S1. Based on the microarray findings, the gene expression in the subset of MafA-overexpressing PDMSCs (step 1) resembled pancreatic-related and islet-like expression patterns more than the parental PDMSCs or vector-control PDMSCs (Fig. 2B). Multidimensional scaling analysis further suggested that expression pattern of MafA-overexpressing PDMSCs was closer to the gene signature of pancreas and pancreatic islet than the expression patterns of parental and vector-control PDMSCs (P < 0.001; Fig. 2C and Fig. S2A). Furthermore, the Q-RT-PCR results showed that expression levels of endodermal-lineage (Sox17 and Foxa2) and pancreatic development-related (Pdx1 and MafA) genes were significantly up-regulated in MafA-overexpressing PDMSCs (step 1) compared to vector control (P < 0.001; Fig. 2D). The results of Western blotting analysis further demonstrated that the levels of MafA, Pdx1; (Fig. 2E), Ptf1/p48 (a marker for pancreatic progenitor-exocrine differentiation and endocrine lineage; Fig. S2B) [15, 16] and Ngn3 (Fig. S2B) proteins in MafA-overexpressing PDMSCs (step 1) were significantly increased compared to those in PDMSCs transfected with mock plasmid. Moreover, immunofluorescent analysis confirmed that the level of Pdx1 protein, a pancreatic progenitor-related marker, was higher in MafA-overexpressing PDMSCs than in the vector control (P < 0.001; Fig. 2F). Taken together, our data indicate that overexpression of MafA in PDMSCs promotes the expression of pancreatic development-related genes in PDMSCs, and also facilitates the specific reprogramming of PDMSCs into pancreatic β-progenitors and islets.
Fig 2.
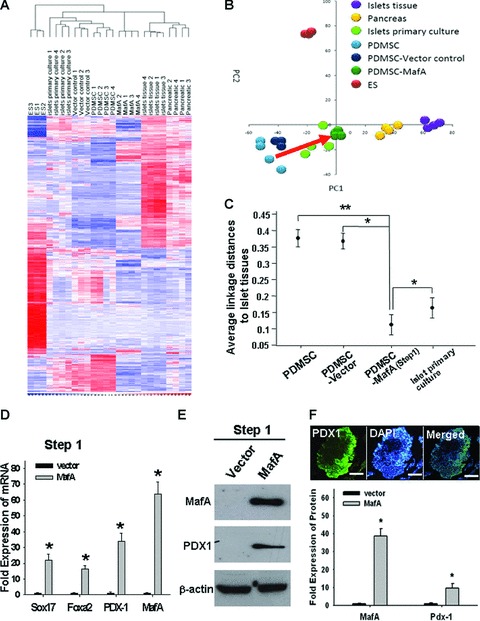
MafA promotes PDMSC reprogramming into pancreatic progenitors. (A) After 7 days of infection, gene expression microarray analysis (Gene tree) showing 500 altered genes differentially expressed in MafA-overexpressing PDMSCs compared to PDMSCs and adult MSCs by a hierarchy heat map (MSC: bone marrow-derived mesenchymal stem cell; EM: endometrium-derived mesenchymal stem cell). The time-dependent changes of the 500 altered genes are presented on a log scale of the expression values provided by the GeneSpring GX software. (B) Principal component analysis demonstrated that the gene signature of MafA–overexpressing PDMSCs was similar to that of islets tissues. (C) Average lineage distances between islet tissues and cell lines derived from PDMSC. (D) The mRNA expression levels of Sox17, FoxA2, PDX1 and MafA in vector control and MafA-overexpressing PDMSCs were detected by Q-RT-PCR. (E) The protein expression levels of MafA and PDX-1 in vector control PDMSCs and MafA-overexpressing PDMSCs were verified by Western blot. (F) By immunofluorescence staining, PDX-1 (green) was found to be highly expressed in the aggregated SB-MafA-overexpressing PDMSCs. Cell nuclei were stained with DAPI. Data shown here are the mean ± S.D. of three independent experiments. *P < 0.001. Bar = 50 μm.
MafA facilitated PDMSC differentiation into pancreatic-lineage cells and increased c-peptide/ insulin secretion in insulin+ cells
To further investigate the process of pancreatic and insulin+ differentiation, on the seventh day of MafA-overexpression PDMSCs (Fig. 1D: step 1) were changed to serum-free modified pancreatic selection medium. After 7 days of culture in modified pancreatic selection medium (Fig. 1D: step 2), the mRNA levels of endodermal progenitor-related genes (Sox17 and Foxa2) were dramatically decreased (P < 0.001; Fig. 3A), but not the levels of Pdx1, Ngn3 and MafA. Consistent with this finding, Western blotting showed a slight decrease in the level of Pdx1 and Ngn3 proteins (step 2; Fig. 3B). In contrast, the levels of MafA protein did not change in MafA-overexpressing PDMSCs after 7 days in pancreatic selection medium (Fig. 3B). We further examined whether the overexpression of MafA can facilitate PDMSC differentiation into mature pancreatic and insulin+ cells. After culture in induction medium for 7 days, quantitative RT-PCR showed a significant increase in the mRNA expression levels of insulin, Glut2, somatostatin, glucagon, Pax4, Nkx2.2, NeuroD and Isl-1 in differentiated MafA-overexpressing PDMSCs compared to PDMSCs control (P < 0.001; Fig. 3C). Furthermore, using a dual immunofluorescence assay, our data showed that the percentages of insulin+ and glucagon+ cells in differentiated, MafA-overexpressing PDMSCs were also significantly higher than those in the PDMSCs control after 7 days of culture in induction medium (P < 0.001; Fig. 3D). Importantly, in contrast to the group of PDMSC with only induction of PDMSC (without overexpression of MafA) [17], data in Fig. 3 supported that this two-step induction (combination of overexpression of MafA with inducing medium for 7 days) can drive PDMSCs toward a pancreatic-lineage cell fate more efficiently, and further significantly promote the islet-like characteristics in PDMSCs.
Fig 3.
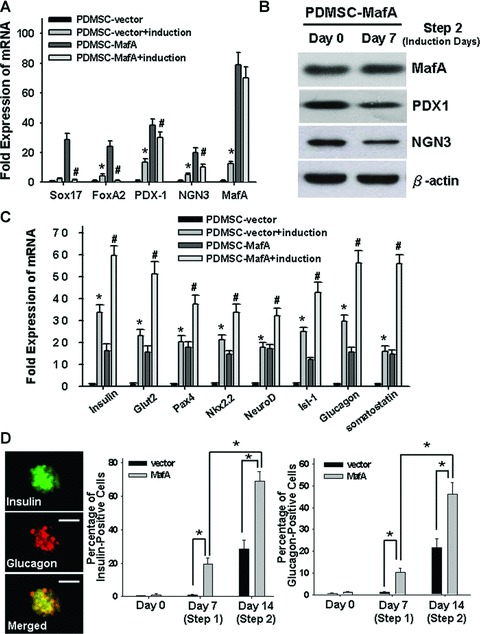
MafA-overexpressing PDMSCs differentiate into insulin+ cells and enhance c-peptide release. After 7 days of culture in pancreatic induction medium, (A) quantitative real-time RT-PCR (Q-RT-PCR) and (B) Western blotting were performed to detect the expression levels of (Sox17, Foxa2, PDX1, NGN3 and MafA) mRNA and (PDX1, NGN3 and MafA) protein in the different groups. (C) After 7 days of induction, the mRNA levels of insulin, Glut2, Pax4, Nkx2.2, NeuroD, Isl-1, somatostatin and glucagon of different treated group were measured by Q-RT-PCR. (D) By immunofluorescence staining, insulin (red) and glucagon (green) were found to be highly expressed in the MafA-overexpressing PDMSCs (step 2) as compared other groups or control. *P < 0.001: PDMSC-MafA (MafA-overexpressing PDMSC; step 1) versus PDMSC-vector (control); #P < 0.001: PDMSC-MafA + induction (PDMSC-MafA treated with modified pancreatic selection medium; step 2) versus PDMSC-MafA (step 1). Data shown here are the mean ± S.D. of three independent experiments. Bar = 50 μm.
The response to glucose-stimulated insulin secretion is the most important function of pancreatic β-cells, essential for maintaining glucose homeostasis [18]. To examine the role of MafA in regulation of insulin secretion in differentiated MafA-overexpressing PDMSCs in response to glucose stimulation, cells were exposed to different glucose levels. In the presence of 5.5 mM and 25 mM glucose, stimulated secretion of insulin in the culture medium of differentiated, MafA-overexpressing PDMSCs (step 2) was significantly higher than secretion in PDMSC control cells (P < 0.001; Fig. 4A). Moreover, the secreted levels of C-peptide (a product of de novo insulin formation) in the culture medium of differentiated MafA-overexpressing PDMSCs (step 2) were significantly higher than those in PDMSC control (P < 0.001, Fig. 4B). The results of immunofluorescence analysis further confirmed that the differentiated MafA-overexpressing PDMSCs exhibited abundant expression of both insulin and C-peptide after 7 days culture of pancreatic selection medium (Fig. 4C). These results suggest that MafA can induce insulin+ cell differentiation, enhance the islet-associated products and promote the functional secretion of insulin.
Fig 4.
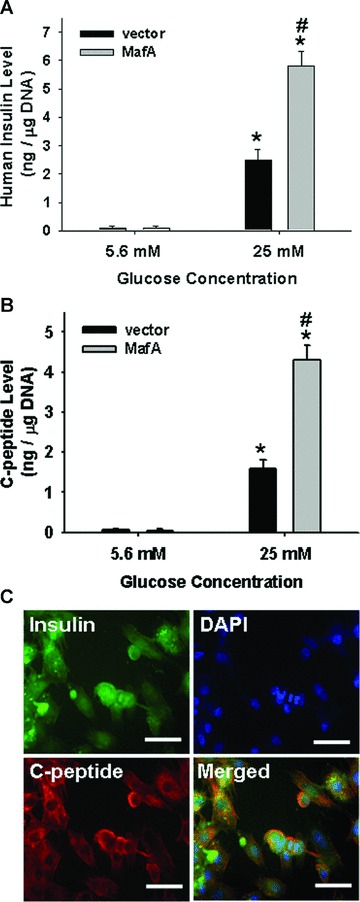
MafA increased c-peptide and insulin secretion in insulin+ cells. (A) Insulin release in response to physiological (5.5 mM) and high (25 mM) glucose concentrations from differentiated MafA-overexpressing PDMSCs (step 2) and control PDMSCs was measured after 7 days of pancreatic induction. (B) C-peptide release in response to glucose stimulation from differentiated MafA-overexpressing PDMSCs (step 2) and control PDMSCs was detected after 7 days of induction. (C) Immunofluorescence analysis was performed for the expression of insulin (green) and C-peptide (red) in differentiated MafA-overexpressing PDMSCs after 7 days of induction. Cell nuclei were stained with DAPI. Data shown here are the mean ± S.D. of three independent experiments. *P < 0.001 (25 mM group compared to 5.5 mM group). #P < 0.001: PDMSC-MafA (step 2) versus PDMSC-vector (control). Bar = 20 μm.
MafA provided resistance to oxidative stress-damage and inhibited IL-1b-induced apoptosis in PDMSC-derived insulin+ cells
To evaluate the anti-oxidant effects and cytoprotective potential of MafA overexpression, cell (step 2) survival rates and reactive oxygen species (ROS) production were measured (Supporting Information ‘Material and methods’). H2O2 treatment significantly decreased cell viability and increased both the percentage of annexin V+ cells and the levels of intracellular ROS production (Fig. 5A–C; P < 0.001). Overexpression of MafA obviously protected PDMSC cells (step 2) from oxidative stress-induced apoptosis and raised the cell survival rate. The anti-oxidant and cytoprotective effects of MafA were confirmed by challenging treated PDMSC cells with additional 25 mM glucose. MafA was found to effectively diminish ROS production and cell death induced by both H2O2 and H2O2 plus high glucose treatments (Fig. 5A–C). Moreover, interleukin-1β (IL-1β) plays a critical role in the pathophysiology of diabetics and severely interferes with insulin production [19, 20]. Thus, we further examined whether up-regulation of MafA could protect β-cells derived from MafA-overexpressing PDMSCs (step 2) against IL-1β-induced apoptosis. In PDMSCs cultured at 22.2 mmol/l glucose and exposed to 50 U/ml of IL-1β, the viability of MafA-overexpressing PDMSCs was significantly higher than that of control cells (P < 0.001; Fig. 5D). The percentage of annexin V+ apoptotic cells in the MafA-overexpressing population was dramatically decreased compared to that of control cells (P < 0.001; Fig. 5E). Furthermore, Western blot analysis confirmed that MafA could efficiently block the IL-1β-induced cleavage of caspase 3 and simultaneously elevate the levels of the Bcl-2 and manganese superoxide dismutase (MnSOD) proteins, but reduced the levels of Bax (Fig. 5F). These data suggest that MafA produces cytoprotective effects in PDMSC-derived insulin-producing cells, and plays a key role in protect cells from oxidative stress-induced damage, glucotoxicity and IL-1β-induced apoptosis.
Fig 5.
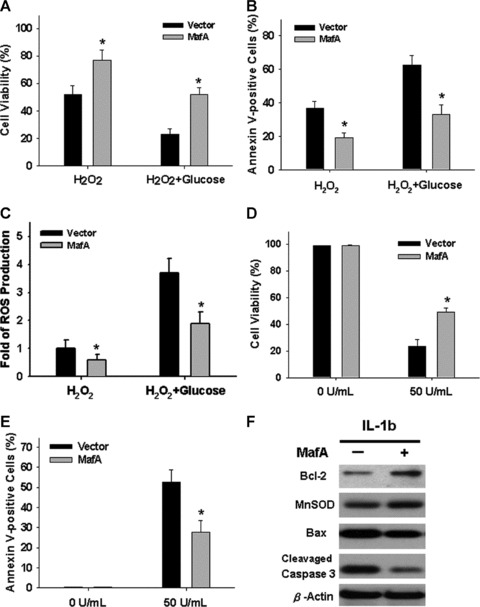
Insulin+, MafA-expressing PDMSCs resist oxidative-stress and IL-1β induced apoptosis. (A–C) Cells at the end of step 2 differentiation were treated with 150 μmol/l H2O2 with or without 25 mM glucose for 6 hrs, followed by MTT assay (A), annexin V staining (B) and the detection of intracellular ROS production (C). Cells were treated with 50 U/ml IL-1β or vehicle control for 18 hrs. Cell viability and apoptotic cells were determined by MTT assay (D) and annexin V staining (E), respectively. (F) Western blots showed the levels of Bcl-2, MnSOD, Bax and cleaved caspase 3 in cells receiving 50 U/ml IL-1β for 18 hrs. Data shown here are the mean ± S.D. of three independent experiments. *P < 0.001.
To further explore the possible mechanism involved in the anti-oxidant effects of MafA-overexpressing PDMSCs, the glutathione (GSH) level and ROS production were measured in IL-1β-treated cells. Noticeably, treatment of 50 U/ml of IL-1β in MafA-overexpressing PDMSC (step 2) significantly inhibited ROS production (Fig. 6; P < 0.001) and increased intracellular GSH level as compared to PDMSC-vector control (Fig. 6B; P < 0.001). Noticeably, MafA overexpression prevented IL-1β-induced ROS production (Fig. 6A; P < 0.001) and increased intracellular GSH level (Fig. 6B; P < 0.001) in MafA-overexpressing PDMSC (step 2). The MafA-overexpressing PDMSC were further pre-treated with an endogenous ROS generator L-S,R-buthionine sulphoximine (BSO; Sigma), a selective inhibitor of γ-glutamylcysteine synthetase, for 24 hrs. The results showed that the BSO could dramatically diminish MafA-derived cytoprotective effects in IL-1β-treated MafA-overexpressing PDMSC (Fig. 6C). Moreover, 24 hrs pre-treatment with 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy (Tempol; Sigma), a membrane-permeable radical scavenger, could effectively protect IL-1β-induced oxidative damage in PDMSC-control group, but only slightly increase the cell survival in IL-1β-induced MafA-overexpressing PDMSC (Fig. 6C). Importantly, under the simultaneous pre-treatment with BSO (ROS agonist) or Tempol (ROS inhibitor), there were no significant difference of oxidative damage and cytoprotective effect between IL-1β-treated MafA-overexpressing PDMSC and IL-1β-treated PDMSC-control group (P > 0.05; Fig. 6C). These data suggested that the cytoprotective effect of MafA on PDMSC-derived insulin-producing cells was mediated mainly by a reduction of oxidative stress.
Fig 6.
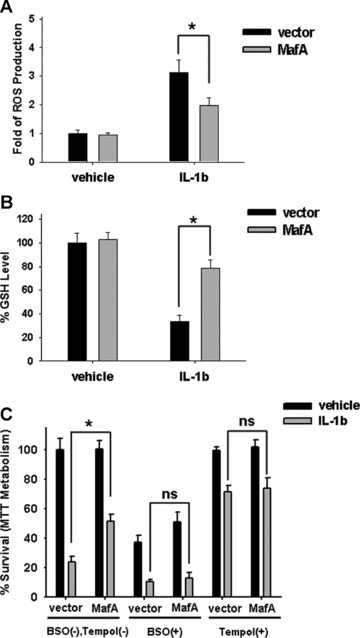
Roles of anti-oxidant effects of MafA in its cytoprotection of PDMSC. (A, B) After 7 days of pancreatic induction (step 2) of PDMSC with or without MafA overexpression, cells were treated with 50 U/ml IL-1β or vehicle control for 18 hrs, followed by detection of ROS production (A, fold of control) and GSH level (B, % of control). (C) After 7 days of pancreatic induction (step 2) of PDMSC with or without MafA overexpression, cells were pre-treated with or without 1 mmol/l BSO (a γ-glutamylcysteine synthetase inhibitor) and 0.5 mmol/l Tempol (a membrane-permeable radical scavenger) for 24 hrs, followed by treatment with 50 U/ml IL-1β or vehicle control. After another 18 hrs, cell survival (% of control) was determined. Data shown here are the mean ± S.D. of three independent experiments. *P < 0.001; ns: not significant (P > 0.05).
MafA-overexpressing PDMSCs improve glucose control and prolong the survival of STZ-treated SCID mice
SCID mice pre-treated with STZ were employed to examine the restoration of normoglycaemia by introduction of differentiated, insulin+ cells derived from MafA-overexpressing PDMSCs through xenotransplantation. PDMSCs and MafA-overexpressing PDMSCs were further transfected using a lentivector combined with the GFP gene (Fig. 7A) [21], and then in vivo GFP imaging and histology were used to monitor stem cell growth (Fig. 7B). The renal subcapsular space (Fig. 7B) in SCID mice has been demonstrated to provide a microenvironment suitable for endocrine cell differentiation [11]. A total of 2 × 105 MafA-overexpressing PDMSCs-GFP (step 2; Fig. 7B; Left part) or control PDMSCs-GFP were implanted into the subcapsular space of the left kidney (n= 6). After 4 weeks, the result of ex vivo GFP imaging revealed that transplanted MafA-overexpressing PDMSCs can proliferate and grow in solid tissues in the subrenal site (Fig. 7B). It indicates that all signals of ex vivo GFP were only detected in the foci of subrenal area and kidney but not in other organs of transplanted mice (Fig. 7B; upper panel). The histology survey demonstrated that diffuse aggregated islet-like clusters were found in the transplanted grafts of MafA-overexpressing PDMSCs (arrows; Fig. 7C). Furthermore, immunofluorescence experiments (Fig. 7C) further confirmed that more strong signals for insulin+ (red fluorescence) and glucagon+ (green fluorescence) staining were detected in the islet-like cluster of MafA-overexpressing, PDMSC-derived graft than in the control PDMSC-derived graft in the subrenal site in SCID mice (Fig. 7D; P < 0.05).
Fig 7.
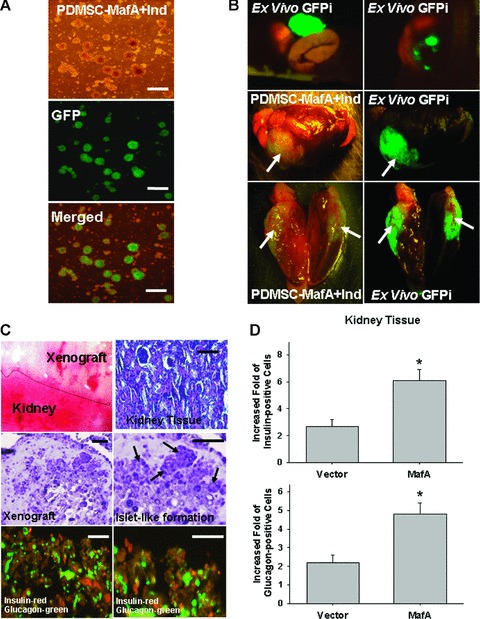
Xenotransplantation of differentiated MafA-overexpressing PDMSCs into STZ pre-treated SCID mice. (A) To effectively monitor the growth conditions of PDMSC xenotransplanted grafts in SCID mice, PDMSCs and MafA-overexpressing PDMSCs in vitro were transfected with GFP by lentivector, and further evaluated by in vitro GFP imaging (Upper-light morphology, Middle: GFP imaging; Bottom: Merged imaging) as well as in vivo GFP imaging (right part photograph). (B) A total of 2 × 105 MafA-overexpressing PDMSCs-GFP or control PDMSCs-GFP were implanted into the subcapsular space of the left kidney (n= 6; each). After 4 weeks, ex vivo biopsies and histology revealed that transplanted MafA-overexpressing PDMSCs-GFP can grow solid tissues in the subrenal site but not in other organs (right part photograph; arrows: PDMSC-GFP xenograft). (C) The histological and immunofluorescent study for reviewing the characteristics of xenograft derived MafA-overexpressing PDMSCs (arrows: the islet-like clusters). (C) and (D) The immunofluorescent analysis revealed that the expression of insulin (red) and glucagon (green) in the graft was significantly higher in mice transplanted with MafA-overexpressing PDMSCs than in those with control PDMSCs. Data shown here are the mean ± S.D. of three independent experiments. *P < 0.05. Bar = 100 μm.
Although the blood glucose was reduced in both groups of transplanted animals in comparison to the untreated control group, a significantly lower blood glucose level was observed in the group of the MafA-overexpressing PDMSC transplanted animals (P < 0.05; Fig. 8A). Importantly, the rebounding levels of blood glucose were noted in MafA-overexpressing PDMSC transplanted animals after the transplanted grafts were removed by nephrectomy (Fig. 8A). Furthermore, the serum level of human insulin was also measured during random feeding. The serum level of human insulin was nearly undetectable before STZ treatment. The insulin levels in STZ-treated mice transplanted with MafA-overexpressing PDMSCs were significantly higher than those of control PDMSCs for one week (P < 0.05; Fig. 8B) and this difference was maintained 10 weeks after transplantation (Fig. 8C). Importantly, no teratoma formation was observed in MafA-overexpressing, PDMSC-derived xenografts 6 months after implantation. These results suggest that MafA can aid in the restoration of insulin to a nearly normal level in STZ pre-treated SCID mice.
Fig 8.
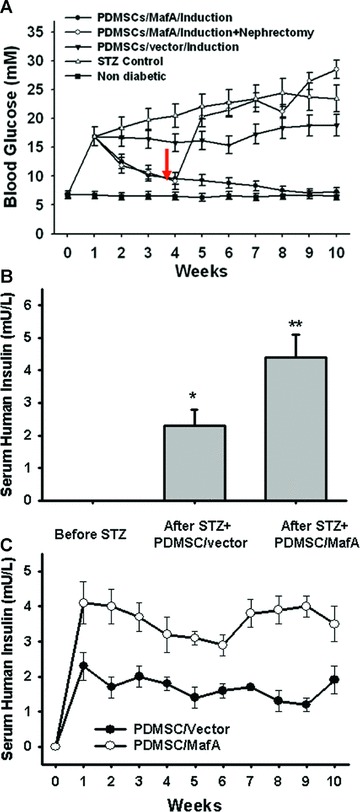
Restoration of normoglycaemia in STZ- pre-treated SCID mice through xenotransplantation of MafA-overexpressing PDMSCs. (A) The blood glucose levels of the group implanted with MafA-overexpressing PDMSCs (step 2) were significantly lower than those implanted with vector control PDMSCs and the STZ-control groups (P < 0.05). After the nephrectomy of transplanted grafts (arrow), the blood glucose levels were significantly increased in the transplanted MafA-overexpressing SCID mice (P < 0.05). (B) Serum human insulin detection 1 week after transplantation in STZ-induced diabetic mice. Random-fed blood glucose levels were measured in all groups. (C) Time-course changes of serum human insulin levels after transplantation (bottom). Data shown here are the mean ± S.D. of three independent experiments. *P < 0.05; **P < 0.001.
Discussion
MafA, a β-cell-specific activator of insulin transcription, maintains β-cell functions by regulating differentiation, maturation, insulin biosynthesis and GLP-1 signalling [22, 23]. It was reported recently that adenoviral MafA overexpression, together with Pdx-1 and Ngn3, markedly induced insulin-producing surrogate β-cells in pancreatic exocrine cells in adult mice [6]. In this study, we overexpressed MafA in PDMSCs using a lentivector, and investigate the role of MafA in the reprogramming of pancreatic lineage cells and the enhancement of insulin-secreting function in PDMSCs. Transcriptome profiles and molecules signatures analyzed using microarrays have suggested that MafA specifically drives PDMSC reprogramming into pancreatic islets (Figs 1 and 2; Table S1). Furthermore, the overexpression of MafA in PDMSCs can effectively activate the endogenous expressions of MafA genes but not embryonic-related genes (data not shown). These data suggest that overexpression of MafA did not result in de-differentiation of treated PDMSCs. Furthermore, the results of Western blotting also supported that the overexpression of MafA in PDMSCs significantly up-regulated the expression of pancreatic progenitor-associated transcriptional factors-Pdx1, Ptf1/p48 and Ngn3 (Fig. 2 and Fig. S2B). Moreover, as compared to PDMSCs carrying vector alone, MafA could efficiently promote PDMSC differentiation into pancreatic-lineage cells, which showed strong expression of both insulin and glucagon at both the mRNA and protein levels (Fig. 3). Our results suggest that MafA can further promote the production of insulin and c-peptide in response to stimulation with different concentrations of glucose in treated PDMSCs (Fig. 4). Finally, our in vivo xenotransplantation study confirmed that MafA facilitated PDMSC differentiation into functional insulin- and glucagon+ cells (Fig. 7), and helped to stably restore normoglycaemia in diabetic SCID mice (Fig. 8). To our knowledge, this is the first report to demonstrate the role of MafA in the reprogramming of PDMSCs into β-islet progenitors but also in increasing the functional secretion of insulin and effective control of blood glucose levels.
That hyperglycaemia itself can decrease insulin secretion has led to the concept of glucose toxicity, which implies the development of irreversible damage to cellular components of the insulin production pathway over time [24, 25]. The effects of the pro-inflammatory cytokine IL-1β have been conclusively shown to impair glucose-stimulated insulin production in mouse, rat and human islets, and to increase β-cell death [19, 20, 26]. In this study, we found that MafA-overexpressing PDMSCs are more resistant to hyperglycaemia-induced or hyperglycaemia and oxidative stress-induced toxicity and damage compared to PDMSCs with the vector control (Fig. 5). Furthermore, our data demonstrate that MafA can further suppress IL-1β-induced apoptosis in treated PDMSCs, in part through activating the expression of anti-apoptotic genes (Bcl-2 and Bcl-xL) and MnSOD (Fig. 5). Pancreatic transcription factors including Pdx-1, FoxO1, sterol regulatory element-binding protein-1c (SREBP-1c) and MafA are known to control different biological processes such as differentiation, proliferation and survival [22]. Nishimura et al. further demonstrated that MafA plays a vital role in regulating the replication, survival and function of β-cells [27]. A recent study demonstrated that MafA had the potential to protect against β-cell failure induced by oxidative stress, which is involved in the regulation of FoxO1, a transcription factor of the forkhead family [28]. Furthermore, using Isl-1 deficient mice as a model, Du et al. demonstrated that MafA was a direct transcriptional target of Isl-1, and Islet-1 is further required for the maturation, proliferation and survival of the endocrine cells of the pancreas [17]. Importantly, our data showed that MafA exhibits the potential to improve the viability of H2O2-treated PDMSCs and makes PDMSC-derived, insulin+ cells more resistant to oxidative stress-induced apoptosis (Fig. 5). Furthermore, in vivo transplantation experiments confirmed that MafA can restore normoglycaemia stably (Fig. 7 and 8), and significantly prolong the survival of the transplanted graft (Fig. 8). In addition, we found that MafA increases Bcl-2 expression and reduces oxidative stress-induced apoptosis in treated PDMSCs (Fig. 5). Furthermore, MafA could dramatically reduce IL-1β-induced ROS accumulation (Fig. 6A) and up-regulate intracellular GSH levels in treated PDMSC (Fig. 6B). Furthermore, the pre-treatment with BSO (GSH inhibitor) and Tempol (ROS scavenger), the study results further indicated that the cytoprotective effect of MafA on PDMSC-derived insulin-producing cells was mediated mainly by a reduction of oxidative stress (Fig. 6C). We will further investigate whether the anti-oxidant effects and the involvement of ROS regulation of MafA play a role in promoting survival and self-renewal of stem cells or β-cells. Taken together, our findings indicate that MafA protects cells from glucotoxicity and oxidative stress-induced damage, but also reveal a novel function of protection against IL-1β-induced apoptosis in PDMSC-derived, insulin-producing cells and promotion of survival of transplanted grafts in STZ-induced diabetic mice.
A recent breakthrough demonstrated that ectopic expression of Yamanaka’s four genes (Oct-4, Sox-2, Klf-4 and c-Myc) is sufficient to reprogram murine and human fibroblasts into inducible pluripotent stem cells (iPS) [29, 30]. The ability to form teratomas in vivo has been a landmark and routine assay for evaluating the pluripotency of embryonic stem cells (ESCs) as well as iPS [31]. However, teratoma formation from pluripotent stem cells is considered a formidable obstacle for the application of stem cell therapy in regenerative medicine [32, 33]. PDMSCs derived from human term placenta preserve the primitive embryonic character and have recently been introduced into clinical trials [21]. In this study, we demonstrated that transduction of a single, critical pancreatic transcriptional factor, MafA, can efficiently convert PDMSCs into functional pancreatic islet-like progenitors without the need to first reprogram them into an ESC or iPS-like state. This strategy overcomes both the ethical concerns associated with the use of ESCs or iPS and the safety concerns related to teratoma formation (Fig. 7; Fig. S3). Although biological roles of MafA in transcriptional regulation and epigenetic programming in islet-like characteristics and functional β-progenitors of human stem cells are needed to be further studied, this application can be further extended and eventually used as an alternative source of stem cells for therapeutic approaches to diabetes mellitus.
Acknowledgments
This study was assisted in part by the Division of Experimental Surgery of the Department of Surgery, Taipei Veterans General Hospital. This study was supported by research grants from NSC-(97–3111-B-075–001-MY3), Taipei Veterans General Hospital (V97B1–006, E1–008, ER2–018, ER3–005, F-001), the Joint Projects of UTVGH (VGHUST 98-G6–6), Yen-Tjing-Ling Medical Foundation, National Yang-Ming University (Ministry of Education, Aim for the Top University Plan), and Technology Development Program for Academia, Department of Industrial Technology (98-EC-17-A-19-S2–0107), Ministry of Economic Affairs, Taiwan.
Supporting Information
(A) PDMSCs were infected with lentiviruswith green fluourescent protein (GFP). After 7 dayspost-transduction, the MafA-overexpressing PDMSC-GFP cells (blueregion) were sorted by FACS. (B) High percentage ofGFP-positive cells were sorted out.
Induced pluripotent stem cells (iPS only)reprogramming from fibroblast by transfection of Yamanakafactors-Oct-4, Sox-2, Klf-4, and c-Myc, and 2 χ 105iPS cells after 7 days culture under induction pancreatic inductionmedium (iPS treated with induction medium) were implanted inot the(A) sub capsular space of the left kidney (n = 6;each). After 4 weeks, (ex vivo biopsies and histologyrevealed that the teratoma formation in the subrenal graft ofeither iPS-only group or iPS treated with induction medium group.Bar = 100 μM.)
The gene list which PDMSC-MafA expression level higher than PDMSC more 4-fold (>4).
The gene list which PDMSC-MafA expression level lower than PDMSC more 4-fold (<1/4)
The sequences for the primers of quantitative RT-PCR
References
- 1.Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 2.Robertson RP. Islet transplantation as a treatment for diabetes – a work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 3.Matsuoka TA, Artner I, Henderson E, et al. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci USA. 2004;101:2930–3. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneto H, Matsuoka TA, Kawashima S, et al. Role of MafA in pancreatic beta-cells. Adv Drug Deliv Rev. 2009;61:489–96. doi: 10.1016/j.addr.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Moriguchi T, Kajihara M, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–76. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen BL, Huang HI, Chien CC, et al. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–9. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 9.Portmann-Lanz CB, Schoeberlein A, Huber A, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664–73. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 10.Chien CC, Yen BL, Lee FK, et al. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 2006;24:1759–68. doi: 10.1634/stemcells.2005-0521. [DOI] [PubMed] [Google Scholar]
- 11.Chang CM, Kao CL, Chang YL, et al. Placenta-derived multipotent stem cells induced to differentiate into insulin-positive cells. Biochem Biophys Res Commun. 2007;357:414–20. doi: 10.1016/j.bbrc.2007.03.157. [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Chen YW, Hsu HS, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–13. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 14.Huang TS, Hsieh JY, Wu YH, et al. Functional network reconstruction reveals somatic stemness genetic maps and dedifferentiation-like transcriptome reprogramming induced by GATA2. Stem Cells. 2008;26:1186–201. doi: 10.1634/stemcells.2007-0821. [DOI] [PubMed] [Google Scholar]
- 15.Afelik S, Chen Y, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–6. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Cooper B, Gannon M, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 17.Du A, Hunter CS, Murray J, et al. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–69. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaney GC, Corkey BE. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia. 2003;46:1297–312. doi: 10.1007/s00125-003-1207-4. [DOI] [PubMed] [Google Scholar]
- 19.Oetjen E, Blume R, Cierny I, et al. Inhibition of MafA transcriptional activity and human insulin gene transcription by interleukin-1beta and mitogen-activated protein kinase kinase kinase in pancreatic islet beta cells. Diabetologia. 2007;50:1678–87. doi: 10.1007/s00125-007-0712-2. [DOI] [PubMed] [Google Scholar]
- 20.Tellez N, Montolio M, Estil-les E, et al. Adenoviral overproduction of interleukin-1 receptor antagonist increases beta cell replication and mass in syngeneically transplanted islets, and improves metabolic outcome. Diabetologia. 2007;50:602–11. doi: 10.1007/s00125-006-0548-1. [DOI] [PubMed] [Google Scholar]
- 21.Brooke G, Rossetti T, Pelekanos R, et al. Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. Br J Haematol. 2009;144:571–9. doi: 10.1111/j.1365-2141.2008.07492.x. [DOI] [PubMed] [Google Scholar]
- 22.Shao S, Fang Z, Yu X, et al. Transcription factors involved in glucose-stimulated insulin secretion of pancreatic beta cells. Biochem Biophys Res Commun. 2009;384:401–4. doi: 10.1016/j.bbrc.2009.04.135. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Brun T, Kataoka K, et al. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia. 2007;50:348–58. doi: 10.1007/s00125-006-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–65. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson RP, Harmon J, Tran PO, et al. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–7. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 26.Montolio M, Biarnes M, Tellez N, et al. Interleukin-1beta and inducible form of nitric oxide synthase expression in early syngeneic islet transplantation. J Endocrinol. 2007;192:169–77. doi: 10.1677/joe.1.06968. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura W, Kondo T, Salameh T, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–39. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes Obes Metab. 2007;9:140–6. doi: 10.1111/j.1463-1326.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 31.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–6. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–5. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 33.Kao CL, Tai LK, Chiou SH, et al. Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev. 2010;19:247–58. doi: 10.1089/scd.2009.0186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) PDMSCs were infected with lentiviruswith green fluourescent protein (GFP). After 7 dayspost-transduction, the MafA-overexpressing PDMSC-GFP cells (blueregion) were sorted by FACS. (B) High percentage ofGFP-positive cells were sorted out.
Induced pluripotent stem cells (iPS only)reprogramming from fibroblast by transfection of Yamanakafactors-Oct-4, Sox-2, Klf-4, and c-Myc, and 2 χ 105iPS cells after 7 days culture under induction pancreatic inductionmedium (iPS treated with induction medium) were implanted inot the(A) sub capsular space of the left kidney (n = 6;each). After 4 weeks, (ex vivo biopsies and histologyrevealed that the teratoma formation in the subrenal graft ofeither iPS-only group or iPS treated with induction medium group.Bar = 100 μM.)
The gene list which PDMSC-MafA expression level higher than PDMSC more 4-fold (>4).
The gene list which PDMSC-MafA expression level lower than PDMSC more 4-fold (<1/4)
The sequences for the primers of quantitative RT-PCR


