Abstract
Tumour-associated fibroblasts (TAFs) are part of the tumour stroma, providing functional and structural support for tumour progression and development. The origin and biology of TAFs are poorly understood, but within the tumour environment, TAFs become activated and secrete different paracrine and autocrine factors involved in tumorigenesis. It has been shown that bone marrow mesenchymal stem cells (MSCs) can be recruited into the tumours, where they proliferate and acquire a TAF-like phenotype. We attempted to determine to what extent TAFs characteristics in vitro juxtapose to MSCs’ definition, and we showed that TAFs and MSCs share immunophenotypic similarities, including the presence of certain cell surface molecules [human leukocyte antigen-DR subregion (HLA-DR), CD29, CD44, CD73, CD90, CD106 and CD117]; the expression of cytoskeleton and extracellular matrix proteins, such as vimentin, α-smooth muscle actin, nestin and trilineage differentiation potential (to adipocytes, chondrocytes and osteoblasts). When compared to MSCs, production of cytokines, chemokines and growth factors showed a significant increase in TAFs for vascular endothelial growth factor, transforming growth factor-β1, interleukins (IL-4, IL-10) and tumour necrosis factor α. Proliferation rate was highly increased in TAFs and fibroblast cell lines used in our study, compared to MSCs, whereas ultrastructural details differentiated the two cell types by the presence of cytoplasmic elongations, lamellar content lysosomes and intermediate filaments. Our results provide supportive evidence to the fact that TAFs derive from MSCs and could be a subset of ‘specialized’ MSCs.
Keywords: mesenchymal stem cells (MSCs), tumour-associated fibroblasts (TAFs), tumour stroma, growth factors, cytokines, pluripotency
Introduction
Beginning with the work of Friedenstein and colleagues almost four decades ago [1], the concept of adult mesenchymal stem cells (MSCs) emerged and progressively evolved into the commonly accepted definition of cells of bone marrow origins, adherent to culture plastic surfaces, with fibroblast-like morphology, long-term proliferation in vitro and self-renewal capacity, and with the ability to differentiate into mineralizing cells (bone), cartilage and fat. Subsequently, the concept expanded, as it proved that MSCs are not only bone marrow resident cells but are also found in many other tissues of the body and have the ability to be recruited at injury sites and tumours. A considerable amount of research has been dedicated during the last decade on the molecular and functional characterization of these cells. However, their in vivo biology is still poorly understood. This is due, at least in part, to the lack of a specific marker or phenotype that would allow easy identification and/or description of any tissue-specific subsets of MSCs. It is widely assumed that such ‘MSC-like’ cells or MSC subsets exist, and although they retain the basic MSC features and functions (i.e. pluripotency and the ability to differentiate into connective tissues), they also exhibit specialized features tailored for each particular tissue. The tumour-associated fibroblasts (TAFs) are considered to be such ‘specialized’ MSCs, mainly located in certain tumours of epithelial and non-epithelial origin (generally solid tumours, such as breast carcinomas, ovarian carcinomas, gliomas, melanomas and Kaposi sarcomas) [2], being a major part of the tumour stroma.
A large body of evidence has been gathered regarding the supportive role of the tumour stroma and its component cells, which contribute to the final texture of the tumour microenvironment. It was even speculated that the stromal cell compartment plays a crucial role in tumorigenesis and invasion by stimulating transformation of normal cells to produce growth factors, cytokines and chemokines that can activate adjacent cells and induce selection and expansion of neoplastic cells [3–5]. Tumour cells are not capable of being maintained in culture conditions without a stromal network, and even so, their in vitro proliferative potential is limited, although the surrounding stromal fibroblasts continue to develop. Recent studies have shown that cancer cells in suspension, injected into immunodeficient animals, are able to generate tumours at the site of injection and tumour growth only when associated with their stromal compartment [6], reinforcing again the importance of the supportive role of the stromal background for the malignant cells [7]. Although the tumour-associated stroma comprises four main components – vasculature, inflammatory cells, extracellular matrix and TAFs [8]– it is the TAFs that offer the dynamic and active support for cancer cell growth.
The origins and the role TAFs play within the tumour are still not entirely clear; however, there is an increasing amount of data showing that these fibroblasts, as a ‘specialized’ subset of MSCs (‘mesenchymal fibroblasts’) [9], are recruited from the bone marrow [10, 11] or from the local healthy tissue during tumour development. For instance, Spaeth et al. [6] were able to induce the ‘activation’ of bone marrow-derived MSCs by tumour-conditioned media, with the expression of TAF-like surface markers and the ability to generate microvascular structures. It is very likely that such a conversion is a crucial step that occurs in vivo during the development of the tumorigenic process. Upon conversion, the activated fibroblasts, now referred to as TAFs, become an integral and necessary part of the tumour, offering support, protection and nutrition for the cancer cells and allowing them to grow, evolve, and, eventually, metastasize.
It is critical to acquire a better understanding of the biology and physiology of TAFs, as these cells could offer some attractive molecular targets to be used in anticancer therapy. For example, specific agents could be tailored to inhibit their ability to secrete growth factors involved in tumoral neoangiogenesis or cytokines with immunosuppressive effects. New cytostatic drugs could be developed to act selectively on TAFs and reduce or block their high proliferation rate, leaving the tumour without its much-needed supplies for survival and growth.
The main purpose of our study was to investigate the phenotype and function of TAFs derived from certain solid tumours (breast cancers) and to establish to what extent they are similar to the ‘genuine’ MSCs derived from bone marrow.
Materials and methods
Cell isolation and culture
Normal human MSCs were obtained from the bone marrow of eight healthy orthopaedic patients undergoing hip replacement surgery. Approximately 10 ml of bone marrow were placed in culture plates, and the fibroblastic-like, plastic adherent fraction was isolated following multiple passages and was used in our experiments. The MSCs were further cultured and expanded in α-minimum essential medium (Gibco BRL, Invitrogen, Carlsbad, CA, USA), supplemented with 10% foetal calf serum (FCS; PromoCell, Heidelberg, Germany) and 2% penicillin/streptomycin mixture (Pen/Strep, 10,000 IU/ml; PromoCell), by incubation at 37°C in 5% CO2 atmosphere. Medium replacement was performed every third day and, upon reaching 80% to 90% confluence, the cells were passed using 0.25% Trypsin-EDTA (ethylenediaminetetraacetic acid) solution (Sigma, St. Louis, MO, USA) followed by centrifugation (10 min., 300 ×g) and were replated in T75 culture flasks at a density of 10,000 cells/cm2 to ensure optimal proliferation. Starting with passage 2, a part of the cells was used for further phenotypical analyses and differentiation assays, whereas MSCs expanded to passages 2 to 5 were used in the subsequent experiments.
Human TAFs were isolated using both the explant and the collagenase type IV-S prepared from Clostridium histolyticum (Sigma), methods and their phenotype was confirmed based on the markers described by Spaeth et al. [6]. Breast cancer surgical pieces of approximately 5 cm2 were obtained from eight female patients, with the histopathological diagnosis of infiltrative ductal mammary carcinoma. Skin-derived fibroblasts isolation involved enzymatic digestion with collagenase type IV-S from Clostridium histolyticum (Sigma). Tissue-isolated cells were washed several times with phosphate-buffered saline (PBS) solution, were passed through 0.70/0.40 μm strainer filters and were replated as single-cell suspension in adherent plastic culture plates.
All tissue samples were obtained after signing the informed consent elaborated under an approved protocol, according to the World Medical Association Declaration of Helsinki.
Cell lines
Fibroblast cell lines used in our study were provided by Lonza (Basel, Switzerland: normal human lung fibroblasts [NHLF]), Cascade Biologics (Invitrogen: human dermal fibroblasts adult [HDFa]) and PromoCell (human pulmonary fibroblasts [HPF-c]), and their morphological and functional characteristics were investigated in parallel with TAFs and MSCs.
All fibroblast types, including TAFs, fibroblasts cell lines and skin-isolated fibroblasts were cultured in Dulbecco’s modified Eagle medium (Sigma) supplemented with 10% FCS (PromoCell) and 2% penicillin/streptomycin solution (PromoCell), and grown at 37°C in humid atmosphere containing 5% CO2.
Osteogenic, chondrogenic and adipogenic differentiation experiments
The trilineage potential of MSCs and TAFs to differentiate into adipogenic, osteogenic and chondrogenic lineages was assessed at different passage levels, starting with passage 2 for MSCs and passage 4 for TAFs. Cells were seeded in 4-well Lab-Tek glass chamber slides (Nunc, Rochester, NY, USA) at a cellular density of 10,000 cells/cm2 in standard growth medium until they reached confluence, which were then stimulated to differentiate under appropriate medium conditions. A non-haematopoietic stem cell medium for the generation of osteoblasts, chondrocytes and adipocytes (Miltenyi Biotec, Bergisch Gladbach, Germany) was used, which was supplemented with 1% penicillin/streptomycin.
MTT assays
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)-based in vitro toxicology assay kit (Tox-1, Sigma) was used to determine proliferation rate of MSCs, TAFs and HDFa cell lines at 24 and 48 hrs. Trypan Blue cell counting and viability assessment were simultaneously performed at the same time intervals for better correlation of the results. Cells were seeded at 2000 cells/well in 96-well plates in quadruplicate, and the average value of specific medium extinction was subtracted from the samples extinction read at 570/655 nm using a benchmark PR 2100 microplate reader from Bio-Rad (Hercules, CA, USA).
ELISA
An ELISA analysis was performed with Quantikine Immunoassay kits (R&D Systems, Minneapolis, MN, USA) specific for interleukin (IL)-4, IL-10, IL-13, TGF-β1, tumour necrosis factor (TNF)-α, interferon (IFN)-γ and vascular endothelial growth factor (VEGF). Most of the TGF-β1 were secreted by the cells in a latent protein complex; however, prior to analysis, these were converted entirely to the activated form, using a dedicated reagent from the ELISA kit. This allowed for an accurate measurement of the actual (both latent and active) TGF-β1 amounts secreted by the various cell types. Supernatants of all MSCs, TAFs, skin-isolated fibroblasts and fibroblast cell line cultures at different passages were collected 24 hrs after medium replacement, filtered through 0.22 μm strainers and immediately stored at −70° C until analysed in 96-well plates on the reader. ELISA readings were normalized to the cell number, for each supernatant collected, in order to provide an accurate comparison of the amounts of cytokines secreted by the various cell types.
Flow cytometry
MSCs, TAFs, skin fibroblasts and fibroblast cell lines in culture reaching 80% to 90% confluence were detached using 0.25% Trypsin-EDTA (Sigma), washed twice with PBS, resuspended in 100 μl PBS at a concentration of 105 cells/ml, and incubated in the dark at room temperature for 30 min. with mouse anti-human fluorochrome-conjugated antibody at a dilution specified in the manufacturer’s protocol. Cells were then washed twice with 1 ml Cell Wash Solution (BD Biosciences, San Jose, CA, USA) and resuspended in 500 μl of the same solution for further analysis on a four-colour capable FACSCalibur (Becton-Dickinson, San Jose, CA, USA) flow cytometer. Conjugated antibodies utilized included PE-conjugated CD14 (BD Pharmingen™, San Jose, CA, USA), CD117 (BD Pharmingen™), α-SMA (BD Biosciences), CD29, CXCR4, Nestin, VEGF-R1 (Flt-1), VEGF-R2 (Kdr), E-Cadherin, TGF-β RII, TGF-β RIII (R&D Systems), as well as FITC-conjugated CD34, CD44, CD45, CD73, CD90, CD106, HLA-DR (BD Pharmingen™), Cytokeratin (R&D Systems) and APC-conjugated CD31 (BD Pharmingen™). Some of the markers (E-Cadherin, Cytokeratin) were located within the cytoplasm and required an additional permeabilization step, which was performed with FACS Permeabilizing Solution 2 - premixed 10X concentrate used to permeabilize cells before intracellular immunofluorescence staining with monoclonal antibodies (BD Biosciences) according to manufacturer’s instructions. Acquisition and data analyses were performed with the CellQuest software (Becton-Dickinson).
Immunohistochemical/immunofluorescence analysis
Immunohistochemistry was performed for MSCs, TAFs, skin-isolated fibroblasts, as well as NHLF, HPF-c and HDFa cell lines. Cells prepared for these analyses were grown in 4-well glass chamber slides, and 3–5 days after plating medium was removed, cells were washed, fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100, and then investigated for expression of the proteins of interest, using the following antibodies for labeling: monoclonal mouse anti-swine vimentin (clone V9), anti-human smooth muscle actin/HRP (clone 1A4), monoclonal anti-human endoglin, CD105 (clone SN6h), monoclonal mouse anti-human cytokeratin (clone MNF116); VEGF and von Willebrand Factor (vWF) presence was also tested on MSCs and TAFs using monoclonal mouse anti-human vascular endothelial growth factor (clone VG1) and monoclonal mouse anti-human von Willebrand factor (clone F8/86), respectively. All primary antibodies were provided by DakoCytomation (Glostrup, Denmark) and were tested for human specificity and cross-reactivity. Staining protocol continued with secondary biotinylated antibody binding, substrate addition and haematoxylin counterstaining of the nuclei (LSAB2 System-HRP, Dako, Carpinteria, CA, USA) following the manufacturer’s procedures.
MSCs and TAFs differentiation experiments towards adipocytes, chondrocytes and osteoblasts were assessed using anti-mFABP4, anti-hAggrecan and anti-hOsteocalcin, respectively, which are primary antibodies from the Human Mesenchymal Stem Cell Functional Identification Kit (R&D Systems), whereas the visualization system was from the LSAB2 System-HRP (Dako). For immunofluorescence, we used AlexaFluor488 and AlexaFluor546 conjugated secondary antibodies (Molecular Probes, Invitrogen) and nuclear counterstaining with 4',6-diamidino-2-phenylindole (DAPI, Sigma). Microscopy analysis was performed on a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan) equipped with adequate fluorescence filters.
RNA extraction and qRT-PCR
Total RNA extraction was conducted at different passages from cultured cells using GenElute™ Mammalian Total RNA Miniprep Kit (Sigma), and RNA concentration was measured on a Nanodrop ND-1000 (Wilmington, DE, USA) spectrophotometer. Total RNA (0.5 μg/reaction) from each sample was reverse transcribed to cDNA (SuperScript III First-Strand Synthesis System; Invitrogen). cDNA samples (10 ng template/reaction) were analysed by quantitative real-time PCR, using a LightCycler480 SYBR Green I Master system (Roche, Florence, SC, USA) and α-SMA primers. Absolute quantification was achieved using the fit points method, with an external standard curve, and α-SMA expression was reported as copy number/sample. The α-SMA primer sequences were: forward 5′-AGGGGGTGATGGTGGGAATG-3′, reverse 5′-GCCCATCAGGCAACTCGTAAC-3′.
Electron microscopy
Transmission electron microscopy (TEM) was used to compare the ultrastructural characteristics of MSCs and TAFs. For TEM analysis, cells were spinned down and immediately fixed with 4% buffered glutaraldehyde. They were then post-fixed with 1% OsO4 in 0.1 M cacodylate buffer, included in agar, ethanol dehydrated and then embedded in Epon 812 at 60°C for 48 hrs. The ultrathin sections were cut using a diamond knife and double stained with uranyl acetate and lead citrate. Ultrathin sections were examined using a Morgagni 286 TEM (FEI Company, Eindhoven, The Netherlands) at 60 kV. Digital electron micrographs were recorded with a MegaView III CCD using iTEM-SIS software (Olympus, Soft Imaging System GmbH, Germany). Parameters examined by TEM were nuclear morphology, lysosome structure and number, smooth and rough endoplasmic reticulum structure, number of mitochondria and intermediate filament content; analysis was performed on at least 10 individual MSCs and 10 individual TAFs.
Statistical analysis
Data were analysed for statistical relevance using Excel Microsoft Office 2003 software (Microsoft Corporation, Redmond, WA, USA). The central tendencies of the variables were expressed as a mean (M), and the dispersion ones as a standard deviation (S.D.). In order to perform the statistical comparisons, Student’s t-test and the ANOVA were conducted for continuous variables. Differences were considered significant for P < 0.05.
Results
Microscopy of MSCs and fibroblasts
As revealed by light microscopy, MSCs and TAFs share a similar morphology, with TAFs being usually larger and more confluent. However, several notable differences between the two cell types have been observed in TEM. Most MSCs have nuclei exhibiting discrete indentations, numerous mitochondria, reduced endoplasmic reticulum, lysosomes, packages of intermediate filaments and short and rare cytoplasmic elongations. On average, their diameter ranges from 10 to 15 μm. On the other hand, almost all TAFs have nuclei with lobulated morphology (large indentations), few mitochondria, highly developed endoplasmic reticulum with dilated cisternae and lamellar content lysosomes. Most TAFs also exhibit numerous thin cytoplasmic elongations tending to group and contain no intermediate filaments. Their average diameter ranges from 15 to 25 μm (Fig. 1).
Fig 1.
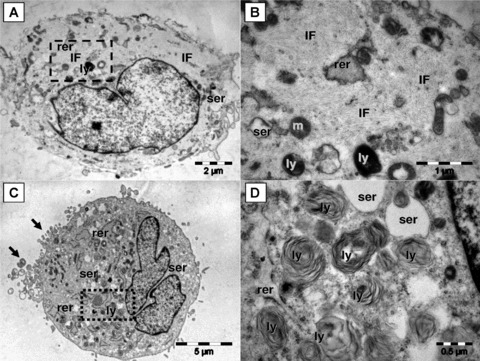
Although, by light microscopy, MSCs and TAFs share a similar morphology, they exhibit major differences in their ultrastructural features. MSCs (A and B) have the cytoplasm loaded with intermediate filaments (f), few smooth (ser) and rough (rer) endoplasmic reticulum cisternae, lysosomes (ly) and mitochondria (m). Square mark in (A) is enlarged in (B). Note in (B) that intermediate filaments are organized in bundles which generate the ‘light’ appearance of the cell. The TAFs (C and D) have abundant rough endoplasmic reticulum (rer), enlarged smooth endoplasmic reticulum (ser), numerous lysosomes (ly) and grouped filopodia (arrows). Numerous lysosomes with multilamellar structure are visible in (D) [enlarged square area from (C)].
Immunophenotype of MSCs, TAFs, HDFa and skin-isolated fibroblasts
Flow cytometry analysis has revealed many phenotypical similarities between the TAFs, MSCs, HDFa cells and the skin fibroblasts employed in our study for comparison purposes. TAFs express all the cell surface markers generally used to characterize the MSCs, such as CD44, CD90, CD73, CD29 and so forth, while being essentially negative for CD34 and CD45 expression (Fig. 2).
Fig 2.
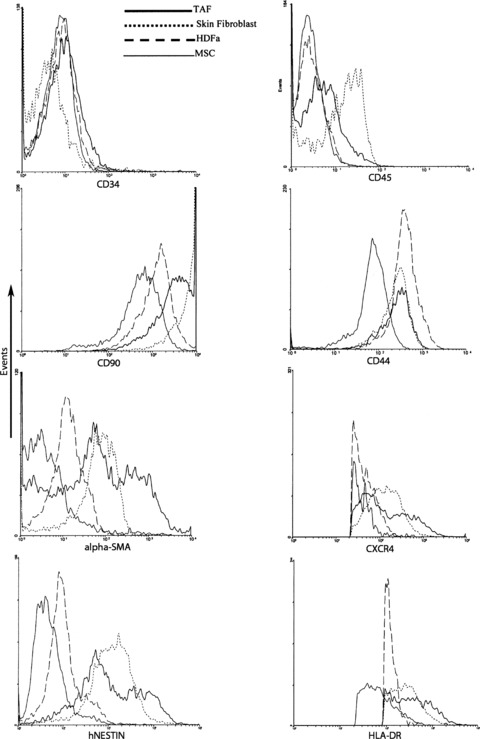
Flow cytometry analysis of cell surface markers expression on TAFs, MSCs, HDFa and skin fibroblasts. TAFs are negative for CD34 and CD45 expression but positive for all the other cell surface markers generally used to characterize MSCs. For flow cytometry cells were trypsinized and stained with specific fluorochrome-labelled monoclonal antibodies.
Although MSCs are essentially defined by their lack of HLA-DR, we have found low levels of expression on both MSC and TAFs at all analysed passages. This is suggestive for their activated status [12–14].
No cytokeratin and E-cadherin expression were detected by either flow-cytometry or immunohistochemistry (IHC).
As revealed by IHC, both TAFs and MSCs strongly expressed vimentin (Fig. 3), with cytoplasmic and perinuclear localization. Vimentin is a defining cytoskeletal protein found in connective tissues and all primitive cell types express vimentin; however, in most non-mesenchymal cells, it is replaced by other intermediate filament proteins during differentiation [15, 16]. It is interesting that vimentin expression does not decrease during the more advanced passages of TAFs (data not shown), suggesting that they retain their ‘primitive’ MSC-like status.
Fig 3.
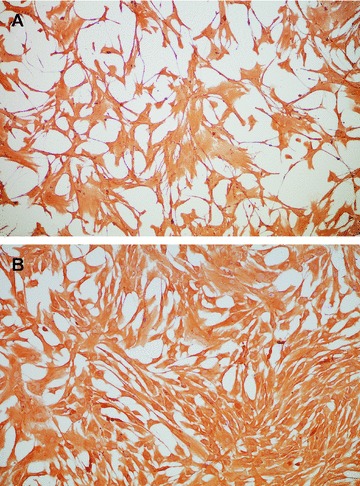
Vimentin expression in TAFs (A) and MSCs (B), as revealed by immunohistochemistry with a specific antibody (clone V9). Vimentin is a generic marker used to define fibroblast cells and TAFs exhibit no visible difference in the expression of this marker compared to MSCs. Magnification, 200x.
Regarding the proliferation rate of MSCs, TAFs and HDFa, the MTT assay showed that the proliferation rate of the MSCs was significantly lower than that of TAFs and HDFa (P < 0.001), and the results were correlated with cell count and viability testing using Trypan Blue vital staining, which was performed in parallel (Fig. 4).
Fig 4.
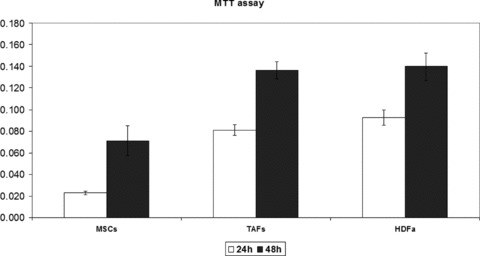
The proliferation rates of TAFs, HDFa cells and MSCs have been quantified using a standard colorimetric MTT assay that measures cellular metabolic activity. TAFs and HDFa cells were grown in culture in 96-well plates for 24 and 48 hrs and were incubated for 3 hrs with the MTT reagent at the end of the specified culture period. Both cell types show a similar proliferation rate at 24 and 48 hrs upon plating, which is higher compared to the proliferation rate of the MSCs, and in good correlation with the Trypan Blue cell viability and proliferation readings.
Plasticity of TAFs compared to MSCs
Adipogenesis was markedly induced in MSCs after exposure to specific culture medium (50% of cells presented lipid drops within the cytoplasm); differentiation of TAFs towards the adipocytic lineage was weaker (30%), although TAFs presented an earlier phase differentiation, with few adipocytes appearing in the culture after 1 week of exposure to differentiation medium. Visualization of TAFs-differentiated adipocytes was performed with primary mouse anti-human FABP4 antibody coupled with AlexaFlour 488 (Fig. 5C). Upon induction of both MSCs and TAFs to differentiate towards osteoblasts and chondrocytes, no differences between the differentiation patterns were observed, as both cell types showed the expression of osteocalcin and aggrecan when immunostained with flourochrome-conjugated primary specific antibody (Fig. 5A and B). We did not perform the differentiation experiments with the other types of fibroblasts (HDFa and skin fibroblasts), their pluripotent abilities being well documented in the literature [17–19].
Fig 5.
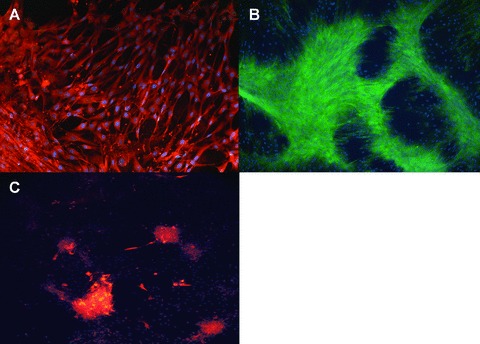
The trilineage differentiation potential of TAFs was assessed by immunofluorescence staining for markers specific for osteoblasts (A– osteocalcin, green fluorescence), chondroblasts (B– aggrecan, red fluorescence) and adipocytes (C– FABP4, red fluorescence). Nuclear counterstaining was performed in all cases with DAPI (blue fluorescence). Both TAFs and MSCs (photos not shown) followed a similar differentiation pattern towards osteoblasts and chondrocytes, whereas differentiation to the adipocytic lineage was 30% less efficient for TAFs compared to MSCs. Magnification, 200x.
TAFs secrete growth factors and cytokines that support tumour cells proliferation
High levels of VEGF were detected in TAFs and HDFa cell culture supernatants whereas almost undetectable levels of VEGF were seen in MSCs. Paired Student’s t-test was used to determine statistical significance of increased VEGF production by TAFs, compared to MSCs (P < 0.001) (Fig. 6).
Fig 6.
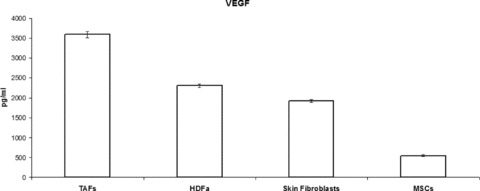
VEGF production by TAFs, as revealed by ELISA on cell culture supernatants, is almost double compared to that of the other fibroblasts studied, and is 5-fold higher compared to that of MSCs.
MSCs secreted much lower amounts of cytokines with direct immunosuppressive effects, such as IL-10, when compared to TAFs (P < 0.001), with their secretion level being similar to HDFa and skin-isolated fibroblasts (Fig. 7). The immunoinhibitory effects of MSCs are well described, and it is not surprising that these become even more potent upon activation and transition to TAFs, in response to signals received within the tumour microenvironment. On the other hand, IL-13 production seems to be similar among the various fibroblasts and insignificantly higher compared to that of MSCs.
Fig 7.
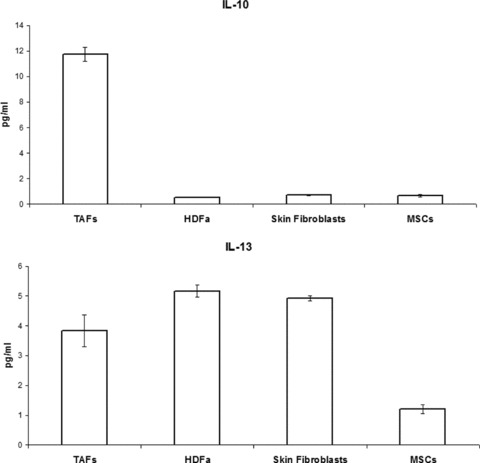
Immunosuppressive cytokine secretion by MSCs and fibroblasts, as quantified by ELISA. TAFs produce, on average, 10-fold higher amounts of IL-10 compared to MSCs and the other types of fibroblasts. IL-13 secretion is similar among the different cell types.
IL-4 and TNF-α secretion by TAFs is significantly higher than MSC production of both cytokines (P < 0.001), and it is similar to that of the other fibroblasts (Fig. 8). As expected, very low and similar levels of IFN-γ were detected for all types of fibroblasts and MSCs (data not shown).
Fig 8.
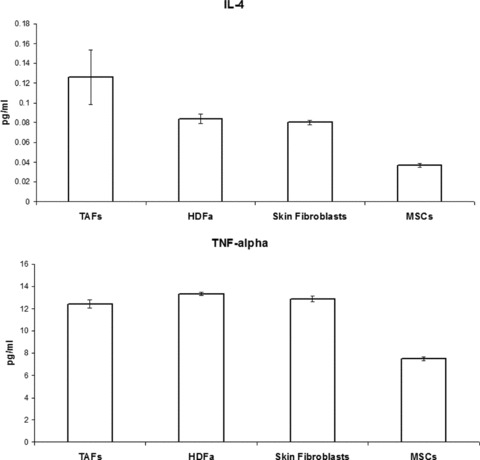
IL-4 and TNF-α secretion by MSCs and fibroblasts measured by ELISA on cell culture supernatants at equivalent passages. TAF production of both cytokines is similar to that of the other fibroblasts and higher compared to MSCs, although the levels of IL-4 secretion were insignificantly low in all cases.
Another cytokine potently secreted by TAFs is TGF-β1, with its quantified level being almost twice as high compared to that of MSCs and HDFa cells (Fig. 9). As our ELISA procedure involves a step that converts all the latent TGF-β1 to the active form that will actually be detected, it is not possible to estimate the amount of the active form, if any, produced ab initio by the cells.
Fig 9.
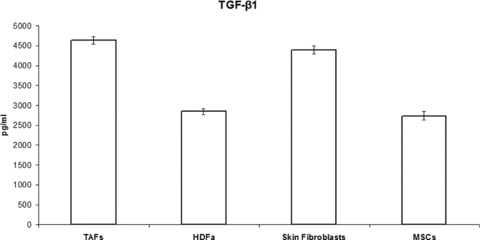
TAFs’ production of TGF-β1 is almost double compared to that of MSCs and HDFa cells, and is similar to the production of skin fibroblasts. Only the activated form of TGF-β1 was measured in our ELISA, and all the latent TGF-β1 from the supernatants was converted to the activated form prior to quantitation.
TAFs activation-related proteins
The α-SMA expression in fibroblasts is variable and generally associated with an activated or ‘aggressive’ phenotype. qRT-PCR analysis has revealed, on average, a 10-fold higher expression of α-SMA in the breast tumour-derived TAFs, compared to the MSCs, suggesting the activated status of these fibroblasts (Fig. 10). This is especially true for the early TAF passages; however, α-SMA expression seems to decrease significantly to the basic level found in MSCs, during the more advanced passages, perhaps in association with the loss of aggressive phenotype and/or the aging process.
Fig 10.
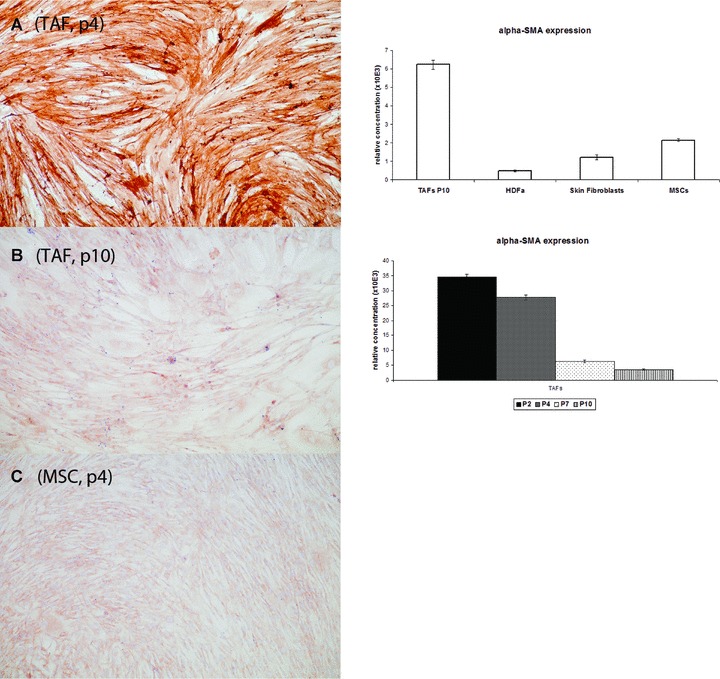
The α-smooth muscle actin (α-SMA) is highly expressed in TAFs at early passages, as revealed by immunohistochemistry (A) on cells grown in 4-well chamber slides and stained with a specific anti-human SMA antibody (clone 1A4), and by qRT-PCR with cDNA samples obtained from total RNA extracted from cells (right panel, bottom graph). However, α-SMA expression decreases significantly in the older passages (B and right panel, bottom graph). α-SMA expression is also much stronger in TAFs at early passages compared to the MSCs (C) and the other fibroblasts (qRT-PCR; right panel, upper graph).
Discussion
The fibroblasts, fibrovascular networks and soluble factors that comprise the tumour stroma, defining the tumoral microenvironment, are critical ingredients of a ‘healthy’ tumour, which is capable of long-term survival, proliferation and evolution. Although many cancer cell types have been thoroughly characterized down to the genetic level, much less has been known about the specific TAFs. In our study, we describe a phenotypic and functional analysis of these fibroblasts, in comparison with other types of fibroblasts and bone marrow- derived MSCs, in order to get a better understanding of the origin and biology of TAFs. TAFs and MSCs share important similarities, suggesting the genealogical relationship between these two types of cells. However, there are some significant differences, mainly functional (i.e. soluble factors production, proliferation rate), that are most likely the result of a specialization process which the converted MSCs go through inside the tumour structure, so they can better ‘serve’ the cancer cell.
VEGF is a well-known proangiogenetic molecule and its high-level production by TAFs is not surprising. In this way, TAFs could not only provide a structural support for the tumour organism but also ensure that the cancer cells are supplied with nutrients and oxygen via neoangiogenesis. Blood vessel neoformation is critical for the long-term survival of many tumours, and VEGF synthesized in large quantities by TAFs may be required for this process. A long-term survival will give the ability of the cancer cells to expand and evolve, with the selection of more aggressive clones that eventually will be able to leave the mother tumour and colonize other organs (metastasis). The absence of VEGF receptors on the cell surface of TAFs (as revealed by us by IHC staining), while VEGF secretion is very high, may suggest that the TAFs are not the cells that transform into vessel-generating structures but some other cells in the tumour stroma which may be involved in tumour neovascularization. TAFs do exhibit continuous moderate expression of α-SMA, which is a protein associated with neovascularization, but no induction method used by us was able to trigger the differentiation of these cells into endothelial-like or muscle-like cells.
Soluble factors secreted by TAFs, such as IL-10 and IL-13 are capable of inhibiting synthesis of pro-inflammatory cytokines (i.e. IFN-γ, IL-2, IL-3, TNF-α and granulocyte macrophage-colony stimulating factor) produced by cells such as macrophages and the type 1 T-helper cells infiltrating the tumour. IL-10 also displays potent abilities to suppress the antigen presentation capacity of antigen-presenting cells.
TGF-β1 is a multifunctional peptide that controls proliferation, differentiation and other functions in many tissues. TGF-β1 inhibits proliferation and can induce apoptosis in various cell types, but on the other hand, most human tumours produce this cytokine whose autocrine and paracrine actions promote cancer cell invasiveness and metastasis, epithelial to mesenchymal transition and controls intratumoral angiogenesis [20]. Enhanced TGF-β1 production by TAFs may be a critical factor in tumour homeostasis, not so much for the cancer cells directly, but for the TAFs themselves, via autocrine mechanisms, allowing them to proliferate and preserve their activated fibroblast behaviour and supportive activity for the tumour. Moreover, TGF-β1 secreted by TAFs could suppress activation, proliferation and differentiation of the cytotoxic T-lymphocytes, NK cells and macrophages recruited within the tumour, impairing immune surveillance and antitumoral immunity. Further studies are needed to clarify these critical issues, as well as to address the intratumoral conversion process of the latent TGF-β complex to the active form, which is capable of binding the cell surface receptors and triggering intracellular signals. TGF-β1 activation mechanisms are complex and only partially understood, and involve a variety of proteases, integrins, reactive oxygen species, and so forth, which act in a tissue-specific or non-specific manner. Some of these activation pathways might be differentially operational in certain tumours, may be controlled by the stromal fibroblasts, and could provide attractive targets for antitumoral therapy.
The function of the peculiar lysosomal structures identified by electron microscopy in TAFs is not understood; however, TAFs might be involved in the capture and sequestration of tumoral-derived antigens, thus becoming unavailable to the immune cells recruited in the antitumoral defence. It is apparent that TAFs, like the genuine MSCs, may have potent immunosuppressive and, in this case, pro-tumoral effects, exerted via immunoinhibitory cytokines secretion and by other mechanisms (tumour-derived antigens ‘depletion’).
Although some of the original depictions of the MSC phenotype have concluded that these cells are negative for the expression of MHC class II molecules (HLA-DR), more recent investigations have proved that, upon activation, MSCs can actually express low levels of both class I and class II molecules [21–23]. MHC class I molecules are ubiquitously expressed on many different cell types, whereas class II molecules have a much more restricted expression on lymphocytes and other cells directly involved in specific immune phenomena, such as antigen presentation (macrophages and dendritic cells, etc.) The mechanisms and the functional implications of this inducible expression are not fully understood. However, it is tempting to speculate that this expression might play some roles in the immunosuppressive effects of the MSCs. It is not surprising to find a similar level of HLA-DR expression on TAFs. It would be interesting to know what the ligand(s) for HLA-DR would be in this case, and if TAFs are capable of direct interaction with the tumour infiltrating lymphocytes (TILs) via HLA-DR; the lack of expressed antigen fragment presented in the context of HLA-DR, like in the case of the specialized antigen-presenting cells, could provide TILs an inhibitory signal upon HLA-DR binding, contributing an additional level of immunosuppression and pro-tumoral effect.
Overall, the similarities between TAFs and MSCs seem to prevail over the differences, reinforcing once again the concept that the tumoral fibroblasts are, in fact, MSCs bearing an activated status. The only difference, nevertheless significant, seems to be the amount of certain secreted cytokines (see also Table 1), reflecting the particular needs of the tumoral cells. We only investigated a limited number of soluble factors produced by the fibroblasts and the MSCs but it is very likely that TAFs secrete a myriad of other proteins that are equally important for the tumour growth and expansion beyond its original borders (metastasis). Moreover, our study was focused on the in vitro biology of TAFs, where there is no homeostatic ‘environmental’ pressure exerted by the organism as a whole; the in vivo differences between MSCs and TAFs, at least in terms of cytokines and other bioactive proteins secretion, could be even more extensive.
Table 1.
Synopsis of MSCs and TAFs similarities and differences
| MSCs | TAFs | |
|---|---|---|
| Pluripotency (trilineage differentiation potential) | Yes | Yes |
| Cell surface molecules | ||
| CD14 | − | – |
| CD29 | + | + |
| CD31 | − | – |
| CD34 | − | – |
| CD44 | + | + |
| CD45 | − | – |
| CD73 | + | + |
| CD90 | + | + |
| CD106 | + | + |
| CD117 | + | + |
| HLA-DR | − | – |
| CXCR4 | − | – |
| VEGF-R1 (Flt-1) | − | – |
| VEGF-R2 (Kdr) | − | – |
| TGF-β RII | − | – |
| TGF-β RII | − | – |
| Cytoskeleton and extracellular matrix proteins | ||
| Vimentin | Yes | Yes |
| α-SMA | Yes | Yes |
| Nestin | Yes | Yes |
| Cytokeratin | No | No |
| E-cadherin | No | No |
| Ultrastructural details | ||
| Cytoplasmic elongations | No | Yes |
| Lamellar content lysosomes | No | Yes |
| Intermediate filaments | Yes | No |
| Cytokines, chemokines and growth factors secretion | ||
| IL-4 | Low | High |
| IL-10 | Low | High |
| IL-13 | Low | High |
| TGF-β1 | Low | High |
| TNF-α | Low | High |
| VEGF | Low | High |
+: expression; −: lack of expression.
Although there are no known specific cell surface or cytoplasmic markers to be expressed by TAFs, therapeutic targeting of these cells should be considered and any future anticancer therapy should take into account the elimination not only of the cancer cells per se but also of the TAFs. It is even likely that targeting only the TAFs in combination with certain immunomodulatory therapies (like anti-IL-10 or anti-IL-13 antibodies), would be sufficient for a long-lasting remission and even cure of those cancers where TAFs have been positively identified and described as a significant component of the tumour anatomy. A tempting target could be the VEGF secretion by TAFs. Anti-angiogenic substances are a current research trend in cancer therapy, and many clinical trials with such agents have been performed, however, with questionable results. Considering that only few and generally minor differences exist between TAFs and fibroblasts from healthy tissues, any efficient therapeutic approach should be focused locally, rather than systemically, and several antitumoral agents should be used in combination with adjuvant therapies for increased efficiency.
Acknowledgments
This work was supported by grants from the Romanian Ministry of Education and Research (grants PNCDII 41-019/2007 and CNCSIS 198/2007) and through the European Union FP6 programme (Integrated Project GENOSTEM no. LSH503161).
References
- 1.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Stagg J. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008;4:119–24. doi: 10.1007/s12015-008-9030-4. [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA, Kohn EC. The microenvironment of the tumor-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 4.Pupa SM, Menard S, Forti S, et al. New insights into the role of extracellular matrix during tumor onset and progression. J Cell Physiol. 2002;192:259–67. doi: 10.1002/jcp.10142. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–49. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Ross SR, Acena M, et al. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992;175:139–46. doi: 10.1084/jem.175.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan NJ, Hall BM. Mesenchymal stem cells in tumor stroma. In: Bagley RG, Teicher BA, editors. Stem cells and cancer. Berlin: Springer; 2009. pp. 29–38. [Google Scholar]
- 9.Haniffa MA, Wang XN, Holtick U, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 10.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 11.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 12.Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 13.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interaction: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–90. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 14.Poggi A, Prevosto C, Massaro AM, et al. Interaction between NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–60. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 15.Goldman RD, Khuon S, Chou Y, et al. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134:971–83. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsumoto T, Mitsushima A, Kurimura T. The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer-graphic reconstruction. Biol Cell. 1990;68:139–46. doi: 10.1016/0248-4900(90)90299-i. [DOI] [PubMed] [Google Scholar]
- 17.Lysy PA, Smets F, Sibille C, et al. Human skin fibroblasts: from mesodermal to hepatocyte-like differentiation. Hepatology. 2007;46:1574–85. doi: 10.1002/hep.21839. [DOI] [PubMed] [Google Scholar]
- 18.Hee CK, Nicoll SB. Induction of osteoblasts differentiation markers in human dermal fibroblasts: potential application to bone tissue engineering. Conf Proc IEEE Eng Med Biol Soc. 2006;1:521–4. doi: 10.1109/IEMBS.2006.259308. [DOI] [PubMed] [Google Scholar]
- 19.Sabatini F, Petecchia L, Tavian M, et al. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005;85:962–71. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 20.Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogenic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 22.Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–34. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–96. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]


