Abstract
The steroid hormone aldosterone regulates sodium and potassium homeostasis. Aldosterone and activation of the mineralocorticoid receptor also causes inflammation and fibrosis of the heart, fibrosis and remodelling of blood vessels and tubulointerstitial fibrosis and glomerular injury in the kidney. Aldosterone and mineralocorticoid-receptor activation initiate an inflammatory response by increasing the generation of reactive oxygen species by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mitochondria. High salt intake potentiates these effects, in part by activating the Rho family member Rac1, a regulatory subunit of reduced NADPH oxidase that activates the mineralocorticoid receptor. Studies in mice in which the mineralocorticoid receptor has been deleted from specific cell types suggest a key role for macrophages in promoting inflammation and fibrosis. Aldosterone can exert mineralocorticoid-receptor-independent effects via the angiotensin II receptor and via G-protein-coupled receptor 30. Mineralocorticoid-receptor antagonists are associated with decreased mortality in patients with heart disease and show promise in patients with kidney injury, but can elevate serum potassium concentration. Studies in rodents genetically deficient in aldosterone synthase or treated with a pharmacological aldosterone-synthase inhibitor are providing insight into the relative contribution of aldosterone compared with the contribution of mineralocorticoid-receptor activation in inflammation, fibrosis, and injury. Aldosterone-synthase inhibitors are under development in humans.
Introduction
During volume depletion or hypoperfusion of the kidney, activation of the renin–angiotensin–aldosterone system leads to vasoconstriction and volume expansion. Aldosterone stimulates sodium reabsorption in the kidney via the sodium–chloride cotransporter (NCC) in the distal convoluted tubule and the epithelial sodium channel (ENaC) in the late distal convoluted tubule, the connecting tubule, and the collecting duct. In the principal cells of the collecting duct, aldosterone, acting at the mineralocorticoid receptor (MR), increases mRNA levels of serum/glucocorticoid-regulated kinase (SGK1).1 SGK1 phosphorylates the ubiquitin-protein ligase neuronal precursor cell expressed developmentally down-regulated protein 4-2 (Nedd4-2), and prevents ubiquitylation and degradation of ENaC.2 Aldosterone also induces the expression of glucocorticoid-induced leucine zipper (GILZ), which inhibits mitogen-activated protein-kinase (MAPK) regulation of ENaC.3 In the distal convoluted tubule, SGK1 phosphorylates Nedd4-2 and WNK4 and attenuates their inhibitory effects on the NCC.4,5 The net effect of aldosterone in the tubule is sodium retention and potassium excretion.
Over the past 20 years, investigators have come to appreciate that aldosterone exerts direct effects on the vasculature, heart and kidney beyond its effects on electrolyte handling in the distal tubule. MRs are expressed in non-epithelial cells such as those of the heart (cardiomyocytes6), vasculature (endothelial cells and vascular smooth muscle cells [VSMCs])7, and kidney (mesangial cells8 and podocytes9), adipocytes,10 and monocytes.11 Seminal studies by the groups of Weber, Hostetter, Safar and many others demonstrated that chronic administration of aldosterone in the setting of high salt intake causes both interstitial and perivascular fibrosis in the heart,12 fibrosis of the aorta,13 and glomerulosclerosis and interstitial fibrosis in the kidney.14 Prior to the development of fibrosis, aldosterone causes monocyte and macrophage infiltration and increased expression of inflammatory markers such as cyclooxygenase-2, monocyte chemoattractant protein 1, and intercellular adhesion molecule 1 (ICAM1) in the heart, vasculature, and kidney.15,16 In the heart, perivascular inflammation is followed by the proliferation of fibroblasts and myofibroblasts, collagen production, perivascular fibrosis, and lastly, interstitial fibrosis.17 The proinflammatory and profibrotic effects of aldosterone are prevented by MR antagonism in most models.13,16,18
Studies in humans confirm that MR activation contributes to cardiovascular fibrosis and remodelling as well as to renal disease. In the Randomized Aldactone Evaluation Study (RALES), spironolactone reduced mortality in heart failure patients who were already being treated with standard therapy including an angiotensin-converting-enzyme (ACE) inhibitor.19 The beneficial effect of spironolactone was associated with a reduction in circulating biomarkers of extracellular matrix turnover, such as procollagen type III N-terminal peptide.20 In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), eplerenone treatment reduced mortality in patients with left ventricular dysfunction following myocardial infarction.21 Eplerenone reduces the combined end point of death and hospitalization in patients with systolic dysfunction and mild symptoms.22 Several small clinical trials have shown a beneficial effect of MR antagonism on proteinuric renal disease in patients already treated with an ACE inhibitor or angiotensin-receptor blocker;23 however, no large outcomes trials have been conducted, in part because of concerns regarding the risk of hyperkalaemia during dual renin–angiotensin–aldosterone system blockade in patients with renal insufficiency.23
This Review discusses the proinflammatory and profibrotic effects of aldosterone and MR activation in the heart, vasculature and kidney. It focuses on recent studies attempting to address the following questions: how is the MR activated when endogenous aldosterone is suppressed, such as during high salt intake? Is activation of the MR in specific cell types required to induce inflammation and fibrosis in the heart, the vasculature, or the kidney? And does aldosterone promote inflammation and/or fibrosis through MR-independent mechanisms? In many cases the answers to these questions are not yet definitive, but the available evidence is discussed.
Aldosterone and MR activation increase ROS
Aldosterone and/or MR activation promote inflammation by stimulating the generation of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide, which activate the proinflammatory transcription factors activator protein (AP)-1 and nuclear factor kappa B (NFκB) (Figure 1).24 In the heart, the aldosterone-induced generation of ROS also activates Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII),25 which contributes to left ventricular remodelling following myocardial infarction.
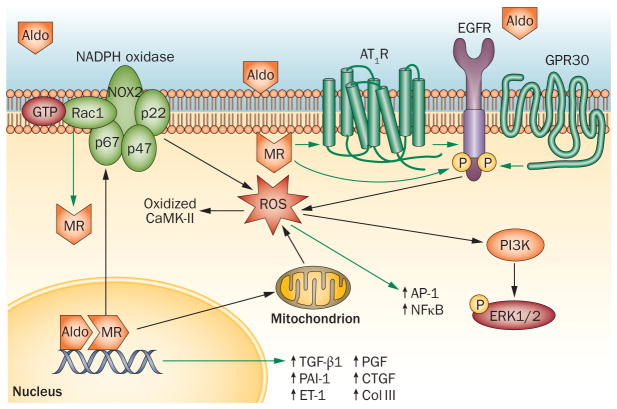
Figure 1.
Mechanisms by which aldosterone and/or MR activation induce oxidative stress, inflammation, and fibrosis. Activation of the MR by aldosterone results in dissociation of chaperone proteins, translocation of the MR to the nucleus and activation of transcription. MR activation induces oxidative stress by both NADPH oxidase and mitochondria. ROS formed by NADPH oxidase oxidize CaMK-II. Oxidative stress triggers the activation of proinflammatory transcription factors such as AP-1 and NFκB. Rac1 can also activate the mineralocorticoid receptor. Aldosterone can induce rapid, nongenomic effects via GPR30. Aldosterone and angiotensin II interact to cause rapid, nongenomic effects via transactivation of the EGFR, resulting in the generation of ROS and phosphorylation of ERK1/2. The green arrows indicate an activating or increasing effect; the black arrows indicate a downstream consequence. See text for references. Abbreviations: Aldo, aldosterone; AP-1, activator protein 1; AT1R, angiotensin II type 1 receptor; CAMK-II, calcium/calmodulin-dependent protein kinase type II subunit gamma; Coll III, collagen III; CTGF, connective tissue growth factor; EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-regulated kinases 1 and 2 (ERK)1/2; ET-1, endothelin 1; GPR30, G-protein-coupled receptor 30; MR, mineralocorticoid receptor; NADPH, nicotinamide adenine dinucleotide phosphate; NFκB, nuclear factor kappa B; P, phosphate; PAI-1, plasminogen activator inhibitor 1; PGF, placental growth factor; PI3K, phosphatidylinositol 3-kinase; ROS, reactive oxygen species; TGF-β1, transforming growth factor-β1.
Aldosterone stimulates the formation of ROS through several mechanisms (Figure 1). Aldosterone increases nicotinamide adenine dinucleotide phosphate [NADP(H)] oxidase activity and oxidative stress in macrophages,26 endothelial cells,27 the heart,28 aorta,26,29 podocytes,9 and mesangial cells.30 MR activation also contributes to angiotensin-II-mediated activation of NADPH oxidase in the heart and aorta.31–33 In the aorta, aldosterone stimulates expression of NOX2 (gp91phox) and p22phox through an MR-dependent mechanism and expression of p47phox through both angiotensin II type 1 (AT1)-receptor-dependent and MR-dependent mechanisms.29 Conversely, p47phox-deficient mice are protected against aldosterone-induced ROS production in the heart, as measured by dihydroethidium fluorescence following myocardial infarction and infusion of aldosterone by the osmotic minipump.25 Aldosterone-stimulated activation of NFκB in the heart is prevented in NOX2-deficient mice.32 In rodent models, antioxidants such as the super-oxide mimetic tempol, the NADPH oxidase inhibitor apocynin, and N-acetylcysteine have been shown to decrease inflammation and injury.29,34–37
In addition, aldosterone decreases the vascular expression of glucose-6-phosphate dehydrogenase, which reduces NADP+ to NADPH.38 The generation of ROS by aldosterone further leads to endothelial dysfunction via the formation of peroxynitrite and the oxidation of 5,6,7,8-tetrahydrobiopterin, an important co-factor for nitric oxide synthase (NOS).39 Aldosterone causes dephosphorylation of Ser1177 of protein phosphatase 2A leading to an ‘uncoupling’ of NOS.39 Aldosterone can contribute further to endothelial dysfunction by inducing swelling and stiffness of endothelial cells through the MR and activation of ENaC.40,41 Increased ambient sodium exacerbates this effect.
Aldosterone also stimulates mitochondrial production of ROS (Figure 1), an effect that has been studied most extensively in renal cells. For example, aldosterone induces epithelial–mesenchymal transformation (EMT) of cultured human proximal tubular cells via MR-dependent phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK)1/2 and phosphorylation of p66Shc, an effector of mitochondrial dysfunction.42 The mitochondrial respiratory chain complex I inhibitor, rotenone, but not the NADPH oxidase inhibitor apocynin, blocks EMT in vitro and in vivo in mice treated with deoxycorticosterone (DOCA) and salt (an animal model of steroid-induced, salt-sensitive hypertension).43 Overexpression of genes encoding regulators of mitochondrial function such as peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α) or silent mating type information regulation 2 homolog 1 (SIRT1) prevents aldosterone- stimulated EMT.42 Similarly, aldosterone-induced proliferation of mesangial cells is inhibited to a greater extent by the mitochondrial respiratory chain inhibitor rotenone than by apocynin.44 Rotenone also reverses aldosterone-induced podocyte injury.45 Rotenone and other inhibitors of mitochondrial ROS generation reduce blood pressure more effectively in DOCA–salt-treated mice than does genetic deletion of NOX2 or p47phox.46 The results of these studies suggest that mitochondrial dysfunction contributes to the aldosterone-dependent or MR-dependent formation of ROS to a greater extent than was previously appreciated.
Aldosterone can also stimulate NFκB activity and the inflammatory pathway directly by activating the MR and increasing the phosphorylation and activity of SGK1 in mesangial cells47 and the cortical collecting duct.48 Genetic deficiency of Sgk1 in mice protects against angiotensin-II-induced macrophage infiltration and cardiac fibrosis,49 as well as albuminuria and renal tubulointerstitial fibrosis.50
Inflammation begets fibrosis
Fibrosis occurs when collagen and matrix production exceeds their degradation by matrix metalloproteinases (MMPs). As noted earlier, aldosterone-induced mitochondrial dysfunction leads to EMT of proximal tubule cells. Aldosterone stimulates the expression of profibrotic molecules, such as transforming growth factor-β1 (TGF-β1), plasminogen activator inhibitor 1 (PAI-1), endothelin 1 (ET-1), placental growth factor (PGF), connective tissue growth factor (CTGF), osteopontin, and galectin-3.
TGF-β1 promotes fibrosis by stimulating cellular transformation of many cells to fibroblasts, by increasing the synthesis of matrix proteins and integrins, and by decreasing the production of MMPs.51 Aldosterone increases the expression of TGF-β1 in cultured cardiomyocytes and treatment with aldosterone plus salt increases cardiac TGF-β1 in the heart in most studies.52 Aldosterone induces TGF-β1 expression via the ERK1/2 pathway in mesangial cells.53 Treatment with aldosterone plus salt increases the renal expression of TGF-β1 in hypertensive rat models and increases the urinary excretion of TGF-β1 in normal rats through an MR-dependent post-transcriptional effect.54
Aldosterone increases expression of PAI-1 in cultured endothelial cells, VSMCs, cardiomyocytes and monocytes.11,55,56 In rat mesangial cells and renal fibroblasts, TGF-β1 and aldosterone act synergistically to increase PAI-1 expression and decrease extracellular degradation.57 PAI-1, a member of the serpin (serine protease inhibitor) superfamily inhibits the activation of plasminogen to plasmin by tissue plasminogen activator. PAI-1 promotes fibrosis and remodelling by preventing plasmin-mediated MMP activation and extra-cellular matrix degradation, but PAI-1 can also exert anti-fibrotic effects by retarding cellular infiltration and impeding urokinase-type plasminogen activator (uPA) or plasmin- mediated activation and the release of latent growth factors.58 PAI-1-deficient mice are protected against aldosterone-induced glomerular injury.59 Aldosterone induces PAI-1 expression in the heart; however, PAI-1 deficiency in the heart enhances aldosterone-induced or angiotensin-II-induced fibrosis.59–61 This finding is consistent with results of studies in senescent mice which have suggested that, in the heart, PAI-1 deficiency results in increased TGF-β activity62 and increased infiltration of uPA-dependent macrophages.63
Increased expression of ET-1 can contribute to cardiovascular fibrosis and hypertrophy stimulated by aldosterone and salt. ET-1 increases collagen synthesis by cardiac fibroblasts, in part via a TGF-β1-dependent mechanism.64 Mineralocorticoid plus salt treatment increases ET-1 expression in the heart and vasculature, whereas treatment with an endothelin-A-receptor blocker prevents aldosterone-induced cardiac and vascular fibrosis.65,66 Endothelin-A-receptor blockade decreases renal injury in spontaneously hypertensive rats.67 In the medullary collecting duct cells of the kidney, aldosterone modulates binding to a steroid-responsive element in the Edn1 gene, which encodes ET-1.68
Jaffe et al. have implicated PGF, a member of the vascular endothelial growth factor (VEGF) family that promotes vascular cell proliferation, in aldosterone-mediated vascular injury.69 Specifically, these investigators demonstrated that aldosterone simulates PGF expression in mouse aortas and atherosclerotic human vessels via an MR response element and found that PGF-deficient mice are protected against aldosterone-induced extracellular matrix deposition in injured carotid arteries.
Osteopontin is a negatively charged extracellular matrix protein that promotes adhesion of inflammatory cells.70 In VSMCs, aldosterone stimulates osteopontin expression through an MR-dependent mechanism involving ERK and p38 MAPK.71 Osteopontin-deficient mice are protected against aldosterone-induced fibrosis but they develop left ventricular dilatation.72 Osteopontin-deficient mice are protected against oxidative stress, macrophage and fibroblast infiltration in the kidney as well as EMT, interstitial fibrosis, and podocyte injury.73
Galectin-3 is a lectin that causes collagen production in cultured VSMCs, cardiac fibroblast proliferation, fibrosis and left ventricular dysfunction.74,75 Aldosterone increases the expression of galectin-3 in the heart.76 Calvier et al. have reported that galectin-3-deficient mice are protected against aldosterone-induced aortic inflammation and fibrosis.75
Nitric oxide and the generation of cyclic GMP moderate many of the profibrotic effects of aldosterone. For example, aldosterone increases PGF expression and protein secretion in mouse aorta only after the endothelium has been denuded.69 Administration of a NOS inhibitor exacerbates activation of the NFκB pathway, proteinuria and renal injury in DOCA–salt-treated rats.77 Mice that lack the guanylyl cyclase-A receptor for natriuretic peptides and are treated with aldosterone and salt develop increased oxidative stress, increased proteinuria, mesangial expansion, and segmental sclerosis, compared with wild-type mice.78
Based on the findings from these studies, it is fair to say that aldosterone increases the expression of a number of profibrotic molecules and that the profile of these molecules is not unique to aldosterone-induced fibrosis.
Nongenomic and MR-independent effects
The classical effect of aldosterone on sodium transport in the renal tubule involves activation of the MR, which results in its dissociation from molecular chaperones, its translocation into the nucleus, and its binding to hormone response elements in the regulatory region of target gene promoters to enhance gene expression.79 Aldosterone can also exert rapid nongenomic effects that are not blocked by inhibitors of transcription. These rapid, nongenomic effects of aldosterone have been most extensively described in vascular cells and tissues, where aldosterone causes a rapid increase in intra-cellular calcium through phosphatidylinositol 3-kinase (PI3 kinase), diacylglycerol and protein kinase C (PKC).80 Aldosterone rapidly activates ERK1/2 in VSMCs, to promote a mitogenic and profibrotic phenotype.71,81 Aldosterone enhances both rapid (10–15 min) and delayed (2 h) activation of ERK1/2 by angiotensin II. Potentiation of the early activation of ERK1/2 involves the transactivation of the epidermal growth factor receptor (EGFR), whereas the late phase involves increased expression of the fibrotic and proliferative small and monomeric GTP-binding protein Ki-ras2A and MAPK.81 Angiotensin-receptor blockade prevents the rapid phosphorylation of ERK1/2 in VSMCs, but MR inhibition does not.81,82 By contrast, spironolactone and inhibitors of transcription and protein synthesis prevent the late effect of aldosterone and angiotensin II.82
These findings highlight the contribution of the EGFR to the cardiovascular effects of aldosterone and to the crosstalk between aldosterone and angiotensin II in particular. In human aortic smooth muscle cells, aldosterone induces EGFR expression via an interaction between the ligand-bound MR and regions 316–163 bp and 163–1 bp of the EGFR promoter.83 Interestingly, studies in mice with genetically decreased EGFR activity suggest that EGFR contributes to angiotensin-II-induced cardiac hypertrophy and aldosterone-induced vascular dysfunction, but not to aldosterone-salt-induced vascular or cardiac fibrosis and remodelling.84,85 Systemic aldosterone administration induces phosphorylation of EGFR and ERK in the kidney within 30 min.86 In cardiomyocytes, aldosterone phosphorylates and enhances activity of the Na+/H+ exchanger (NHE-1) via transactivation of the EGFR; this effect is blocked by MR antagonists but not by inhibitors of transcription (that is, it is MR-dependent and nongenomic).87 In rabbit heart, aldosterone increases Na+/K+/2Cl− co-transporter activity and decreases Na+/K+ pump activity through a nongenomic effect on PKC epsilon type,88 which stimulates NFκB activation via MAPKs.89
Lemarie et al. used small interfering RNA to explore the specific contributions of the AT1a and AT1b receptors and MR to crosstalk between aldosterone and angiotensin II in mouse VSMCs.90 Their data suggest that activation of NFκB by either angiotensin II or aldosterone requires both the AT1 receptor and the MR. Aldosterone can stimulate activation of ERK1/2 through an AT1-dependent, MR-independent mechanism, but requires AT1 and MR to activate c-Jun NH2-terminal kinase (JNK).90 VSMC-specific knockout of the MR protects mice from angiotensin-II-induced vascular superoxide production, contraction of resistance blood vessels and hypertension, without affecting vascular structure.91
Identification of the receptor mediating the rapid, nongenomic effects of aldosterone has proven difficult. Some rapid effects of aldosterone cannot be blocked by the MR antagonist spironolactone, but may be blocked by its open-ring water-soluble metabolite, canrenoate, or by eplerenone.92 Studies on fibroblasts derived from MR-deficient mice and on MR-transfected human embryonic kidney (HEK) cells suggest that the MR contributes to the nongenomic effects of aldosterone on the ERK1/2 and JNK pathway, but not to its rapid effects on calcium.93,94 In addition, the nongenomic effects of aldosterone may facilitate classical MR-mediated effects.88
Recent studies suggest a new candidate receptor for the nongenomic effects of aldosterone. G-protein-coupled receptor (GPR) 30, an ‘orphan’ G-protein-coupled receptor expressed on the cell surface, acts via members of the G-protein family of GTP-binding proteins (Figure 1).95 Gros et al. found that, in VSMCs, aldosterone activates both GPR30 and the MR to activate PI3 kinase, ERK and myosin light chain phosphorylation.95 The contribution of GPR30 to the effects of aldosterone depends on the relative expression of GPR30 versus MR in cells; importantly, the authors reported that GPR30 expression declines in VSMCs when they are cultured, suggesting that studies in cultured VSMCs may underestimate the rapid, MR-independent effects of aldosterone in vivo. The authors further observed that spironolactone and eplerenone partially blocked the effects of a GPR30 agonist in cells that lacked the MR, consistent with prior studies suggesting that these drugs could prevent the rapid effects of aldosterone.95 In a later paper, Batenburg et al. reported that either an EGFR antagonist or a GPR30 antagonist prevented potentiation of angiotensin II-induced vasoconstriction by aldosterone.96
Key role of salt and MR activation
The observation that MR antagonism decreases inflammation and fibrosis during conditions of high salt intake, which is associated with suppression of circulating aldosterone concentrations, suggests that ligands other than aldosterone might activate the MR. As noted earlier, angiotensin II stimulates the MR via transactivation of the EGFR. Cortisol may activate the MR in cells that lack the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD2). Cortisol circulates at concentrations 100-fold to 1,000-fold higher than aldosterone and binds to the MR with equal affinity. In epithelial cells, expression of 11β-HSD2 converts cortisol (or corticosterone in rodents) to an 11-ketometabolite that is inactive at the MR.97 Some non-epithelial cells, such as endothelial cells,98 also express 11β-HSD2, but cardiomyocytes and macrophages do not.99,100 In the glomerulus, both mesangial cells and podocytes express 11β-HSD2.101,102
Because cardiomyocytes lack 11β-HSD2, the MR is typically occupied by glucocorticoids. Funder and co-workers have proposed that glucocorticoids activate the MR under conditions of oxidative stress. In a Langendorff model of myocardial infarction, researchers found that cortisol increased cardiac infarct size and that this effect was blocked by spironolactone or tempol, but not by the glucocorticoid-receptor (GR) antagonist, RU486.103 The GR agonist dexamethasone also increased infarct size in this model and, paradoxically, so did the GR antagonist RU486.103 In a study investigating rats treated with the mineralocorticoid deoxycorticosterone plus salt, RU486 did not reverse cardiac inflammation and fibrosis; however, interpretation of this study is confounded by the fact that RU486 increased blood pressure.104 Administration of corticosterone and salt to adrenalectomized rats did not increase mRNA expression of osteopontin, NOX4 or p22phox in the heart unless 11β-HSD2 was inhibited, suggesting that activation of MR by corticosterone does not cause cardiac inflammation under physiological conditions.105 In summary, the case that activation of local cardiac MRs by glucocorticoids leads to inflammation has not been made beyond a reasonable doubt.
Compelling evidence suggests that the Rho family member Rac1, a regulatory subunit of reduced NADPH oxidase, activates the MR. Fujita and co-workers have reported that mice in which Rac1 activity is increased due to the loss of a regulatory factor, RhoGDIa, have increased MR activity and MR-dependent gene expression in the kidney, and develop focal sclerosing glomerulosclerosis.106 Administration of either a Rac1 inhibitor or an MR antagonist prevents kidney injury in this model, even though aldosterone concentration is not increased. Likewise, overexpression of constitutively active Rac1 potentiates MR-dependent transcription in vitro in HEK293 cells, even in the absence of aldosterone.
Increased Rac1 activity may contribute to MR activation during high salt intake. Shibata et al. have reported that high salt intake induces Rac1 overexpression in the salt-sensitive Dahl rat but not in salt-resistant Dahl rats.107 Furthermore, they showed that Tiam1, a Rac guanine nucleotide exchange factor that promotes the transition of inactive GDP-bound Rac to active GTP-bound Rac, mediates salt-induced Rac1 activation. Although aldosterone concentrations are suppressed in both salt-sensitive and salt-resistant Dahl rats, eplerenone or adrenalectomy prevented Rac1 activity being induced by a high salt intake in the Dahl salt-sensitive rat, suggesting some dependence of Rac1 activity on aldosterone or MR activation. Rac1-dependent activation also contributes to kidney injury in mice treated with angiotensin II and salt.108
More recently, Nagase et al. have reported that Rac1 and ROS contribute to activation of the MR in rat cardiomyocytes.109 Treatment of cardiomyocytes with an inhibitor of glutathione synthesis increased ROS and increased translocation of MR into the nucleus, even when the ligand binding site was mutated. The anti-oxidant N-acetylcysteine blocked activation of the MR in cultured myocytes.
In short, the Rac1 pathway may account for MR activation under conditions such as high salt intake, when endogenous aldosterone is decreased.
Effects of cell-specific MR activation
MR antagonism prevents cardiovascular and renal injury in rodent models and in clinical trials. Because systemic administration of an MR antagonist increases sodium excretion and reduces blood pressure in rodent models, it can be difficult to distinguish the effects of systemic MR activation, sodium retention and hypertension on inflammation and fibrosis from effects of local MR activation.
In order to dissect the effects of aldosterone from the effects of systemic hypertension on the expression of profibrotic genes in the heart, Azibani et al. crossed hypertensive global renin (Ren) transgenic mice with normotensive cardiac-specific aldosterone synthase (AS) transgenic mice.76 Hypertension was associated with increased cardiac expression of genes encoding fibronectin, CTGF and TGF-β1 and there was no difference in the cardiac expression of these profibrotic genes between Ren and Ren-AS mice. By contrast, local overexpression of aldosterone synthase increased cardiac expression of genes encoding monocyte chemoattractant protein-1, osteopontin, galectin-3, and the macrophage marker CD68, independent of blood pressure (that is, expression of these genes was increased in AS and Ren-AS mice but not in Ren mice). Interestingly, expression of brain natriuretic peptide and bone morphogenic protein (BMP)-4, known to antagonize the effect of TGF-β1, was decreased by expression of aldosterone synthase in the heart.
Studies in mice in which the MR has been selectively deleted from specific cell types highlight the important contribution of inflammatory cell MR activation to inflammation and fibrosis, and will be discussed below.
As noted earlier, McCurley et al. have reported that VSMC-specific knockout of the MR protects mice from ageing-induced and angiotensin-II-induced hypertension, as well as from angiotensin-II-induced oxidative stress.91 These mice are not protected against hypertension induced by aldosterone plus salt, suggesting that renal sodium retention is the primary driver of aldosterone-induced hypertension in this model. The central effects of MR activation on salt appetite, vasopressin release and the sympathetic nervous system110 could also contribute to the development of hypertension in these VSMC-specific knockout mice.
Role of cardiomyocyte MR
Cardiomyocyte-specific knockout of the gene encoding the MR protects mice against ventricular dilatation and dysfunction following myocardial infarction.111 Infiltration by inflammatory cells is increased in cardiomyocyte-MR-deficient mice following infarction (Figure 2). At 8 weeks, cardiac expression of NADPH subunits NOX2 and NOX4 and of profibrotic genes such as CTGF, fibronectin, and collagen, is decreased, as is the formation of ROS. Lother et al. reported that cardiomyocyte-specific knockout of the gene encoding the MR protected mice against left ventricular dilation and dysfunction during pressure overload caused by aortic banding, whereas fibroblast-specific knockout of the gene encoding the MR did not have a beneficial effect.112 In addition, neither cardiomyocyte-specific nor fibroblast-specific knockout of the gene encoding the MR prevented macrophage infiltration, inflammatory gene expression in the heart or cardiac hypertrophy or fibrosis in this model. The data from these two studies indicate that the activation of MR on cells other than cardiomyocytes contribute importantly to inflammation in the heart.
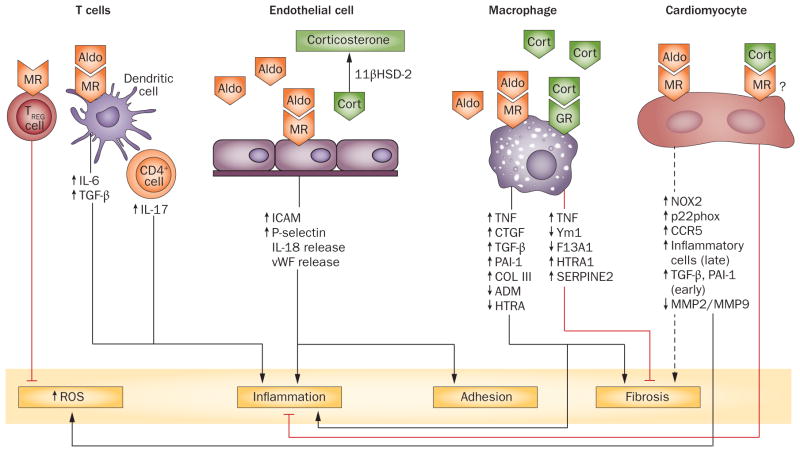
Figure 2.
Effects of cell-specific activation of the MR. Aldosterone activates the MR on macrophages to produce inflammation, hypertrophy, and fibrosis in the heart. At low concentrations, cortisol can also cause an inflammatory response via activation of glucocorticoid receptors. At higher concentrations, cortisol induces alternative activation of macrophages. Activation of MRs in cardiomyocytes increases the formation of ROS and might promote fibrosis. Studies in which 11βHSD-2 is overexpressed in cardiomyocytes suggest that cortisol opposes these effects. In endothelial cells, MR activation by aldosterone promotes inflammation and adhesion of leukocytes; endothelial cells produce 11βHSD-2, rendering cortisol inactive at the mineralocorticoid receptor. Stimulation of the MR on dendritic cells increases production of IL-6 and TGF-β1, primes CD8+ T cells, and induces TH17 production by CD4+ cells. The dotted line indicates that the positive effect is not consistent across studies (see text). The red lines indicate a decreasing effect. See text for references. Abbreviations: 11βHSD-2, 11β-hydroxysteroid dehydrogenase type II; ADM, adrenomedullin; Aldo, aldosterone; CCR5, C-C chemokine receptor type 5; Cort, cortisol; COL III, collagen III; CTGF, connective tissue growth factor; F13A1, coagulation factor XIII, A1 polypeptide; GR, glucocorticoid receptor; HTRA, high temperature requirement factor A1; ICAM1, intercellular adhesion molecule 1; IL, interleukin; MMP, matrix metalloproteinase; MR, mineralocorticoid receptor; PAI-1, plasminogen activator inhibitor 1; ROS, reactive oxygen species; TGF-β, transforming growth factor-β; TNF, tumour necrosis factor; TREG, regulatory T cell; vWF, von Willebrand factor; Ym1, a secretory protein produced by activated macrophages.
Role of macrophage MR
The macrophage plays a key role in cardiovascular inflammation, fibrosis and remodelling induced by aldosterone and high salt intake. Aldosterone at physiological concentrations increases the expression of pro-inflammatory genes in cultured macrophages, an effect that is prevented by MR antagonism.99 MR antagonism decreases macrophage expression of the profibrotic genes TGF-β1 and PAI-1 and increases expression of anti-fibrotic genes such as HTRA1, which encodes a member of the trypsin family of serine proteases (Figure 2).99 Because macrophages do not express 11β-HSD2, physiological concentrations of corticosterone may also be expected to activate the MR; however, although a low concentration (10 nmol/l) of corticosterone induces the expression of the gene encoding tumour necrosis factor in cultured macrophages, this effect is prevented by blocking the GR, not by blocking the MR.99 Moreover, a higher concentration of cortisol (1 μmol/l) induces alternative activation of macrophages.
Two groups have reported that macrophage-specific knockout of the gene encoding the MR protects mice against interstitial and perivascular cardiac fibrosis induced by DOCA plus salt113 or by chronic NOS inhibition and high salt intake (L-NAME plus salt).99,114 Young and co-workers reported that mice with macrophage-specific knockout of the gene encoding the MR have lower blood pressure but are not protected against cardiac hypertrophy induced by DOCA plus salt or by L-NAME plus salt, whereas Usher et al. reported that mice with macrophage-specific knockout of the gene encoding the MR have increased blood pressure during treatment with L-NAME plus salt and are protected against cardiac hypertrophy.99,114 The two groups report differing effects of macrophage-specific knockout of the gene encoding the MR on the recruitment of macrophages to the heart. The reason for this difference is not readily apparent, but may reflect differences in the models used (for example, differences in duration of L-NAME treatment) or differences in the derivation of the macrophage-specific knockouts.
Role of endothelial cell MR
The endothelial cell promotes macrophage adhesion and infiltration via an MR-dependent mechanism. Endothelial cells express functional MRs as well as 11βHSD-2.115 Aldosterone stimulates expression of ICAM1 by endothelial cells (Figure 2) and promotes adhesion of leukocytes to endothelial cells.115 Jeong et al. found that physiological concentrations of aldosterone stimulate the release of von Willebrand factor and interleukin (IL)-18 from human aortic endothelial cells, without stimulating tissue plasminogen activator (t-PA) release.116 In this study, aldosterone enhanced the adherence of leukocytes to endothelial cells by increasing P-selectin expression. The authors reported that aldosterone stimulates endothelial exocytosis through a nongenomic, MR-dependent mechanism. Aldosterone stimulated endothelial exocytosis within 10 min and exocytosis was not blocked by actinomycin, but was blocked by MR inhibition.
Other immune-cell-specific MR effects
T-cell activation contributes to the inflammatory response to MR stimulation. Adoptive transfer of T cells into mice lacking T cells and B cells increases the inflammatory and hypertensive response to DOCA plus salt.117,118 In addition, aldosterone modulates interactions between antigen-presenting cells (such as dendritic cells) and T cells (Figure 2). Dendritic cells express MRs, and aldosterone increases activation of the MAPK pathway and secretion of IL-6 and TGF-β1 from dendritic cells.119 Aldosterone-treated dendritic cells activate CD8+ T cells and enhance IL-17 excretion by CD4+ T cells, through an MR-dependent pathway.119 On the other hand, regulatory T cells decrease vascular injury induced by aldosterone plus salt. Specifically, Kasal et al. found that adoptive transfer of CD24+CD25+ cells prevented oxidative stress, inflammation, endothelial dysfunction, and remodelling in mesenteric arteries from mice with high salt intake treated with aldosterone.120
Endogenous aldosterone vs MR activation
Because endogenous aldosterone concentrations are suppressed during high salt intake and as ligands other than aldosterone can activate the MR, the contribution of endogenous aldosterone to MR-mediated inflammation and fibrosis has been the subject of interest.
Messaoudi et al. sought to clarify the role of aldosterone-specific activation of the MR in the heart by identifying genes that were upregulated in mice overexpressing the cardiomyocyte MR, and further upregulated by 7 days of treatment with low-dose aldosterone, but not by treatment with corticosterone.121 They identified 43 genes regulated by aldosterone-mediated activation of cardiac MR (23 that were upregulated and 20 that were downregulated), including the genes encoding the growth factors CTGF and hepatocyte growth factor, several potassium and calcium channel genes, and genes encoding cell adhesion molecules such as cadherin 4 and integrin β6.
The availability of mice lacking active aldosterone synthase, as well as pharmacological aldosterone-synthase inhibitors, is enabling investigators to study the contribution of endogenous aldosterone to inflammation and fibrosis. Lee et al. disrupted the mouse gene encoding aldosterone synthase (Cyp11b2) by replacing its first two exons with sequences coding for enhanced green fluorescent protein.122 These mice exhibit mild hypotension, as well as hyperkalaemia and increases in renin that are both corrected by high salt intake.123
Using aldosterone-deficient (AS−/−) mice, Luther et al. determined that the induction of Pai-1 and Et-1 expression in the heart by acute angiotensin II administration required endogenous aldosterone, whereas acute angiotensin-II-induced expression of these profibrotic genes in the aorta was aldosterone-independent. The investigators later compared the effect of pharmacological blockade of the MR with spironolactone with the effect of genetic aldosterone-synthase deficiency on chronic end-organ damage induced by angiotensin II and salt in mice.124 They found that blockade of the MR with spironolactone and genetic aldosterone synthase deficiency were associated with similar reductions in cardiac and aortic fibrosis in mice treated with angiotensin II plus salt, but MR antagonism prevented glomerular injury whereas aldosterone deficiency did not. Therefore, in the kidney, angiotensin II caused injury via aldosterone-independent activation of the MR. Rafiq et al. have reported that hydrocortisone also causes renal injury via activation of the MR.125
Trials of aldosterone-synthase inhibition
FAD286, the D-enantiomer of the aromatase inhibitor fadrozole, was developed as an aldosterone-synthase inhibitor after it was recognized that fadrozole reduced aldosterone concentrations without affecting cortisol concentrations in patients with breast cancer. Fiebeler and co-workers demonstrated that either treatment with FAD286 or adrenalectomy significantly reduced mortality, cardiac hypertrophy, albuminuria, and cardiac and renal inflammation in rats doubly transgenic for the human genes encoding renin and angiotensinogen.126,127 FAD286 also reduces inflammation and atherosclerosis in apolipoprotein-E-deficient mice.128
LCI699 is the first orally available aldosterone-synthase inhibitor to be developed for use in humans. In a proof-of-concept study, LCI699 decreased plasma aldosterone concentrations by approximately 70% and increased concentrations of the aldosterone precursor 11-DOC by more than 700% in patients with primary hyperaldosteronism.129 LCI699 had no effect on basal concentrations of cortisol. LCI699 reduced office and ambulatory systolic blood pressure and increased serum potassium concentration. In a subsequent study, LCI699 at doses of 0.5 mg or 1.0 mg twice daily was less effective in reducing blood pressure in patients with primary hyperaldosteronism than was eplerenone 50–100 mg twice daily, even though LCI699 decreased aldosterone concentrations by 70–80%.130
Calhoun et al. tested the effect of 8 weeks of therapy with LCI699 (0.25 mg once daily, 0.5 mg once daily, 1.0 mg once daily and 0.5 mg twice daily) on blood pressure in a double-blind, placebo-controlled and eplerenone-controlled trial in patients with essential hypertension.131 LCI699 induced a dose-dependent reduction in systolic and diastolic blood pressure, with the 1.0 mg per day dose having a similar effect on blood pressure as a 50 mg twice daily dose of eplerenone. LCI699 and eplerenone both increased plasma renin activity. At the highest dose (0.5 mg twice daily), LCI699 significantly decreased plasma aldosterone concentration, whereas eplerenone (50 mg twice daily) increased plasma aldosterone concentration. Paradoxically, the 0.5 mg twice-daily dose of LCI699 had less effect on blood pressure than did the 1.0 mg per day dose, even though the 0.5 mg twice-daily dose had a greater inhibitory effect on aldosterone synthesis. This finding that LCI699 0.5 mg twice daily suppressed aldosterone but did not decrease blood pressure has been attributed to increases in precursor mineralocorticoids.132 In Calhoun et al.’s study, one individual in each LCI699 treatment group had a recorded potassium concentration of >6 mmol/l, which resolved on repeat testing without discontinuation of study medication.131 Overall, the effect of LCI699 on serum potassium concentration was similar to the effect of eplerenone. Although LCI699 had no effect on basal cortisol concentrations, the aldosterone-synthase inhibitor significantly blunted the cortisol response to adrenocorticotropic hormone. To date, the effects of aldosterone-synthase inhibition on inflammation or on cardiovascular or renal injury have not been reported in humans.
Conclusions
In conclusion, aldosterone stimulates the production of ROS, inflammation and fibrosis of the heart, vasculature, and kidney through both MR-dependent and MR-independent mechanisms. GPR30 may mediate MR-independent effects of aldosterone in vascular cells. In addition, during conditions of high salt intake, Rac1 activates the MR to produce oxidative stress. Findings in mice with cell-specific deficiency of the MR suggest that activation of local MRs (for example, on macrophages) induces fibrosis. The MR may be activated or transactivated by ligands other than aldosterone. Studies in mice treated with aldosterone synthase or aldosterone synthase inhibitors suggest that aldosterone is the primary ligand involved in cardiac and vascular fibrosis, but that MR antagonism may be necessary to prevent renal injury. Human studies suggesting that aldosterone-synthase inhibitors may be less effective in reducing blood pressure than are MR antagonists also suggest that ligands other than aldosterone activate the MR in the kidney.
Key points.
Aldosterone or mineralocorticoid-receptor activation trigger the formation of reactive oxygen species by NADPH oxidase and mitochondria that, in turn, induce a proinflammatory and profibrotic phenotype
Under conditions of high salt intake, Rac1 activates the mineralocorticoid receptor and increases the formation of reactive oxygen species
Aldosterone exerts rapid, transcription-independent effects (nongenomic effects) that may be mediated by G-protein-coupled receptor 30 and transactivation of the epithelial growth factor receptor
Studies in mice in which the mineralocorticoid receptor has been selectively deleted on specific cells indicate that systemic mineralocorticoid-receptor activation is not necessary to induce local inflammation and fibrosis
Aldosterone-synthase inhibition or deficiency prevents inflammation and fibrosis in many rodent models of cardiovascular or renal injury
Review criteria.
PubMed was searched for articles published between 1960 and the present using the terms “aldosterone”, “mineralocorticoid”, and/or “mineralocorticoid receptor” together with the terms “oxidative stress”, “inflammation” and/or “fibrosis”. Full-text English-language papers were reviewed and the bibliographies of these papers were searched for additional leads. Focused searches were also conducted regarding topic areas identified in the initial search. Emphasis was placed on papers published from 2009 onwards.
Acknowledgments
The author has received grant support from the National Institutes of Health (HL060906).
Footnotes
Competing interests
The author declares associations with the following companies: Novartis, Shire Pharmaceuticals. See the article online for full details of the relationships.
References
- 1.Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G. sgk is an aldosterone-induced kinase in the renal collecting duct Effects on epithelial na+ channels. J Biol Chem. 1999;274:16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 2.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 3.Bhalla V, Soundararajan R, Pao AC, Li H, Pearce D. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am J Physiol Renal Physiol. 2006;291:F714–F721. doi: 10.1152/ajprenal.00061.2006. [DOI] [PubMed] [Google Scholar]
- 4.Rozansky DJ, et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009;119:2601–2612. doi: 10.1172/JCI38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arroyo JP, et al. Nedd4–2 modulates renal Na+-Cl− cotransporter via the aldosterone-SGK1-Nedd4–2 pathway. J Am Soc Nephrol. 2011;22:1707–1719. doi: 10.1681/ASN.2011020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato A, Funder JW. High glucose stimulates aldosterone-induced hypertrophy via type I mineralocorticoid receptors in neonatal rat cardiomyocytes. Endocrinology. 1996;137:4145–4153. doi: 10.1210/endo.137.10.8828470. [DOI] [PubMed] [Google Scholar]
- 7.Lombes M, et al. Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res. 1992;71:503–510. doi: 10.1161/01.res.71.3.503. [DOI] [PubMed] [Google Scholar]
- 8.Terada Y, et al. Aldosterone stimulates proliferation of mesangial cells by activating mitogen-activated protein kinase 1/2, cyclin D1, and cyclin A. J Am Soc Nephrol. 2005;16:2296–2305. doi: 10.1681/ASN.2005020129. [DOI] [PubMed] [Google Scholar]
- 9.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 10.Guo C, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calo LA, et al. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J Clin Endocrinol Metab. 2004;89:1973–1976. doi: 10.1210/jc.2003-031545. [DOI] [PubMed] [Google Scholar]
- 12.Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 13.Lacolley P, et al. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats effects of eplerenone. Circulation. 2002;106:2848–2853. doi: 10.1161/01.cir.0000039328.33137.6c. [DOI] [PubMed] [Google Scholar]
- 14.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha R, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–H1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 16.Blasi ER, et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 17.Gerling IC, et al. Aldosteronism: an immunostimulatory state precedes proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H813–H821. doi: 10.1152/ajpheart.00113.2003. [DOI] [PubMed] [Google Scholar]
- 18.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol. 1993;25:563–575. doi: 10.1006/jmcc.1993.1066. [DOI] [PubMed] [Google Scholar]
- 19.Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 20.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES) Rales Investigators Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 22.Zannad F, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 23.Volk MJ, Bomback AS, Klemmer PJ. Mineralocorticoid receptor blockade in chronic kidney disease. Curr Hypertens Rep. 2011;13:282–288. doi: 10.1007/s11906-011-0202-2. [DOI] [PubMed] [Google Scholar]
- 24.Fiebeler A, et al. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension. 2001;37:787–793. doi: 10.1161/01.hyp.37.2.787. [DOI] [PubMed] [Google Scholar]
- 25.He BJ, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keidar S, et al. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- 27.Iwashima F, et al. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149:1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, et al. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirono Y, et al. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148:1688–1696. doi: 10.1210/en.2006-1157. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009;296:F1323–F1333. doi: 10.1152/ajprenal.90428.2008. [DOI] [PubMed] [Google Scholar]
- 31.Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 33.Stas S, et al. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of NADPH oxidase and cardiac remodeling. Endocrinology. 2007;148:3773–3780. doi: 10.1210/en.2006-1691. [DOI] [PubMed] [Google Scholar]
- 34.Matsui H, et al. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension. 2008;52:287–294. doi: 10.1161/HYPERTENSIONAHA.108.111815. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida K, et al. Excess aldosterone under normal salt diet induces cardiac hypertrophy and infiltration via oxidative stress. Hypertens Res. 2005;28:447–455. doi: 10.1291/hypres.28.447. [DOI] [PubMed] [Google Scholar]
- 36.Rude MK, et al. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension. 2005;46:555–561. doi: 10.1161/01.HYP.0000176236.55322.18. [DOI] [PubMed] [Google Scholar]
- 37.Yuan J, Jia R, Bao Y. Aldosterone up-regulates production of plasminogen activator inhibitor-1 by renal mesangial cells. J Biochem Mol Biol. 2007;40:180–188. doi: 10.5483/bmbrep.2007.40.2.180. [DOI] [PubMed] [Google Scholar]
- 38.Leopold JA, et al. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagata D, et al. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–171. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- 40.Oberleithner H, et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korte S, et al. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch. 2012;463:269–278. doi: 10.1007/s00424-011-1038-y. [DOI] [PubMed] [Google Scholar]
- 42.Yuan Y, et al. Mitochondrial dysfunction accounts for aldosterone-induced epithelial-to-mesenchymal transition of renal proximal tubular epithelial cells. Free Radic Biol Med. 2012;53:30–43. doi: 10.1016/j.freeradbiomed.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol. 2007;293:F723–F731. doi: 10.1152/ajprenal.00480.2006. [DOI] [PubMed] [Google Scholar]
- 44.Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009;296:F1323–F1333. doi: 10.1152/ajprenal.90428.2008. [DOI] [PubMed] [Google Scholar]
- 45.Zhu C, et al. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARgamma. Am J Pathol. 2011;178:2020–2031. doi: 10.1016/j.ajpath.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang A, Jia Z, Wang N, Tidwell TJ, Yang T. Relative contributions of mitochondria and NADPH oxidase to deoxycorticosterone acetate-salt hypertension in mice. Kidney Int. 2011;80:51–60. doi: 10.1038/ki.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terada Y, et al. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clin Exp Nephrol. 2012;16:81–88. doi: 10.1007/s10157-011-0498-x. [DOI] [PubMed] [Google Scholar]
- 48.Leroy V, et al. Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol. 2009;20:131–144. doi: 10.1681/ASN.2008020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang M, et al. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1675–1686. doi: 10.1161/ATVBAHA.112.248732. [DOI] [PubMed] [Google Scholar]
- 50.Artunc F, et al. Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J Mol Med (Berl) 2006;84:737–746. doi: 10.1007/s00109-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 51.Noble NA, Harper JR, Border WA. In vivo interactions of TGF-beta and extracellular matrix. Prog Growth Factor Res. 1992;4:369–382. doi: 10.1016/0955-2235(92)90017-c. [DOI] [PubMed] [Google Scholar]
- 52.Chun TY, Bloem LJ, Pratt JH. Aldosterone inhibits inducible nitric oxide synthase in neonatal rat cardiomyocytes. Endocrinology. 2003;144:1712–1717. doi: 10.1210/en.2002-220956. [DOI] [PubMed] [Google Scholar]
- 53.Han JS, Choi BS, Yang CW, Kim YS. Aldosterone-induced TGF-beta1 expression is regulated by mitogen-activated protein kinases and activator protein-1 in mesangial cells. J Korean Med Sci. 2009;24 (Suppl):S195–S203. doi: 10.3346/jkms.2009.24.S1.S195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juknevicius I, Segal Y, Kren S, Lee R, Hostetter TH. Effect of aldosterone on renal transforming growth factor-beta. Am J Physiol Renal Physiol. 2004;286:F1059–F1062. doi: 10.1152/ajprenal.00202.2003. [DOI] [PubMed] [Google Scholar]
- 55.Brown NJ, et al. Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab. 2000;85:336–344. doi: 10.1210/jcem.85.1.6305. [DOI] [PubMed] [Google Scholar]
- 56.Chun TY, Pratt JH. Aldosterone increases plasminogen activator inhibitor-1 synthesis in rat cardiomyocytes. Mol Cell Endocrinol. 2005;239:55–61. doi: 10.1016/j.mce.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 57.Huang W, et al. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol. 2008;294:F1287–F1295. doi: 10.1152/ajprenal.00017.2008. [DOI] [PubMed] [Google Scholar]
- 58.Rerolle JP, Hertig A, Nguyen G, Sraer JD, Rondeau EP. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000;58:1841–1850. doi: 10.1111/j.1523-1755.2000.00355.x. [DOI] [PubMed] [Google Scholar]
- 59.Ma J, et al. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney Int. 2006;69:1064–1072. doi: 10.1038/sj.ki.5000201. [DOI] [PubMed] [Google Scholar]
- 60.Oestreicher EM, et al. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation. 2003;108:2517–2523. doi: 10.1161/01.CIR.0000097000.51723.6F. [DOI] [PubMed] [Google Scholar]
- 61.Weisberg AD, et al. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- 62.Ghosh AK, et al. Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation. 2010;122:1200–1209. doi: 10.1161/CIRCULATIONAHA.110.955245. [DOI] [PubMed] [Google Scholar]
- 63.Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE, Dichek DA. Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res. 2004;95:637–644. doi: 10.1161/01.RES.0000141427.61023.f4. [DOI] [PubMed] [Google Scholar]
- 64.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27:2130–2134. doi: 10.1093/cvr/27.12.2130. [DOI] [PubMed] [Google Scholar]
- 65.Park JB, Schiffrin EL. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens. 2002;15:164–169. doi: 10.1016/s0895-7061(01)02291-9. [DOI] [PubMed] [Google Scholar]
- 66.Seccia TM, et al. Cardiac fibrosis occurs early and involves endothelin and AT-1 receptors in hypertension due to endogenous angiotensin II. J Am Coll Cardiol. 2003;41:666–673. doi: 10.1016/s0735-1097(02)02860-7. [DOI] [PubMed] [Google Scholar]
- 67.Tostes RC, Touyz RM, He G, Ammarguellat F, Schiffrin EL. Endothelin A receptor blockade decreases expression of growth factors and collagen and improves matrix metalloproteinase-2 activity in kidneys from stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;39:892–900. doi: 10.1097/00005344-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 68.Stow LR, et al. Aldosterone modulates steroid receptor binding to the endothelin-1 gene (edn1) J Biol Chem. 2009;284:30087–30096. doi: 10.1074/jbc.M109.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaffe IZ, et al. Placental growth factor mediates aldosterone-dependent vascular injury in mice. J Clin Invest. 2010;120:3891–3900. doi: 10.1172/JCI40205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Isoda K, et al. Osteopontin plays an important role in the development of medial thickening and neointimal formation. Circ Res. 2002;91:77–82. doi: 10.1161/01.res.0000025268.10302.0c. [DOI] [PubMed] [Google Scholar]
- 71.Fu GX, Xu CC, Zhong Y, Zhu DL, Gao PJ. Aldosterone-induced osteopontin expression in vascular smooth muscle cells involves MR, ERK, and p38 MAPK. Endocrine. 2012;42:676–683. doi: 10.1007/s12020-012-9675-2. [DOI] [PubMed] [Google Scholar]
- 72.Sam F, et al. Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens. 2004;17:188–193. doi: 10.1016/j.amjhyper.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Irita J, et al. Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am J Physiol Renal Physiol. 2011;301:F833–F844. doi: 10.1152/ajprenal.00557.2010. [DOI] [PubMed] [Google Scholar]
- 74.Morrow DA, O’Donoghue ML. Galectin-3 in cardiovascular disease: a possible window into early myocardial fibrosis. J Am Coll Cardiol. 2012;60:1257–1258. doi: 10.1016/j.jacc.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 75.Calvier L, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 76.Azibani F, et al. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension. 2012;59:1179–1187. doi: 10.1161/HYPERTENSIONAHA.111.190512. [DOI] [PubMed] [Google Scholar]
- 77.Kimura K, Ohkita M, Koyama M, Matsumura Y. Reduced NO production rapidly aggravates renal function through the NF-kappaB/ET-1/ETA receptor pathway in DOCA-salt-induced hypertensive rats. Life Sci. 2012;91:644–650. doi: 10.1016/j.lfs.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 78.Ogawa Y, et al. Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury. J Am Soc Nephrol. 2012;23:1198–1209. doi: 10.1681/ASN.2011100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hellal-Levy C, Fagart J, Souque A, Rafestin-Oblin ME. Mechanistic aspects of mineralocorticoid receptor activation. Kidney Int. 2000;57:1250–1255. doi: 10.1046/j.1523-1755.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 80.Christ M, Meyer C, Sippel K, Wehling M. Rapid aldosterone signaling in vascular smooth muscle cells: involvement of phospholipase C, diacylglycerol and protein kinase C alpha. Biochem Biophys Res Commun. 1995;213:123–129. doi: 10.1006/bbrc.1995.2106. [DOI] [PubMed] [Google Scholar]
- 81.Min LJ, et al. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005;97:434–442. doi: 10.1161/01.RES.0000180753.63183.95. [DOI] [PubMed] [Google Scholar]
- 82.Mazak I, et al. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation. 2004;109:2792–2800. doi: 10.1161/01.CIR.0000131860.80444.AB. [DOI] [PubMed] [Google Scholar]
- 83.Grossmann C, et al. Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am J Physiol Endocrinol Metab. 2007;292:E1790–E1800. doi: 10.1152/ajpendo.00708.2006. [DOI] [PubMed] [Google Scholar]
- 84.Griol-Charhbili V, et al. Epidermal growth factor receptor mediates the vascular dysfunction but not the remodeling induced by aldosterone/salt. Hypertension. 2011;57:238–244. doi: 10.1161/HYPERTENSIONAHA.110.153619. [DOI] [PubMed] [Google Scholar]
- 85.Messaoudi S, et al. The epidermal growth factor receptor is involved in angiotensin II but not aldosterone/salt-induced cardiac remodelling. PLoS ONE. 2012;7:e30156. doi: 10.1371/journal.pone.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sinphitukkul K, Eiam-Ong S, Manotham K, Eiam-Ong S. Nongenomic effects of aldosterone on renal protein expressions of pEGFR and pERK1/2 in rat kidney. Am J Nephrol. 2011;33:111–120. doi: 10.1159/000322965. [DOI] [PubMed] [Google Scholar]
- 87.De Giusti VC, et al. Aldosterone stimulates the cardiac Na(+)/H(+) exchanger via transactivation of the epidermal growth factor receptor. Hypertension. 2011;58:912–919. doi: 10.1161/HYPERTENSIONAHA.111.176024. [DOI] [PubMed] [Google Scholar]
- 88.Mihailidou AS, Mardini M, Funder JW. Rapid, nongenomic effects of aldosterone in the heart mediated by epsilon protein kinase C. Endocrinology. 2004;145:773–780. doi: 10.1210/en.2003-1137. [DOI] [PubMed] [Google Scholar]
- 89.Li RC, et al. PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1679–H1689. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- 90.Lemarie CA, et al. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res. 2009;105:852–859. doi: 10.1161/CIRCRESAHA.109.196576. [DOI] [PubMed] [Google Scholar]
- 91.McCurley A, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michea L, et al. Eplerenone blocks nongenomic effects of aldosterone on the Na+/H+ exchanger, intracellular Ca2+ levels, and vasoconstriction in mesenteric resistance vessels. Endocrinology. 2005;146:973–980. doi: 10.1210/en.2004-1130. [DOI] [PubMed] [Google Scholar]
- 93.Haseroth K, et al. Rapid nongenomic effects of aldosterone in mineralocorticoid-receptor-knockout mice. Biochem Biophys Res Commun. 1999;266:257–261. doi: 10.1006/bbrc.1999.1771. [DOI] [PubMed] [Google Scholar]
- 94.Grossmann C, et al. Human mineralocorticoid receptor expression renders cells responsive for nongenotropic aldosterone actions. Mol Endocrinol. 2005;19:1697–1710. doi: 10.1210/me.2004-0469. [DOI] [PubMed] [Google Scholar]
- 95.Gros R, et al. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Batenburg WW, Jansen PM, van den Bogaerdt AJ, AHJD Angiotensin II-aldosterone interaction in human coronary microarteries involves GPR30, EGFR, and endothelial NO synthase. Cardiovasc Res. 2012;94:136–143. doi: 10.1093/cvr/cvs016. [DOI] [PubMed] [Google Scholar]
- 97.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 98.Gong R, Morris DJ, Brem AS. Variable expression of 11beta hydroxysteroid dehydrogenase (11beta-HSD) isoforms in vascular endothelial cells. Steroids. 2008;73:1187–1196. doi: 10.1016/j.steroids.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 99.Usher MG, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qin W, et al. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003;93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5. [DOI] [PubMed] [Google Scholar]
- 101.Lee SH, et al. Activation of local aldosterone system within podocytes is involved in apoptosis under diabetic conditions. Am J Physiol Renal Physiol. 2009;297:F1381–F1390. doi: 10.1152/ajprenal.00101.2009. [DOI] [PubMed] [Google Scholar]
- 102.Terada Y, et al. Aldosterone stimulates proliferation of mesangial cells by activating mitogen-activated protein kinase 1/2, cyclin d1, and cyclin a. J Am Soc Nephrol. 2005;16:2296–2305. doi: 10.1681/ASN.2005020129. [DOI] [PubMed] [Google Scholar]
- 103.Mihailidou AS, Loan Le TY, Mardini M, Funder JW. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension. 2009;54:1306–1312. doi: 10.1161/HYPERTENSIONAHA.109.136242. [DOI] [PubMed] [Google Scholar]
- 104.Rickard AJ, Funder JW, Morgan J, Fuller PJ, Young MJ. Does glucocorticoid receptor blockade exacerbate tissue damage after mineralocorticoid/salt administration? Endocrinology. 2007;148:4829–4835. doi: 10.1210/en.2007-0209. [DOI] [PubMed] [Google Scholar]
- 105.Young MJ, Morgan J, Brolin K, Fuller PJ, Funder JW. Activation of mineralocorticoid receptors by exogenous glucocorticoids and the development of cardiovascular inflammatory responses in adrenalectomized rats. Endocrinology. 2010;151:2622–2628. doi: 10.1210/en.2009-1476. [DOI] [PubMed] [Google Scholar]
- 106.Shibata S, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–1376. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 107.Shibata S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–3243. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kawarazaki W, et al. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol. 2012;23:997–1007. doi: 10.1681/ASN.2011070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagase M, et al. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension. 2012;59:500–506. doi: 10.1161/HYPERTENSIONAHA.111.185520. [DOI] [PubMed] [Google Scholar]
- 110.Oki K, Gomez-Sanchez EP, Gomez-Sanchez CE. Role of mineralocorticoid action in the brain in salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2012;39:90–95. doi: 10.1111/j.1440-1681.2011.05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fraccarollo D, et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation. 2011;123:400–408. doi: 10.1161/CIRCULATIONAHA.110.983023. [DOI] [PubMed] [Google Scholar]
- 112.Lother A, et al. Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension. 2011;57:746–754. doi: 10.1161/HYPERTENSIONAHA.110.163287. [DOI] [PubMed] [Google Scholar]
- 113.Rickard AJ, et al. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 114.Bienvenu LA, et al. Macrophage mineralocorticoid receptor signaling plays a key role in aldosterone-independent cardiac fibrosis. Endocrinology. 2012;153:3416–3425. doi: 10.1210/en.2011-2098. [DOI] [PubMed] [Google Scholar]
- 115.Caprio M, et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeong Y, et al. Aldosterone activates endothelial exocytosis. Proc Natl Acad Sci USA. 2009;106:3782–3787. doi: 10.1073/pnas.0804037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guzik TJ, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vinh A, et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Herrada AA, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184:191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 120.Kasal DA, et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 121.Messaoudi S, et al. Aldosterone-specific activation of cardiomyocyte mineralocorticoid receptor in vivo. Hypertension. 2013;61:361–367. doi: 10.1161/HYPERTENSIONAHA.112.198986. [DOI] [PubMed] [Google Scholar]
- 122.Lee G, et al. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656. doi: 10.1210/en.2004-1102. [DOI] [PubMed] [Google Scholar]
- 123.Makhanova N, et al. Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol. 2006;290:F61–F69. doi: 10.1152/ajprenal.00257.2005. [DOI] [PubMed] [Google Scholar]
- 124.Luther JM, et al. Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin II-induced cardiac, renal, and vascular injury. Kidney Int. 2012;82:643–651. doi: 10.1038/ki.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rafiq K, et al. Effects of mineralocorticoid receptor blockade on glucocorticoid-induced renal injury in adrenalectomized rats. J Hypertens. 2011;29:290–298. doi: 10.1097/hjh.0b013e32834103a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fiebeler A, et al. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
- 127.Fiebeler A, et al. Aldosterone synthase inhibitor FAD286 ameliorates angiotensin II-induced end-organ damage. Hypertension. 2004;44:514–515. [Google Scholar]
- 128.Gamliel-Lazarovich A, et al. FAD286, an aldosterone synthase inhibitor, reduced atherosclerosis and inflammation in apolipoprotein E-deficient mice. J Hypertens. 2010;28:1900–1907. doi: 10.1097/HJH.0b013e32833c2197. [DOI] [PubMed] [Google Scholar]
- 129.Amar L, et al. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension. 2010;56:831–838. doi: 10.1161/HYPERTENSIONAHA.110.157271. [DOI] [PubMed] [Google Scholar]
- 130.Amar L, Azizi M, Menard J, Peyrard S, Plouin PF. Sequential comparison of aldosterone synthase inhibition and mineralocorticoid blockade in patients with primary aldosteronism. J Hypertens. 2013;31:624–629. doi: 10.1097/HJH.0b013e32835d6d49. [DOI] [PubMed] [Google Scholar]
- 131.Calhoun DA, et al. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo- and active-controlled phase 2 trial. Circulation. 2011;124:1945–1955. doi: 10.1161/CIRCULATIONAHA.111.029892. [DOI] [PubMed] [Google Scholar]
- 132.Azizi M, Amar L, Menard J. Aldosterone synthase inhibition in humans. Nephrol Dial Transplant. 2013;28:36–43. doi: 10.1093/ndt/gfs388. [DOI] [PubMed] [Google Scholar]