Abstract
Several hypolipidemic drugs and certain industrial plasticizers induce proliferation of peroxisomes, enhance the activity of peroxisome-associated beta-oxidation of fatty acids, and produce hepatocellular carcinomas in the livers of rodents. Because these chemicals themselves are not mutagens and do not covalently modify DNA, unlike the majority of chemical carcinogens, we proposed that the persistent proliferation of peroxisomes, and the induction of associated peroxisomal oxidases, caused a sustained increase in intracellular H2O2 or other reduced oxygen species, which would then introduce mutagenic DNA damage. In the present study, we investigated the ability of peroxisomes purified from the livers of normal and hypolipidemic drug-treated rats to induce DNA strand scission in vitro. Gradient-purified peroxisomes from livers of hypolipidemic drug-treated rats produced a 30- to 70-fold increase in H2O2 generation when compared to controls. The levels of H2O2 generated in incubations containing control or hypolipidemic drug-induced peroxisomes correlated well with the induction of single strand breaks in supercoiled simian virus 40 DNA molecules that were included in these reconstituted peroxisome incubations. Addition of excess catalase to peroxisome incubations failed to prevent strand breaks, suggesting that other reduced oxygen species may be rapidly generated from H2O2. These experimental results are consistent with a mechanism of hepatocarcinogenesis in which hepatocellular genetic damage is introduced by the by-products of peroxisomal fatty acid beta-oxidation, an oxidative pathway that is dramatically increased in hypolipidemic drug-treated livers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Erickson L. C. Comparison of the effects of hydrogen peroxide and x-ray irradiation on toxicity, mutation, and DNA damage/repair in mammalian cells (V-79). Biochim Biophys Acta. 1981 Jun 26;654(1):135–141. doi: 10.1016/0005-2787(81)90146-5. [DOI] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Demyan W. F., Lalwani N. D., Reddy J. K., Roy A. K. Reversible alteration of hepatic messenger RNA species for peroxisomal and non-peroxisomal proteins induced by the hypolipidaemic drug Wy-14,643. Biochem J. 1983 Sep 15;214(3):879–883. doi: 10.1042/bj2140879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. 5,6-Saturated thymine lesions in DNA: production by ultraviolet light or hydrogen peroxide. Nucleic Acids Res. 1982 Jun 25;10(12):3781–3789. doi: 10.1093/nar/10.12.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahl W. E., Lalwani N. D., Reddy M. K., Reddy J. K. Induction of peroxisomal enzymes in livers of neonatal rats exposed to lactating mothers treated with hypolipidaemic drugs. Role of drug metabolite transfer in milk. Biochem J. 1983 Mar 15;210(3):875–883. doi: 10.1042/bj2100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Chemical carcinogenesis. N Engl J Med. 1981 Dec 3;305(23):1379–1389. doi: 10.1056/NEJM198112033052304. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Furuta S., Hashimoto T., Miura S., Mori M., Tatibana M. Cell-free synthesis of the enzymes of peroxisomal beta-oxidation. Biochem Biophys Res Commun. 1982 Mar 30;105(2):639–646. doi: 10.1016/0006-291x(82)91482-6. [DOI] [PubMed] [Google Scholar]
- Hajra A. K., Bishop J. E. Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxyacetone phosphate pathway. Ann N Y Acad Sci. 1982;386:170–182. doi: 10.1111/j.1749-6632.1982.tb21415.x. [DOI] [PubMed] [Google Scholar]
- Hess R., Stäubli W., Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965 Nov 27;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- Hirota N., Yokoyama T. Enhancing effect of hydrogen peroxide upon duodenal and upper jejunal carcinogenesis in rats. Gan. 1981 Oct;72(5):811–812. [PubMed] [Google Scholar]
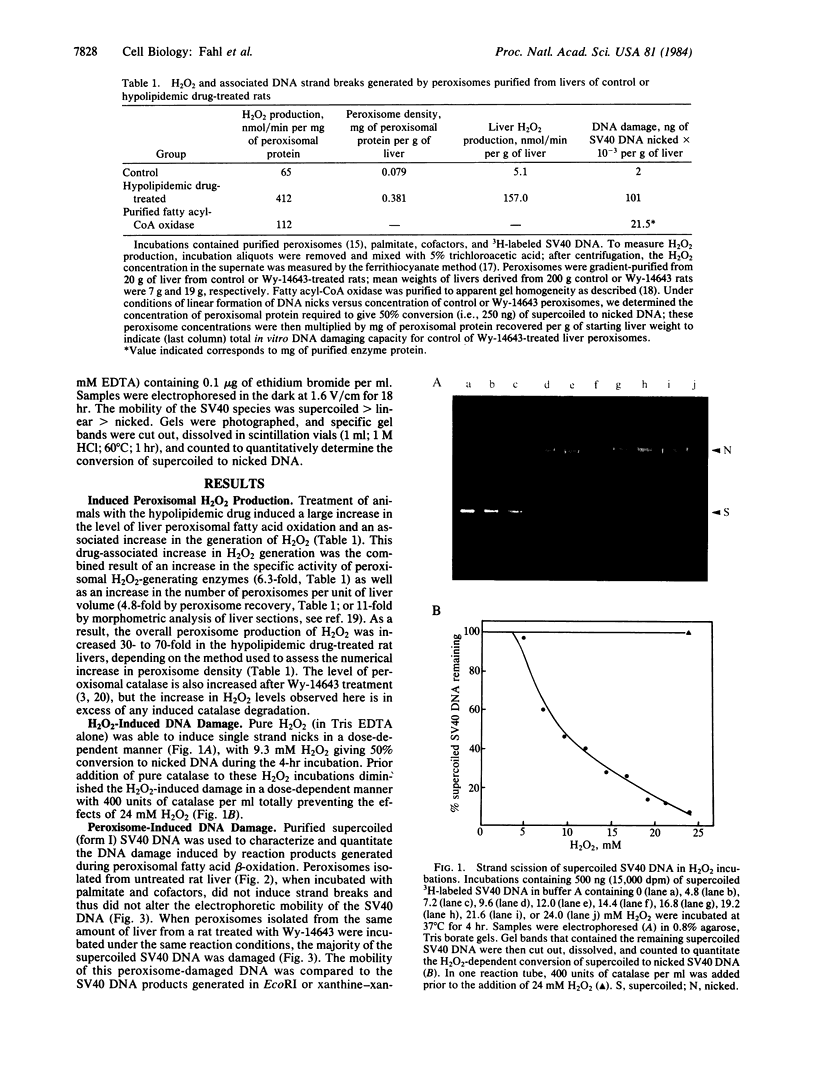
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kong S., Davison A. J. The relative effectiveness of .OH, H2O2, O2-, and reducing free radicals in causing damage to biomembranes. A study of radiation damage to erythrocyte ghosts using selective free radical scavengers. Biochim Biophys Acta. 1981 Jan 8;640(1):313–325. doi: 10.1016/0005-2736(81)90555-1. [DOI] [PubMed] [Google Scholar]
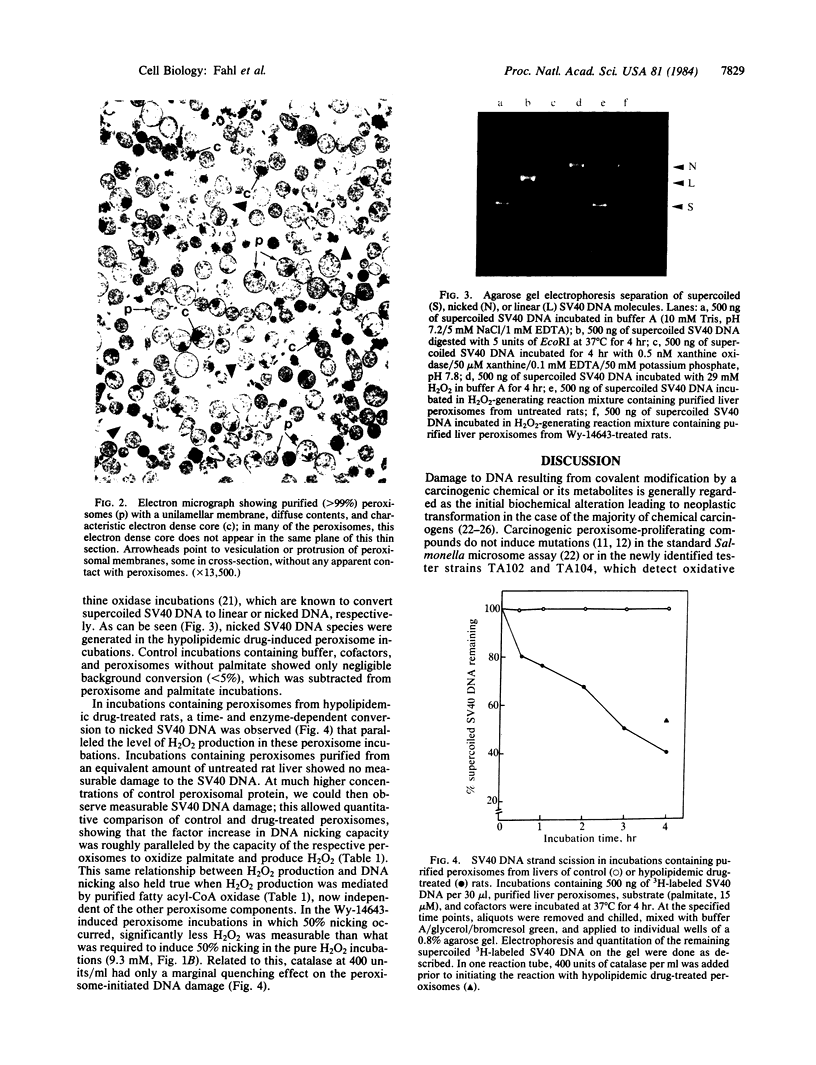
- Lalwani N. D., Reddy M. K., Qureshi S. A., Moehle C. M., Hayashi H., Reddy J. K. Noninhibitory effect of antioxidants ethoxyquin, 2(3)-tert-butyl-4-hydroxyanisole and 3,5-di-tert-butyl-4-hydroxytoluene on hepatic peroxisome proliferation and peroxisomal fatty acid beta-oxidation induced by a hypolipidemic agent in rats. Cancer Res. 1983 Apr;43(4):1680–1687. [PubMed] [Google Scholar]
- Lalwani N. D., Reddy M. K., Qureshi S. A., Sirtori C. R., Abiko Y., Reddy J. K. Evaluation of selected hypolipidemic agents for the induction of peroxisomal enzymes and peroxisome proliferation in the rat liver. Hum Toxicol. 1983 Jan;2(1):27–48. doi: 10.1177/096032718300200103. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Hollstein M., Christman M. F., Schwiers E. A., Ames B. N. A new Salmonella tester strain (TA102) with A X T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie H. R., Samis H. V., Baird M. B. The kinetics of degradation of DNA and RNA by H 2 O 2 . Biochim Biophys Acta. 1972 Jul 31;272(4):539–548. doi: 10.1016/0005-2787(72)90509-6. [DOI] [PubMed] [Google Scholar]
- McCormick J. P., Fischer J. R., Pachlatko J. P., Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science. 1976 Feb 6;191(4226):468–469. doi: 10.1126/science.1108203. [DOI] [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 1982 May;79(9):2855–2859. doi: 10.1073/pnas.79.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T., Ui N. Purification and properties of acyl-CoA oxidase from rat liver. J Biochem. 1980 Jun;87(6):1735–1746. doi: 10.1093/oxfordjournals.jbchem.a132918. [DOI] [PubMed] [Google Scholar]
- Pitot H. C. Chemicals and cancer: initiation and promotion. Hosp Pract (Off Ed) 1983 Jul;18(7):101–113. doi: 10.1080/21548331.1983.11702589. [DOI] [PubMed] [Google Scholar]
- Poole B. Diffusion effects in the metabolism of hydrogen peroxide by rat liver peroxisomes. J Theor Biol. 1975 May;51(1):149–167. doi: 10.1016/0022-5193(75)90145-9. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Azarnoff D. L., Hignite C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980 Jan 24;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Krishnakantha T. P. Hepatic peroxisome proliferation: induction by two novel compounds structurally unrelated to clofibrate. Science. 1975 Nov 21;190(4216):787–789. doi: 10.1126/science.1198095. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Lalwai N. D. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12(1):1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Lalwani N. D., Qureshi S. A., Reddy M. K., Moehle C. M. Induction of hepatic peroxisome proliferation in nonrodent species, including primates. Am J Pathol. 1984 Jan;114(1):171–183. [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Lalwani N. D., Reddy M. K., Qureshi S. A. Excessive accumulation of autofluorescent lipofuscin in the liver during hepatocarcinogenesis by methyl clofenapate and other hypolipidemic peroxisome proliferators. Cancer Res. 1982 Jan;42(1):259–266. [PubMed] [Google Scholar]
- Reddy J. K., Scarpelli D. G., Subbarao V., Lalwani N. D. Chemical carcinogens without mutagenic activity: peroxisome proliferators as a prototype. Toxicol Pathol. 1983;11(2):172–180. doi: 10.1177/019262338301100209. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Warren J. R., Reddy M. K., Lalwani N. D. Hepatic and renal effects of peroxisome proliferators: biological implications. Ann N Y Acad Sci. 1982;386:81–110. doi: 10.1111/j.1749-6632.1982.tb21409.x. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Wang R. J., Ananthaswamy H. N., Nixon B. T., Hartman P. S., Eisenstark A. Induction of single-strand DNA breaks in human cells by H2O2 formed in near-uv (black light) irradiated medium. Radiat Res. 1980 May;82(2):269–276. [PubMed] [Google Scholar]
- Warren J. R., Simmon V. F., Reddy J. K. Properties of hypolipidemic peroxisome proliferators in the lymphocyte [3H]thymidine and Salmonella mutagenesis assays. Cancer Res. 1980 Jan;40(1):36–41. [PubMed] [Google Scholar]
- Weisburger J. H., Williams G. M. Carcinogen testing: current problems and new approaches. Science. 1981 Oct 23;214(4519):401–407. doi: 10.1126/science.7291981. [DOI] [PubMed] [Google Scholar]
- von Däniken A., Lutz W. K., Schlatter C. Lack of covalent binding to rat liver DNA of the hypolipidemic drugs clofibrate and fenofibrate. Toxicol Lett. 1981 Feb;7(4-5):305–310. doi: 10.1016/0378-4274(81)90053-9. [DOI] [PubMed] [Google Scholar]