Abstract
ObjectivesTo measure geographic variations in treatment costs for specific conditions, explore the consistency of these patterns across conditions, and examine how service mix and population health factors are associated with condition-specific and total area costs.
Data SourcesMedicare claims for 1.5 million elderly beneficiaries from 60 community tracking study (CTS) sites who received services from 5,500 CTS Physician Survey respondents during 2004–2006.
Study DesignEpisodes of care for 10 costly and common conditions were formed using Episode Treatment Group grouper software. Episode and total annual costs were calculated, adjusted for price, patient demographics, and comorbidities. We correlated episode costs across sites and examined whether episode service mix and patient health were associated with condition-specific and total per-beneficiary costs.
Principal FindingsAdjusted episode costs varied from 34 to 68 percent between the most and least expensive site quintiles. Area mean costs were only weakly correlated across conditions. Hospitalization rates, surgery rates, and specialist involvement were associated with site episode costs, but local population health indicators were most related to site total per-beneficiary costs.
ConclusionsPopulation health appears to drive local per-beneficiary Medicare costs, whereas local practice patterns likely influence condition-specific episode costs. Reforms should be flexible to address local conditions and practice patterns.
Keywords: Geographic cost variation, Medicare costs, physician practice patterns, episodes of care
New forms of payment for health care services, such as the use of global payment models for accountable care organizations (ACOs), encourage sponsoring organizations to identify opportunities to improve quality and reduce costs. Cost savings can be achieved through systemic care process reforms but are likely to involve identification of specific clinical conditions where local care practices can be improved. However, the general impression one draws from the geographic variations literature is that that there are high-and low-cost areas where nearly all health care is provided inefficiently and efficiently, respectively. Indeed, some have asserted that if higher spending areas could change their practices to mimic lower spending areas, up to 30 percent could be saved without reducing care quality (Wennberg, Fisher, and Skinner 2002; Skinner and Fisher 2010).
Yet other research suggests that geographic variations in medical care are more complex. Service mix delivered to Medicare patients varied considerably across both high-and low-cost areas (Reschovsky et al. 2012); geographic variations in the cost of treating Medicare and privately insured patients were not highly correlated (Chernew et al. 2010); and the costs of six condition-specific episodes of care varied considerably across 13 areas, high cost for some conditions and low cost for others (Medicare Payment Advisory Commission [MedPAC] 2006). Thus, emerging research suggests that there is substantial heterogeneity within as well as across regions, and that more nuanced approaches will be needed to control spending, rather than just focusing on regions that are high cost, overall. This mirrors preliminary results from an Institute of Medicine report to be published later this year (Newhouse and Garber 2013).
In this study, we extend MedPAC’s study by both expanding the number of conditions and local markets examined, and exploring the associations between service mix and patient health factors with geographic variations in the costs of treating specific episodes. We also explore how area episode costs are related to overall costs in an area.
We confirm and extend MedPAC’s finding of little consistency in geographic cost variations across treatment of different conditions. We also show that episode cost variation across sites suggests differing practice patterns for those particular conditions. However, when explaining why some sites have higher per-beneficiary costs than others, we offer evidence that variation in population health, not physician diagnostic or treatment patterns, appears to be the major driver. Our results focus only on the cost side of the “value” question as quality information is not available. Nevertheless, cost variations are likely to substantially affect the efficiency by which care is provided across areas.
Background
Medical care is characterized by incomplete and asymmetric information on the best treatment alternatives for particular conditions, which leads to a principal–agent relationship: the principal (the patient) entrusts an agent (the physician) with responsibility to make treatment recommendations affecting the principal. However, the recommendations may be influenced by the agent’s self-interest.
Wennberg, Fisher, and Skinner (2002) build on the principal–agent problem to group health services into categories that can explain geographic cost variations: “effective care” for which well-established evidence of efficacy (and agreement on correct treatment approach) exists, “preference-sensitive” care in which the choice of treatment options is sensitive to patient preferences (but also that of physicians), and “supply-sensitive” services, where service provision is correlated with provider supply, implying provider-induced demand. In practice, demarcations among these categories are often unclear. While this typology acknowledges both demand-and supply-side factors, it presupposes that other patient demand factors, especially patient health status, have been controlled. Without robust control for patient health (case mix adjustment), correlations between provider supply and “supply-sensitive” service provision cannot distinguish between patient health-induced or provider-induced demand for medical care (Bernstein, Reschovsky, and White 2011). There is disagreement as to the best methods for case mix adjustment, and recent work suggests that population health may explain a larger portion of geographic variations than previously thought (Sutherland, Fisher, and Skinner 2009; Zuckerman et al. 2010; MedPAC 2011; Reschovsky, Hadley, and Romano 2013). This study sheds new light on the complexity of geographic variations in health care delivery by examining the consistency in relative treatment costs in an area among a variety of conditions. While descriptive and unable to disentangle supply-and demand-side factors definitively, it offers evidence on their likely relative importance in explaining overall cost variations in Medicare.
Data and Methods
Data
The Medicare claims data for this study were drawn from patients treated by about 5,500 physicians who responded to the 2004–2005 Community Tracking Study Physician Survey (Strouse et al. 2009). Conducted by telephone, the survey interviewed nonfederal physicians who have completed residency training and spend at least 20 hours per week in direct patient care (response rate = 52 percent). The survey sample was drawn from 60 local health care markets, whose populations are representative of the continental United States. Survey weights account for sample design and survey nonresponse.
The patient sample consisted of elderly, non-ESRD Medicare beneficiaries who were enrolled in the traditional fee-for-service Medicare program and who received at least one service (Medicare claim) from a physician survey respondent during the 3-year period 2004–2006. We obtained complete Part A and B claims submitted by all Medicare providers for the entire time period for each patient, resulting in 4,448,612 beneficiary/year observations.1 CTS physician survey data and Medicare claims were linked by matching Medicare’s Unique Physician Identifier Number (UPIN), which was present on all claims submitted by physicians.2 Weights were assigned to beneficiaries to make them representative of the elderly, non-ESRD population nationally.3
Creating Episodes of Care
We used the Symmetry Episode Treatment Groups (ETG), Version 6, to combine clinically related services (e.g., visits, tests, hospitalizations) delivered to a patient for a given condition into distinct episodes of care. The duration of acute condition episodes is defined by “clean periods” of no service, whereas chronic condition episodes are defined as calendar years.
MaCurdy et al. (2008) analyzed ETG and another commercial “grouper” on Medicare beneficiaries, finding that assigning or allocating costs for services such as hospitalizations to specific conditions posed difficulties for people with multiple comorbidities. Moreover, coding of principal diagnoses used in grouping claims may be inconsistent across care settings (e.g., hospitals and skilled nursing facilities).
These and other limitations led CMS to initiate development of a Medicare-specific episode grouper, which is due to be released in 2015. We therefore used the ETG grouper with several adaptations to make it more appropriate for our sample. The software subclassifies episodes by severity (i.e., surgeries, hospitalizations, and complications). We used broader episode definitions because severity may be sensitive to local practice behavior and coding. We also adjusted episode costs for patient health and combined similar defined episodes into broader categories to reduce potential variation attributable to discretionary coding as well as to address the comorbidity problem identified by MaCurdy et al. (2008). Specifically, we combined heart disease episodes (ischemic heart disease, chronic heart failure, atrial fibrillation/flutter, and valvular disorder episodes), COPD and asthma, and joint degeneration of the neck and back. Although these adjustments fail to address all concerns about use of a commercial grouper, robustness tests with and without case mix adjustment provided similar patterns of results, suggesting potential inaccuracies associated with the ETG grouper are unlikely to be sensitive to health or diagnostic testing differences across local health care markets.
We selected 10 frequent or costly clinical conditions for the analysis: two acute conditions (bacterial lung infections and inflammation of the esophagus) and eight chronic conditions (heart disease, joint degeneration of the knee or lower leg, joint degeneration of the back or neck, pulmonary disease [COPD and asthma], diabetes, cataracts, nonmalignant neoplasm of the prostrate, and chronic sinusitis). Episode sample sizes range from 103,903 to 1,149,943.
We calculated price-and case mix–adjusted site mean costs per condition-specific episode and formed quintiles of sites based on site mean costs. Sites were arrayed separately for each condition, as well as by total Medicare cost (for all services and conditions) per beneficiary. The smallest number of beneficiaries in a condition-specific quintile is 14,257. Quintile sample sizes are reported in Appendix Tables S4 and S5.
Episode costs were calculated as the sum of standardized costs for each specific service assigned to the episode by the grouper. Standardization eliminates payment differences in allowed charges embedded in Medicare rules for locality adjustments, provider payments to achieve other social goals (e.g., indirect medical education), annual reimbursement updates, and differing payment systems for identical services (e.g., cost-based vs. prospective reimbursement for classes of hospitals). See Appendix for details.
Controlling for Health Status
We controlled for patient health when calculating episode and total annual beneficiary costs by regressing costs on the variables from the Hierarchical Condition Category (HCC) risk adjustment model. These variables include age, sex, Medicaid status, indicators for 70 conditions, and interaction terms indicating coexisting conditions. Severity of beneficiaries’ specific conditions is not incorporated into the model. Claims-based case mix control in geographic variation studies, including use of the HCC, has been criticized for reflecting local diagnostic and coding patterns as distinct from underlying patient health (Fisher et al. 2003; Song et al. 2010; Welch et al. 2011). Consequently, we used a subset of previously identified HCC condition variables that omits or combines condition indicators possibly subject to physician testing and diagnostic discretion (Reschovsky, Hadley, and Romano 2013). The Appendix contains details on the full and modified HCC models. In robustness checks, we found that results were insensitive to whether case mix control was applied or the choice of HCC model. We report results using the modified HCC model. Detailed results with adjustment using the modified HCC and results without any case mix control are included in the Appendix.
Analyses
We first described beneficiary characteristics for the sample and by condition. Then we presented the geographic cost variation for each condition and for total costs per beneficiary, expressed as the ratio of health-adjusted mean costs among beneficiaries in the most expensive quintile relative to the least expensive quintile of sites. Specific CTS sites making up site quintiles vary across conditions. We then investigated whether CTS sites are consistently high or low cost across conditions by calculating a correlation matrix of adjusted site mean costs across the 10 conditions and examining the consistency of rank orderings of episode costs within individual sites.
We next examined correlates of episode-specific site costs by exploring whether a more expensive mix of services—consistent with greater preference-sensitive or supply-sensitive service use—and patient health were related to area costs of treating specific medical conditions. We measured service mix by the percent of evaluation and management visits provided by specialists, the percent of physician costs attributable to procedures and tests, and the percentages of episodes involving a hospitalization or surgery. We measured patient health by each condition’s annual site prevalence and by a patient’s comorbidity index, constructed from coefficients on site dummies from regressions of total costs on modified HCC variables (excluding those directly related to the condition in question) and site fixed effects. Finally, for chronic conditions, we measured the percent of annual episodes ending in death.4
To test associations, we assessed whether each measure was significantly different among beneficiaries in the most expensive quintile of sites as compared with beneficiaries in the least expensive quintile for each condition. We also examined whether relationships were monotonically increasing or decreasing across quintiles. Finally, using the same approach, we examined the association between episode costs and total costs.
Significance tests accounted for the presence of multiple observations on individual beneficiaries within our analysis sample and the physician sample’s complex design. Costs are expressed in 2006 dollars.
Results
Sample and Episode Characteristics
Table 1 shows weighted beneficiary characteristics. In our sample, 37 percent were aged 75–84 and 13 percent aged 85 or older, 60 percent were female, and 11 percent were racial or ethnic minorities. Mortality rates were under 5 percent annually.
Table 1.
Sample Characteristics
| Analysis Sample* | ||
|---|---|---|
| Unweighted Number of Observations | Percent (Weighted)† | |
| Age | ||
| 65–74 | 1,991,226 | 50.2 |
| 75–84 | 1,773,981 | 37.2 |
| 85+ | 589,217 | 12.6 |
| Sex | ||
| Male | 1,755,313 | 41.0 |
| Female | 2,599,111 | 59.0 |
| Race/ethnicity | ||
| White | 3,894,719 | 88.7 |
| Black | 322,401 | 7.9 |
| Other | 137,304 | 3.4 |
| % dual eligible | 458,091 | 10.8 |
| Region | ||
| Northeast | 925,657 | 18.8 |
| South | 1,661,201 | 40.2 |
| Midwest | 1,080,968 | 23.3 |
| West | 686,598 | 17.7 |
| Mortality rate (%) | 224,866 | 4.6 |
The analysis sample includes elderly Medicare beneficiaries who were enrolled in Parts A and B during the entire 2004–2006 period in which they were living, lived in the 60 Community Tracking Study sites, and were not living in a long-term care institution. Observations consist of beneficiary/year dyads.
Survey weights account for sample design and survey nonresponse.
Table 2 presents information on the 10 study conditions. Heart disease was the most expensive, averaging $5,163 per episode, followed by bacterial lung infections (pneumonia) ($3,439) and joint degeneration of the knee/lower leg ($3,433). Cataracts (23.4 percent) and heart disease (17.6 percent) were most prevalent. Total cost per condition (episode cost times prevalence) was highest for heart disease ($1.3 billion annually), over six times that of the next most expensive condition, joint degeneration of the knee and lower leg ($249 million). The percent of costs directly attributable to physicians varied widely among conditions, ranging from 16 to 72 percent. Patient disease burden (average modified HCC comorbidity index) varied across conditions, from 0.99 for cataracts to 2.25 for bacterial lung infections.
Table 2.
Correlation Matrix of Mean Medicare Costs for Ten Clinical Conditions and Total of All Conditions across Sixty Areas
| Mean Quintile Cost* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Sample Ave. Annual Treatment Costs ($Mill) | Annual Prevalence (%) | Ave. HCC Score | Percent Physician Costs | Ave. No. of Physicians Involved | Mean Adjusted Cost | Lowest Cost Quintile | Highest Cost Quintile | Ratio (High to Low) | |
| Acute conditions | |||||||||
| Bacterial lung infection | 74 | 1.5 | 2.25 | 16.0 | 4.1 | 3,050 | 2,453 | 3,735 | 1.52 |
| Inflammation of the esophagus | 17 | 2.1 | 1.05 | 54.3 | 2.0 | 487 | 406 | 572 | 1.41 |
| Chronic conditions | |||||||||
| Heart disease | 1,347 | 17.6 | 1.67 | 23.3 | 4.5 | 4,245 | 3,538 | 5,171 | 1.46 |
| Joint degeneration of the knee/lower leg | 249 | 4.9 | 1.02 | 21.5 | 2.4 | 3,227 | 2,513 | 3,927 | 1.56 |
| Joint degeneration of back or neck | 229 | 7.9 | 1.14 | 38.8 | 2.8 | 1,752 | 1,506 | 2,009 | 1.33 |
| COPD/asthma | 222 | 5.2 | 1.58 | 20.0 | 3.5 | 2,228 | 1,768 | 2,977 | 1.68 |
| Cataract | 221 | 23.4 | 0.99 | 51.2 | 1.3 | 611 | 507 | 737 | 1.45 |
| Diabetes | 150 | 9.9 | 1.21 | 46.2 | 2.4 | 866 | 755 | 1,008 | 1.34 |
| Nonmalignant neoplasm of the prostrate | 106 | 12.3 | 1.00 | 64.1 | 1.6 | 551 | 422 | 687 | 1.63 |
| Chronic sinusitis | 20 | 3.15 | 1.08 | 71.6 | 1.7 | 414 | 314 | 531 | 1.69 |
| Total beneficiary costs | – | – | 1.00 | – | – | 8,811 | 6,971 | 10,991 | 1.58 |
Costs were adjusted for patient health status using the modified Hierarchical Condition Category (HCC) model. Mean costs are per beneficiary and have been adjusted for Medicare prices and health status (comorbidities, age, and sex).
Episode and Total Beneficiary Cost Variation
Table 2 also shows mean costs in the highest and lowest cost quintiles of sites for each condition and total beneficiary costs. The ratio of adjusted costs in the most expensive quintile of sites to the lowest quintile ranged from 1.33 (joint degeneration of back/neck) to 1.69 (chronic sinusitis). The ratio for total cost per beneficiary was 1.58.5
Consistency of Relative Episode Treatment Intensity within Geographic Areas
Spearman correlations across condition-specific adjusted site cost means are shown in Table 3. Most (39 of 45) correlations were positive but relatively low in magnitude (<0.3). The largest correlation was 0.63, between COPD/asthma and bacterial lung infections, but the latter is often a complication of the former and both are likely treated by the same types of physicians. Costs per episode between the two orthopedic conditions, joint degeneration of the neck/back and knee/lower leg, had a correlation coefficient of 0.40. Correlations between condition-specific costs per episode and total cost per beneficiary were generally small and half were negative. Correlations of site means unadjusted for patient health provided similar results.
Table 3.
Correlations of Community Tracking Study Site Mean Costs per Episode of Treating Ten Clinical Conditions and Total Costs per Beneficiary
| Bact. Lung Infect. | Inflam. Esophagus | Heart Disease | Jt. Degen: Knee/L Leg | Jt. Degen: Neck/Back | COPD/Asthma | Cataract | Diabetes | Nonmal. Prostate | Chronic Sinusitis | Tot. Site Costs/Bene. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bact. lung inf. | 1 | ||||||||||
| Inflam. esophagus | 0.29 | 1 | |||||||||
| Heart disease | 0.43 | 0.39 | 1 | ||||||||
| Jt. degen: knee/L. leg | 0.19 | 0.52 | 0.51 | 1 | |||||||
| Jt. degen: back/neck | 0.21 | 0.48 | 0.27 | 0.40 | 1 | ||||||
| COPD/asthma | 0.63 | 0.19 | 0.59 | 0.28 | 0.09 | 1 | |||||
| Diabetes | 0.00 | 0.20 | −0.12 | −0.17 | 0.26 | 0.05 | 1 | ||||
| Cataract | 0.17 | 0.04 | 0.05 | 0.10 | 0.13 | 0.32 | 0.15 | 1 | |||
| Nonmal. prostrate | −0.07 | 0.19 | 0.08 | 0.23 | 0.08 | 0.10 | 0.10 | 0.06 | 1 | ||
| Chronic sinusitis | 0.02 | 0.25 | −0.16 | 0.12 | 0.20 | −0.06 | −0.01 | 0.14 | 0.05 | 1 | |
| Tot. site costs/bene. | −0.11 | 0.15 | −0.14 | −0.33 | 0.06 | −0.21 | −0.06 | 0.44 | 0.06 | 0.03 | 1 |
Note Bolded correlation coefficients are 0.4 or greater in value.
No site was in the most costly quintile for all 10 conditions or in the least costly quintile for all 10 conditions. Most sites had at least one condition in its most costly episode-specific quintile and another in its least expensive quintile. Only four of the 60 sites had a majority of the 10 conditions falling in their respective highest or lowest cost quintiles (data not shown).
Correlates of Site Episode Costs
The top half of Table 4 summarizes the relationships between service mix and health factors and cost per episode for each condition. (Detailed tables are in the Appendix.) The sign indicates whether the factor was statistically significantly greater (+) or lower (−) at p ≤ 0.05, among patients in the highest cost quintile of sites relative to the lowest cost quintile. The letter “M” indicates whether the relationship was monotonic across cost quintiles (i.e., steadily increasing or decreasing in value). Quintiles were specific to each condition.
Table 4.
Associations of Quintile Costs with Selected Service Mix and Patient Health Factors
| Ave. Episode Cost | Annual Prevalence | Ave. Modified HCC Score | % Episodes Ending in Death | Episode Includes Hospitalization | Episode Includes Surgery | % Specialist Visits | Percent % Physician Costs Testing/Procedural | |
|---|---|---|---|---|---|---|---|---|
| Quintiles formed by specific episode risk-adjusted costs | ||||||||
| Bacterial lung infection | +M | N/A | +M | +M | ||||
| Inflam. of the esophagus | +M | + | N/A | + | + | +M | ||
| Heart disease | +M | − | + | +M | +M | |||
| Jt. degen. knee/lower leg | +M | − | +M | +M | +M | |||
| Jt. degen. back or neck | +M | + | − | +M | +M | |||
| COPD/asthma | +M | + | +M | +M | ||||
| Cataract | +M | −M | +M | +M | ||||
| Diabetes | +M | +M | + | +M | + | |||
| Nonmalig. prostrate neoplasm | +M | + | +M | − | ||||
| Chronic sinusitis | +M | + | +M | + | + | |||
| Quintiles formed by total beneficiary risk-adjusted costs | ||||||||
| Bacterial lung infection | + | + | +M | N/A | + | +M | − | |
| Inflam. of the esophagus | + | +M | N/A | − | +M | + | ||
| Heart disease | +M | +M | − | − | + | + | ||
| Jt. degen. knee/lower leg | − | + | +M | − | − | − | ||
| Jt. degen. back or neck | +M | +M | − | +M | ||||
| COPD/asthma | +M | +M | +M | |||||
| Cataract | +M | + | ||||||
| Diabetes | +M | +M | + | + | +M | |||
| Nonmalig. prostrate neoplasm | +M | +M | + | + | ||||
| Chronic sinusitis | +M | + | +M | + | ||||
+ or − indicate the direction of the association with episode specific or total costs and that the mean among beneficiaries in the highest cost quintile of sites is significantly different from the mean among beneficiaries in the lowest cost quintile. M indicates that the relationship is monotonically rising or falling across quintiles. HCC, hierarchical condition category.
Episodes that included hospitalizations and surgery were most consistently positively and monotonically associated with higher cost of treating conditions. For a smaller number of episodes, costs were also associated with the percentage of evaluation and management visits by specialists and, relatedly, the percentage of physician costs that are procedural and testing in nature. Costs were mostly negatively, but insignificantly associated with episode prevalence in the site, and only monotonically and significantly associated with the patient comorbidity index for bacterial lung infections and diabetes. For the three major chronic conditions, heart disease, diabetes, and COPD, mortality rates were significantly and positively related to episode cost, but the relationships were not monotonic. In general, there was little consistency across conditions in the patterns of associations observed.
Correlates of Area Total Medical Cost per Beneficiary
The bottom half of Table 4 shows how the same set of episode and patient characteristics were associated with total cost per beneficiary. Quintiles were formed by total cost per beneficiary instead of condition-specific costs. Figure 1 illustrates these relationships for three factors: mean cost per episode, patient comorbidity index, and episode prevalence.
Figure 1.
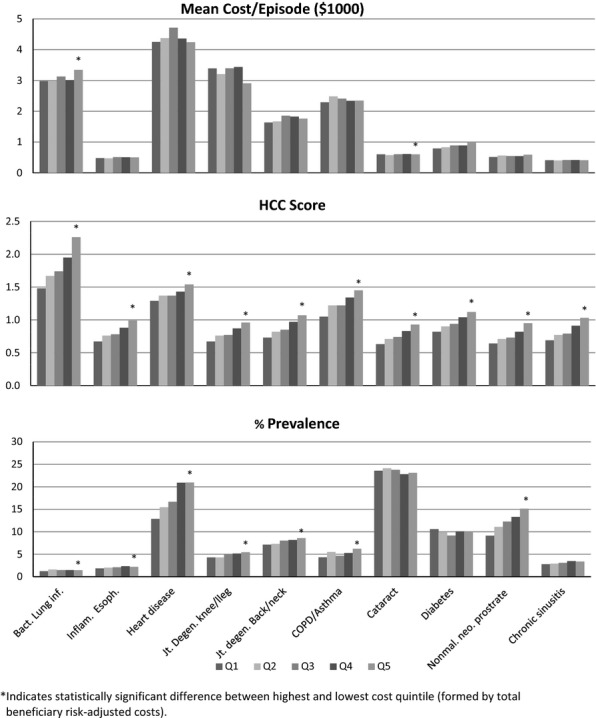
Average Episode Cost, Hierarchical Condition Category Score, and Annual Prevalence Rate for Ten Episodes of Care, among Quintiles of Community Tracking Study Sites, Ordered by Average Total Costs per Medicare Beneficiary
Consistent with the low and sometimes negative correlations in Table 2, when the sites were grouped by total per-beneficiary costs, cost per episode in quintile 5 was significantly greater than in quintile 1 for only two of the 10 conditions (diabetes and bacterial lung infection), significantly lower for one condition (joint degeneration of the knee/lower leg), and not significantly different for the other seven conditions. Only diabetes showed a monotonic and positive trend across total cost quintiles.
The only service mix variable clearly associated with total adjusted area costs was the percentage of specialist visits, but not hospital and surgery episode rates. The strongest association, however, was with the patient comorbidity index, which was positively, monotonically, and significantly associated with adjusted area per-beneficiary costs for all 10 conditions. This indicates that sites with higher average episode costs—though adjusted for comorbidities and patient demographics—also have populations that are, on average, sicker. The comorbidity indices (HCC scores) in Table 4 and Figure 1 exclude conditions directly related to the condition(s) represented by each particular episode type, so they represent the disease burden associated with comorbidities and patient age. Similarly, the prevalence of most conditions increased between low to high total cost quintiles for most of the conditions, although the relationships were significant and monotonic for only four of the 10 conditions.
Discussion
Many other studies demonstrate geographic variations in the cost of health care, which were largely thought to be related to differences in practice patterns. Our study adds to a growing body of literature that shows that area patterns of geographic variations in medical costs are far more complex than generally thought (MedPAC 2006; Chernew et al. 2010; Reschovsky et al. 2012; Newhouse and Garber 2013). Although the cost of treating specific conditions varies considerably across areas, cost patterns in the treatment of specific conditions within areas were not very consistent and were only weakly related to total costs in an area. Most communities were relatively expensive in the treatment of some conditions and inexpensive in the treatment of others.
Despite intrasite inconsistency across conditions, there were strong associations with the cost of treating specific conditions that apply to most or all of the 10 conditions examined here. Areas with high average adjusted episode costs for specific conditions often had higher rates of hospitalizations and (where relevant) surgeries for those conditions and, to a lesser degree, greater specialist involvement. Health-adjusted mean episode costs were not associated with the condition-specific HCC comorbidity index. We cannot discount the possibility that the associations were confounded by unmeasured variations in patient severity of the specific conditions. However, the insensitivity of our results to case mix adjustment (which includes age) point to practice pattern variations that were more inpatient and specialist oriented.
We found little relationship between the area-level costliness of treating specific conditions and the overall costliness of the site, despite the fact that treatment of most conditions in higher total cost areas was more specialist oriented. This could lead one to conclude that expensive and inexpensive practice patterns for different conditions offset each other within a site. However, there remains the considerably geographic variations in total adjusted cost per beneficiary (58 percent) reported in Table 2.
Our results suggest that beneficiaries in geographic areas with higher total cost per beneficiary are sicker and therefore have more episodes of care. The prevalence of most conditions we examined was significantly greater in high-cost areas, consistent with our finding of higher comorbidity (HCC) scores in those areas.
Alternatively, this could also reflect the proclivity of physicians to diagnose conditions (Fisher et al. 2003; Song et al. 2010; Welch et al. 2011). Greater diagnostic frequency could be a marker for local practice patterns leading to earlier diagnoses. If so, we would expect that average episode-specific costs would be negatively associated with prevalence of the condition in claims. That was only found for one of 10 conditions examined—cataracts. Thus, for the other conditions, more frequent diagnosis is not necessarily indicative of these conditions being diagnosed at an earlier less severe stage. Cataracts, in contrast, are frequently diagnosed but do not require surgical treatment until interfering with vision. Thus, it is not surprising that areas with a higher prevalence of cataract diagnoses also have lower average costs for these cases because a lower percentage require surgical treatment. Our findings were robust to whether costs were adjusted for patient health and across alternative case mix adjustment methods.
Although inferences drawn from cross-sectional descriptive comparisons are not conclusive, our findings are consistent with long-standing inferences drawn from the Dartmouth Atlas of Healthcare, and other research, that condition-specific episode costs may be related to local practice patterns. But evidence from treatment of specific conditions cannot be generalized to all medical care within geographic areas. Rather, practice patterns appear to be specific to the type of condition in question. Despite this, there still may remain considerable practice variations across physicians treating specific conditions within a community. For instance, previous research showed that individual physicians did not have a consistent pattern of use across a set of discretionary services (Landon et al. 2001).
Future research should attempt to determine factors that influence variations in local treatment patterns for specific conditions. For example, are treatment patterns related to the specialty training of physicians involved, the physicians’ practice organization, norms among physicians within referral networks, or the specific training of physicians? Do patient preferences, unmeasured patient severity, local reimbursement rates, and medical care market conditions specific to the treatment of the condition play a role?
Our work has several limitations. First, our sample of beneficiaries was indirectly drawn based on respondents to the CTS survey. Nonetheless, weighted sample characteristics closely matched national administrative data published by the federal government. Second, the commercial episode grouper proprietary software used to define episodes of care is not perfectly suited for the Medicare population (MaCurdy et al. 2008). We took steps to address the limitations, but other biases may exist. Our results could be affected if these biases were sensitive to local population or diagnostic differences. Results of robustness tests around patient health adjustment argue that this is not a strong possibility. Third, we investigated variations in costs but lack information on clinical outcomes. Therefore, we cannot address differences in the efficiency by which medical care is delivered across areas. High costs do not necessarily indicate inefficient care delivery. Indeed, the important role population health appears to play in explaining area cost variations suggests that conclusions from prior descriptive research that greater spending does not produce better patient outcomes may be confounded by the failure to adequately control for patient health status (Wennberg, Fisher, and Skinner 2002; Fisher et al. 2003; Baicker and Chandra 2004; Bernstein, Reschovsky, and White 2011; Reschovsky, Hadley, and Romano 2013). Finally, this is an observational descriptive study so causal inferences cannot be drawn. Results should be regarded as suggestive and future research should apply more rigorous methods to isolate demand and supply-side factors affecting medical use.
Policy Implications
Our results have implications for current payment and practice organization reforms. The predominant theme of current reform efforts is to link payment to value, that is, to both cost and quality. Although quality measurement has made great strides in recent years, data structures (e.g., electronic health record data) have constrained the extent to which clinical quality can be measured on a wide scale. In the near term, many reform policies will need to focus largely on costs of care. The Physician Feedback/Value-Based Modifier Program is beginning to send quality and resource use reports to physicians and practices treating Medicare patients, with payment tied to performance in future years. This may focus physician attention on the costs of treatment for episodes of common conditions that they have a primary or contributory role in treating. Informing physicians of their relative standing on cost and quality metrics with respect to treatment of specific conditions could motivate changes and more consistency in medical practice. This might be important because it is likely that within-area differences will be more important than between-area differences. A bundled payment demonstration being implemented by CMS is designed to incentivize Medicare providers to integrate their actions to reduce the cost of specific episodes of care, variously defined. Our results suggest that focusing attention on the treatment of a limited number of conditions may be an effective strategy to achieve delivery reform. On the other hand, bundled payments, which start with or are defined by inpatient admissions, do not provide incentives to avoid initial hospitalizations, which our results suggest are a key correlate to the cost of most episodes.
Finally, ACOs are designed to reward/punish groups of providers for the outcomes and costs of a defined population of Medicare beneficiaries. How well the local ACOs will work to identify specific conditions where cost savings are most readily achievable and whether they will be able to affect physician treatment patterns remain to be seen. Our results at the regional level are likely to carry over to individual ACOs. These results suggest that even high-performing ACOs with respect to overall costs will be able to identify specific areas of care where there may be potential savings because even low spending regions were high spending for some types of care (and vice versa). Thus, to achieve savings, ACOs will need a nuanced approach to target numerous treatment areas as well as across-the-board efforts to improve upon their care management and data infrastructures.
As indicated, future research should rigorously focus on the underlying reasons for practice variations in the treatment of specific conditions, both across areas as well as within local markets. Variations in the mix of services, costs, and outcomes offer opportunities to assess best clinical practices.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors wish to thank Cynthia Saiontz-Martinez of Social and Scientific Systems, Inc. for her excellent programming and data management support. This study was funded by a grant from the National Institutes of Aging (1R01AG027312) to Harvard University (Bruce Landon, PI). The Community Tracking Study, including the 2004–2005 Physician Survey, was funded by the Robert Wood Johnson Foundation.
Disclosures
None.
Disclaimers
None.
Notes
Part-year, Medicare Advantage, permanently institutionalized, and those not living in the 60 CTS sites were excluded from beneficiary/year observations.
UPINs were obtained for all respondents from survey’s sample frame, the AMA Masterfile. These served as a finder file in constructing the claims database.
Beneficiary weights adjusted the survey weight of the physician respondent that had contact with beneficiary for the number of unique physicians seen by each beneficiary across 2004–2006. Weighted beneficiary sample characteristics closely matched federal Medicare spending and mortality statistics, and site-level mean spending was highly correlated with CMS data.
Acute conditions death rates were not provided because of the “clean periods” used to define these episodes.
Median site cost variation was similar.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Author Matrix.
Table S1: Provides Description of the CMS-HCC Model and the Modified Version Developed by Reschovsky, Hadley, and Romano (2012).
Table S2: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Condition-specific costs.
Table S3: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Total Beneficiary Costs.
Table S4: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Condition-Specific Costs,Not Adjusted for Patient Health Status.
Table S5: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Total Beneficiary Costs, Not Adjusted for Patient Health Status (includes quintile sample sizes).
References
- Baicker K, Chandra A. Medicare Spending, the Physician Workforce, and Beneficiaries’ Quality of Care. Health Affairs. 2004 doi: 10.1377/hlthaff.w4.184. [accessed 6/8/13]. Available at http://content.healthaffairs.org/content/early/2004/04/07/hlthaff.w4.184.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- Bernstein J, Reschovsky JD, White C. Washington, DC: National Institute for Health Care Reform; 2011. [accessed on September 13, 2011]. Available at http://www.nihcr.org/Geographic-Variation.html Geographic Variation in Health Care: Changing Policy Directions.” Policy Analysis No. 4. [Google Scholar]
- Chernew ME, Sabik LM, Chandra A, Gibson TB, Newhouse JP. Geographic Correlation between Large-Firm Commercial Spending and Medicare Spending. American Journal of Managed Care. 2010;16(2):131–8. [PMC free article] [PubMed] [Google Scholar]
- Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The Implications of Regional Variations in Medicare Spending. Part 1: The Content, Quality, and Accessibility of Care. Annals of Internal Medicine. 2003;138(4):273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- Landon BE, Reschovsky JD, Reed M, Blumenthal D. Personal, Organizational, and Market Level Influences on Physicians’ Practice Patterns: Results of a National Survey of Primary Care Physicians. Medical Care. 2001;39(8):889–905. doi: 10.1097/00005650-200108000-00014. [DOI] [PubMed] [Google Scholar]
- MaCurdy T, Kerwin J, Gibbs J, Lin E, Cotterman C, O’Brien-Strain M. Evaluating the Functionality of the Symmetry ETG and Medstat MEG Software in Forming Episodes of Care Using Medicare Data. Contractor’s Report to the Centers for Medicare and Medicaid Studies. Burlingame, CA: Accumen, LLC; 2008. [Google Scholar]
- Medicare Payment Advisory Commission. Report to Congress: Regional Variation in Medicare Service Use. Washington, DC: MedPAC; 2011. [Google Scholar]
- Medicare Payment Advisory Commission (MedPAC) Report to the Congress: Increasing the Value of Medicare. Washington, DC: MedPAC; 2006. ; June. [Google Scholar]
- Newhouse JP, Garber AM. Geographic Variation in Medicare Services. New England Journal of Medicine. 2013;368:1465–8. doi: 10.1056/NEJMp1302981. doi: 10.1056/NEJMp1302981 (March 23, 2013) [DOI] [PubMed] [Google Scholar]
- Reschovsky JD, Hadley J, Romano PS. Geographic Variation in Fee-for-Service Medicare Beneficiaries’ Medical Costs Is Largely Explained by Disease Burden. Medical Care Research and Review. 2013 doi: 10.1177/1077558713487771. epub ahead of print. doi: 10.1177/1077558713487771. [DOI] [PubMed] [Google Scholar]
- Reschovsky JD, Ghosh A, Stewart KA, Chollet DJ. Durable Medical Equipment and Home Health among the Largest Contributors to Area Variations in Use of Medicare Services. Health Affairs. 2012;31(5):956–64. doi: 10.1377/hlthaff.2011.0243. [DOI] [PubMed] [Google Scholar]
- Skinner J, Fisher E. “Reflections on Geographic Variations in the U.S. Health Care.”. The Dartmouth Institute for Health Policy and Clinical Practice [accessed on February 16, 2013] 2010. Available at http://www.dartmouthatlas.org/downloads/press/Skinner_Fisher_DA_05_10.pdf.
- Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional Variations in Diagnostic Practices. New England Journal of Medicine. 2010;363:45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse RC, Potter F, Davis T, Hall J, Williams S, Herbold E, Walsh J, Boukus E, Reschovsky JD. Washington, DC: Center for Studying Health System Change; 2009. Available at http://www.hschange.org/CONTENT/1085/1085.pdf HSC 2008 Health Tracking Physician Survey Methodology ReportTechnical Publication No. 99. [Google Scholar]
- Sutherland JM, Fisher ES, Skinner JS. Getting Past Denial–The High Cost of Health Care in the United States. New England Journal of Medicine. 2009;361:1227–30. doi: 10.1056/NEJMp0907172. [DOI] [PubMed] [Google Scholar]
- Welch HG, Sharp SM, Gottlieb DJ, Skinner JS, Wennberg JE. Geographic Variation in Diagnosis Frequency and Risk of Death among Medicare Beneficiaries. Journal of the American Medical Association. 2011;305(11):1113–8. doi: 10.1001/jama.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg JE, Fisher ES, Skinner JS. Geography and the Debate over Medicare Reform. Health Affairs. 2002;23:W96–114. doi: 10.1377/hlthaff.w2.96. [DOI] [PubMed] [Google Scholar]
- Zuckerman S, Waidmann T, Berenson R, Hadley J. Clarifying Sources of Geographic Differences in Medicare Spending. New England Journal of Medicine. 2010;363:54–62. doi: 10.1056/NEJMsa0909253. ; July 1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Matrix.
Table S1: Provides Description of the CMS-HCC Model and the Modified Version Developed by Reschovsky, Hadley, and Romano (2012).
Table S2: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Condition-specific costs.
Table S3: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Total Beneficiary Costs.
Table S4: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Condition-Specific Costs,Not Adjusted for Patient Health Status.
Table S5: Detailed Treatment Cost and Other Factor Means among Beneficiaries in Sites Organized by Quintiles Formed by Total Beneficiary Costs, Not Adjusted for Patient Health Status (includes quintile sample sizes).


