Abstract
ObjectiveTo examine the roles of facility-and state-level factors in treatment facilities’ adoption and diffusion of pharmaceutical agents used in addiction treatment.
Data SourcesSecondary data from the National Survey of Substance Abuse Treatment Services (N-SSATS), Substance Abuse and Mental Health Services Administration (SAMHSA), Centers for Medicare and Medicaid Services, Alcohol Policy Information System, and Kaiser Family Foundation.
Study DesignWe estimate ordered logit and multinomial logit models to examine the relationship of state and treatment facility characteristics to the adoption and diffusion of three pharmaceutical agents over 4 years when each was at a different stage of adoption or diffusion.
Data CollectionN-SSATS data with facility codes, obtained directly from SAMHSA, were linked by state identifiers to the other publicly available, secondary data.
Principal FindingsThe analysis confirms the importance of awareness and exposure to the adoption behavior of others, dissemination of information about the feasibility and effectiveness of innovations, geographical clustering, and licensing and accreditation in legitimizing facilities’ adoption and continued use of pharmacotherapies in addiction treatment.
ConclusionsPolicy and administrative levers exist to increase the availability of pharmaceutical technologies and their continued use by substance abuse treatment facilities.
Keywords: Substance abuse treatment, diffusion, policy
A substantial body of research confirms the effectiveness of addiction medications for alcohol and opioid use disorders, yet the adoption of their use in treatment continues at a slow pace (Brown and Flynn 2002; Thomas and McCarty 2004; Knudsen and Roman 2012). In addition, treatment practices are being strongly influenced by managed care and related economic pressures to limit services, which further diminishes interest in adopting new evidence-based practices that are viewed as increasing pressure to “do more with less” (Drake et al. 2001; Goldman et al. 2001; Simpson 2002). Research suggests that barriers or supports to transferring research and evidence-based technologies to practice increasingly operate at policy, environmental, and organizational levels (e.g., regulatory and financial pressures, insurance coverage, inter-organizational linkages, organizational climate, staffing and resources, management structures, and philosophies) (Backer 1995; Harris and Thomas 2004; Fuller et al. 2005; Kovas et al. 2007; Simpson, Joe, and Rowan-Szal 2007).
Research that explores the adoption and diffusion of evidence-based innovations in addiction treatment has largely focused on the application of alternative methodologies of technology transfer (e.g., staff training) and other program and staff characteristics associated with adoption and diffusion (Simpson 2002; Roman, Ducharme, and Knudsen 2006). In this study, we examine the adoption and diffusion of three evidence-based addiction medications1 —disulfiram, naltrexone, and buprenorphine—in substance abuse treatment facilities over time, estimating the influence of state policies and other external factors, as well as internal treatment facility characteristics.
Each of these three addiction medications varies in terms of the particular condition it is intended to treat and its stage of adoption. Disulfiram has been used for decades to treat alcohol dependence; naltrexone was first approved by the Food and Drug Administration (FDA) in 1984 as a treatment for opioid dependence and then later (in 1994) as an adjunct to the treatment of alcohol dependence; and buprenorphine was approved by the FDA for treatment of opioid dependence in 2002. To date, studies of these addiction medications have focused primarily on treatment programs as the unit of analysis, exploring the relationships of internal organization characteristics (size, age, staff education and training, ownership status, affiliations, services, etc.) to the adoption or use of these medications. Although this research confirms that treatment facility attributes are important predictors of the use of evidence-based addiction medications (Roman and Johnson 2002; Fuller et al. 2005; Knudsen et al. 2005b; Knudsen, Ducharme, and Roman 2006; Roman, Ducharme, and Knudsen 2006; Ducharme et al. 2007), few studies have explicitly considered the role of interorganizational and other external professional, policy, and environmental factors on adoption and diffusion. In addition, a majority of existing studies use a single cross-section of data in their empirical analyses, which, along with limited samples of treatment organizations, have contributed to some initial conflicting findings in the literature (Schmidt et al. 2012).
The broader theoretical literature on the adoption and diffusion of innovations identifies important roles for external pressures and environmental influences, in addition to organizational characteristics (Berry and Berry 1990; Damanpour 1991; Borins 1998; Rogers 2003). Summarizing key theoretical tenets, awareness (“early knowing”) and salience of innovations is an important first step, and dissemination of evidence showing that the use of an innovation is feasible and effective is critical. Research across substantive areas suggests that exposure to this type of information and the adoption behavior of others through direct ties and indirect, structural correspondence (i.e., “environmental scanning”) influences the adoption of new practices, with the threshold for knowledge transfer lower in more collaborative settings. In other words, the social and communication structures in which treatment organizations operate and the norms (or patterns of behavior) they establish will affect the rate of adoption and diffusion (Rogers 2003). Both the number of adopters, as well as the proximity of adopting entities, also influences the likelihood that a particular organization will decide to implement an innovation (Walker 1969; Berry and Berry 1990, 1992).
As more organizations adopt over time, social pressures to conform increase, along with the perceived legitimacy of adopting, which in turn reduces costs associated with evaluating evidence on technologies. As innovations and related policies gain legitimacy and are adopted in neighboring jurisdictions, they are more likely to be viewed as viable solutions to problems, even if politically or socially contentious (Jensen 2003). States thus have the potential to play important roles in allocating resources for disseminating research evidence and in encouraging and legitimizing adoption of addiction medications, through, for example, their identification on state Medicaid drug formularies and other Medicaid/managed care policies that promote their use (Harris and Thomas 2004). Licensing, accreditation, and oversight bodies likewise have a role in encouraging innovation by requiring evidence of quality improvement, reinforcing professional norms that support adoption of innovations, and influencing the capacity-building efforts of potential adopters (Oser and Roman 2007). Learning over time can also reduce the level of uncertainty surrounding an innovation and improve conditions for adoption (Mooney 2001). In addition, third-party initiatives intended to increase interorganizational connections and treatment facilities’ familiarity with these medications, such as the Clinical Trials Network, Drug-Free Coalitions, and Screening and Brief Intervention efforts, may also hold promise for increasing their adoption and diffusion (Oser and Roman 2007; Rieckmann, Kovas, and Rutkowski 2010).
Timing and rate are other elemental variables in the modeling of diffusion of innovations. Among the addiction medications in the time period we examine, none had reached a “majority” adopters stage, and the adoption rates of the medications in use longer (disulfiram and naltrexone) leveled off at relatively low levels. Still, the low or stable year-to-year levels of adoption of addiction medications obscure variation in facilities’ offering of them over time. Once a facility has adopted an addiction medication—that is, it reports offering the medication to patients at the time we observe it in our sample—what factors contribute to continued availability of this medication at the facility in subsequent years, or to the adoption of this same medication by other facilities that were not offering the medication at the time we first observed them (i.e., diffusion)? Research suggests that the compatibility of innovations—the degree to which they are believed to be consistent with values, objectives, capabilities, and client needs—and the costs and difficulty of using them are important determinants of their adoption and continued availability (Rogers 2003).
An important limitation of prior research is a lack of information on policy and environmental factors and how changes in external factors influence adoption and diffusion over time. Among the different variables identified above as important to diffusion (e.g., public policies and incentives for investments in innovations and their dissemination, monitoring and legitimization, environmental scanning and interorganizational ties/networks, and other structural and environmental factors), few studies have constructed measures of these factors at a level other than the organization/treatment center. For example, Knudsen and Roman (2004) defined “environmental scanning” based on treatment center administrators’ estimates of the extent to which their staff drew their knowledge of treatment innovations from publications and professional associations. National Treatment Center Study researchers measured the diffusion of buprenorphine using counselor reports of their knowledge of the effectiveness of the treatment at single point in time; a “don’t know” answer was coded as indicating “lack of diffusion” (Knudsen et al. 2005a; Roman, Ducharme, and Knudsen 2006).
Others have also shown how public policies and factors such as increased cost containment pressures and shifts in ownership and funding interact with organizational characteristics to influence the internal environment of substance abuse treatment programs and treatment approaches (Zarkin et al. 1995; Schmidt and Weisner 1993; Heinrich and Fournier 2004). These studies suggest that state governments have the potential to influence the adoption of addiction medications and increase access to clinically proven, cost-effective treatments for substance abuse.
Data and Methods
We obtained access to 4 years (2002–2005) of data from the National Survey of Substance Abuse Treatment Services (N-SSATS), which, for these years only, include facility codes that allow us to track the adoption (observed offering) of addiction medications by treatment facilities and their diffusion (subsequent offering by facilities not initially offering the medication) within and across states over time.2 Since 2002, the N-SSATS has added new questions that enable us to overcome some limitations of prior research. We use the majority of facility-level measures as they appear in the original N-SSATS datasets (comprising a total of more than 54,000 facility observations over the 4-year study period).
The N-SSATS data were linked with state-level measures of factors identified as important to adoption and diffusion, including policy-led diffusion, incentives for investment in innovation (including coverage and financing), exposure to and legitimization of innovations, the number and proximity of adopters, interorganizational ties and professional networks, administrative structures and managerial/technical capacity, compatibility (with norms, values/culture, resources, etc.), and other environmental (political, social, and economic) factors. The primary sources for these data are the Centers for Medicare and Medicaid Services, SAMHSA, Alcohol Policy Information System, and the Kaiser Family Foundation. Table 1 shows descriptive statistics and data sources for measures included in the models presented in this study.3
Table 1.
Descriptive Measures and Data Sources for Measures Used in Analyses
| Data Sources | Variable | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| National Survey of Substance Abuse Treatment Services | Number of years antabuse offered | 0.687 | 1.282 | 0 | 4 |
| Number of years naltrexone offered | 0.494 | 1.087 | 0 | 4 | |
| Number of years buprenorphine offered | 0.205 | 0.616 | 0 | 3 | |
| Comprehensive assessment | 0.931 | 0.254 | 0 | 1 | |
| Group therapy | 0.904 | 0.295 | 0 | 1 | |
| Individual therapy | 0.949 | 0.221 | 0 | 1 | |
| Relapse prevention | 0.813 | 0.390 | 0 | 1 | |
| Outpatient substance abuse services | 0.807 | 0.395 | 0 | 1 | |
| Hospital inpatient substance abuse services | 0.078 | 0.267 | 0 | 1 | |
| Nonhospital residential substance abuse services | 0.275 | 0.446 | 0 | 1 | |
| Uses sliding fee scale | 0.637 | 0.481 | 0 | 1 | |
| Accepts Medicare | 0.357 | 0.479 | 0 | 1 | |
| Accepts Medicaid | 0.549 | 0.498 | 0 | 1 | |
| Contracts w/MCOs | 0.498 | 0.500 | 0 | 1 | |
| % clients treated for alcohol abuse only | 21.533 | 22.617 | 0 | 100 | |
| Accredited by JCAHO | 0.233 | 0.423 | 0 | 1 | |
| Licensed by State Mental Health Department | 0.306 | 0.461 | 0 | 1 | |
| Licensed by State Public Health Department | 0.390 | 0.488 | 0 | 1 | |
| Licensed by hospital licensing authority | 0.084 | 0.278 | 0 | 1 | |
| Midwest | 0.240 | 0.427 | 0 | 1 | |
| South | 0.270 | 0.444 | 0 | 1 | |
| West | 0.270 | 0.444 | 0 | 1 | |
| Primary focus: mental health services | 0.079 | 0.270 | 0 | 1 | |
| Primary focus: substance abuse/mental health mix | 0.266 | 0.442 | 0 | 1 | |
| Primary focus: general health care | 0.019 | 0.135 | 0 | 1 | |
| Primary focus: other | 0.014 | 0.116 | 0 | 1 | |
| For-profit | 0.266 | 0.442 | 0 | 1 | |
| Nonprofit | 0.592 | 0.491 | 0 | 1 | |
| Centers for Medicare and Medicaid Services | Medicaid state preferred drug list | 0.727 | 0.445 | 0 | 1 |
| Medicaid policy: generics required | 0.806 | 0.396 | 0 | 1 | |
| Medicaid policy: lower generic copays | 0.494 | 0.500 | 0 | 1 | |
| Medicaid policy: generics on PDL/formulary | 0.367 | 0.247 | 0 | 1.7 | |
| Substance Abuse and Mental Health Services Administration (SAMHSA) | Percent state budget spent on prevention, treatment, research | 0.503 | 0.500 | 0 | 1 |
| Number of drug-free coaltion grantees | 15.432 | 8.901 | 1 | 32 | |
| Number of clinical trials network organizations | 4.811 | 4.783 | 0 | 14 | |
| Alcohol tax collections for treatment | 0.118 | 0.323 | 0 | 1 | |
| Alcohol Policy Information System | Health insurers must offer coverage | 0.578 | 0.494 | 0 | 1 |
| Cannot impose greater copayments | 0.379 | 0.485 | 0 | 1 | |
| Health insurers exempt from some/all parity provisions | 0.055 | 0.227 | 0 | 1 | |
| Insurers prohibited from denying payment of insurance benefits | 0.089 | 0.285 | 0 | 1 | |
| SAMHSA | Screening and brief intervention grant | 0.291 | 0.454 | 0 | 1 |
| State websites | State agency director appointed by governor | 0.288 | 0.453 | 0 | 1 |
| Richard Fording | Citizen ideology index* | 54.905 | 11.239 | 28.24 | 91.24 |
| GIS computations | % of facilities per county adopting the pharmacotherapy | 9.517 | 13.361 | 0 | 100 |
Citizen Ideology (2006) by Richard Fording: http://www.uky.edu/~rford/stateideology.html.
Some recoding was required for several state-level measures, and not every measure was available for each state. The most common reasons for missing data were as follows: (1) the measure was not applicable to the state or the measure indicated the existence of a specific type of state law and the state did not have any law on the subject or (2) the state did not participate in the survey used to collect the information. In the first case, we recoded data to generate measures indicating that a given state had a law prohibiting a particular policy or allowing an exemption to a policy, whereas “0” in these cases indicated that the law did not prohibit the policy, did not allow an exemption, or was missing. In the second case, we assigned missing values when no information was available on a state policy for any year in a given state.4
Analyses of treatment facilities’ adoption (i.e., offering) of addiction medications over time in our sample show ranges of 16.3–17.5 percent for disulfiram, 11.7–12.9 percent for naltrexone, and 5.4–7.8 percent for buprenorphine (the latter for 2003–2005)—see the rows in bold in Table 2. However, these apparently stable year-to-year rates of offering these medications mask some variation in facility-level reports of their offering from year to year. Although it is possible that some of this variation could be attributed to changes in survey respondents (i.e., who completed the questionnaire each year), we further investigated this in our analysis.5 For example, in each year and for each medication, a nonnegligible fraction (28–42 percent) of facilities that were offering a given medication in the prior year did not report offering it in the subsequent year (see Table 2 again). Disulfiram has been available the longest and appears to be the most stable of the three medications in terms of the number of facilities that continue to offer it from year to year (approximately 70 percent). Although Table 2 shows that the fraction of treatment facilities that are new adopters from year to year is more variable, we also observe that the percent of facilities continuing to offer these treatments from year to year increases over time, especially for buprenorphine.
Table 2.
Rates of Adoption and Continuation/Discontinuation of Pharmacotherapies by Treatment Facilities
| Disulfiram | Naltrexone | Buprenorphine | |
|---|---|---|---|
| % of facilities adopting in 2002 | 17.52 (n = 11,592) | 12.92 (n = 11,518) | n.a. |
| % of 2002 adopters discontinuing in 2003 | 31.91 | 38.44 | n.a. |
| % of adopters that are new in 2003 | 30.22 | 35.49 | n.a. |
| % of facilities adopting in 2003 | 17.00 (n = 11,747) | 12.27 (n = 11,656) | 5.41 (n = 11,582) |
| % of 2003 adopters discontinuing in 2004 | 31.00 | 36.99 | 42.26 |
| % of adopters that are new in 2004 | 28.00 | 33.85 | 56.39 |
| % of facilities adopting in 2004 | 16.41 (n = 11,687) | 11.69 (n = 11,593) | 7.08 (n = 13,210) |
| % of facilities discontinuing in 2005 | 27.89 | 31.29 | 34.22 |
| % of adopters that are new in 2005 | 27.63 | 36.54 | 40.18 |
| % of facilities adopting in 2005 | 16.35 | 12.65 | 7.78 |
The period over which we examine the adoption and diffusion of addiction medications also includes variation in state Medicaid policies that we use to assess policy-led diffusion. In general, states were moving from 2002 to 2005 to make addiction medications more readily available, with more states requiring generics (up from 63 to 80 percent); establishing state preferred drug lists (PDLs)/formularies (58–73 percent) or including generics on PDLs (38–49 percent); lowering copays (41–49 percent) and paying generic rates for brand names (67–75 percent), while also limiting refills (56–63 percent) and restricting access to brand name drugs (61–100 percent), presumably to hold down Medicaid costs. Other state policies we examine include five measures of state health insurance parity laws (for alcohol-related treatment) that we expect would influence incentives for coverage and financing of substance abuse treatment (i.e., requiring coverage or the offer of coverage, prohibiting higher copays or the denial of benefit payments or exempting insurers from parity provisions). Prior research has shown lower rates of health insurance coverage for individuals discharged from hospitals with a principal diagnosis of alcohol-related mental and behavioral disorder. Research by Yi et al. (2007) found that although the risk of having no insurance coverage is five times higher for these individuals, this risk is reduced by approximately half for discharges in states that have “must cover” parity provisions.
Some of the state-level variables that we identified to measure external pressures to conform or encourage the adoption of these medications were only available for 1 or 2 years.6 For example, we measure the numbers or presence of organizations or support networks in states that might influence (encourage or legitimize) the adoption of addiction medications by treatment facilities, such as the number of Drug-Free Coalition Grantees and Clinical Trials Network Members in 2005, community treatment programs in 2004, operating drug courts, and Screening and Brief Intervention Grantees in 2004 and 2005. The implication is that we are limited in our ability to examine the role of these factors in diffusion (i.e., newly observed offerings of addiction medications over time), although we might still explore their association with the level of adoption by treatment facilities in the states.
We also constructed measures of the geographical proximity and density of treatment facilities adopting addiction medications over time to measure conformity pressures or supports for diffusion. Figures 1 and 2 present maps showing the density of adopters from year to year for naltrexone and buprenorphine (the same maps for disulfiram are omitted due to space constraints but are available from the authors).7 Counties with facilities offering the medication are shaded on a green-to-blue scale in increments of 10 percent (according to the percentage of facilities offering the medication); counties with no facilities offering the treatment are in white.8 Overall, our analyses and mapping of adoption density confirm that there is diffusion of these addiction medications taking place over time, and that the rate of new adoptions and continued availability varies both over time and across the three medications we study.
Figure 1.
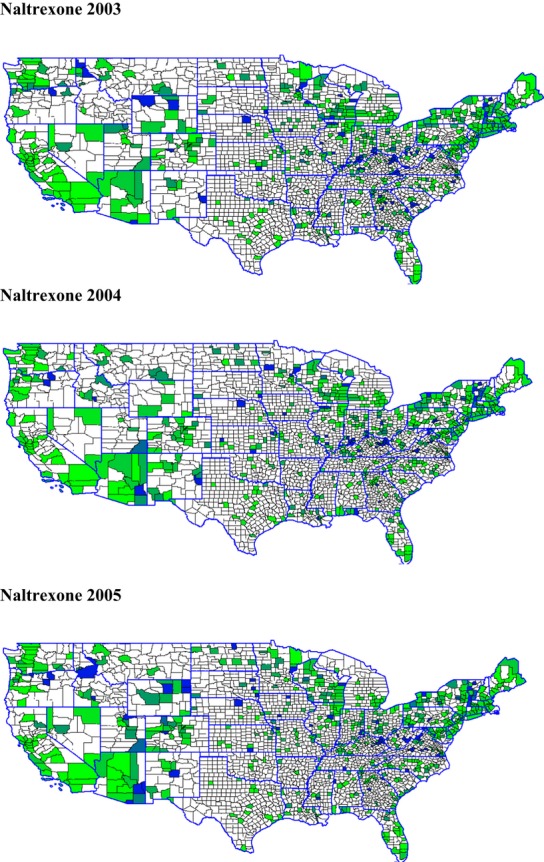
Percentage of Counties Adopting Naltrexone in the States, 2003, 2004, and 200511
Figure 2.
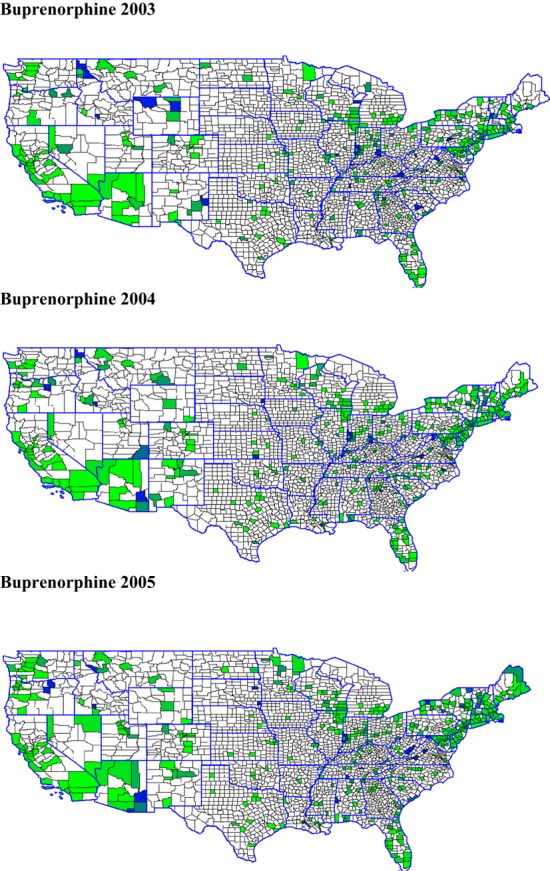
Percentage of Counties Adopting Buprenorphine in the States, 2003, 2004, and 2005
As the relationships we explore in the adoption and diffusion of addiction medications span multiple levels (substance abuse treatment facilities within states), we employed three estimation strategies that account for facilities’ clustering within states. We first predicted the number of years (over 2002–2005) that facilities offered these medications in ordered logit models and in multilevel models.9 We then estimated multinomial logit models, which allowed us to distinguish factors that predict facilities’ initial adoption (or adoption for just 1 year) from those that influence the offering of addiction medications over multiple years. As these three estimation strategies produced findings that were substantively very similar, our discussion focuses on the results of the multinomial logit models.
Results and Discussion
Treatment Facility-Level Findings
Table 3 presents the results of multinomial logit models that predict the adoption of addiction medications for 1, 2, 3, or 4 years, relative to the reference category of 0 years (i.e., not offered in 2002–2005), reported in odds ratios. Our expectations for relationships of a number of the facility-level variables to the adoption of these medications in substance abuse treatment are largely confirmed and are fairly consistent across the three medications (Friedmann, Alexander, and D’Aunno 1999; Fuller et al. 2005; Knudsen et al. 2005a,b2005b; Knudsen, Ducharme, and Roman 2006; Roman, Ducharme, and Knudsen 2006; Ducharme et al. 2007; Heinrich and Hill 2008). For example, one of the strongest, statistically significant relationships observed across the models is a positive association of accreditation of substance abuse treatment facilities by the Joint Commission on Accreditation of Health Care Organizations (JCAHO) and the adoption of these medications for 1 or more years. The odds of facilities adopting these treatments for just 1 (vs. 0) year are 52–76 percent higher if they are JCAHO-accredited; for 2 years versus 0 years, the odds are 112–178 percent higher, and for adoption over 3 or 4 years versus 0 years, the odds are 194–376 percent higher for JCAHO-accredited facilities. JCAHO accreditation is seen as a signal of higher quality services and may contribute to the perceived legitimacy of making these medications available (Oser and Roman 2007). In addition, licensing/certification by a hospital licensing authority (relative to a state substance abuse licensing agency) is likewise positively associated with adoption of all three medications over these years, which is expected given the well-known, important role of medical personnel in supporting the use of these medications in treatment. As with JCAHO accreditation, the associations with hospital licensing/certification are stronger (i.e., the odds of adopting or continuing to offer the medication are higher) for successive years of adoption.
Table 3.
Multinomial Logit Model of Number of Years Facilities Offered Addiction Medications
| Years Offered Disulfiram | Years Offered Naltrexone | Years Offered Buprenorphine | Years Offered Disulfiram | Years Offered Naltrexone | Years Offered Buprenorphine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 6,522 | n = 6,433 | n = 7,227 | n = 6,522 | n = 6,433 | n = 7,227 | |||||||
| Facility and State Characteristics (Effects Reported in Odds Ratios) | Offered 1 Year versus 0 Years | Offered 2 Years versus 0 Years | ||||||||||
| Comprehensive assessment | 0.680 | 0.301 | 1.338 | 0.196 | 1.742 | 0.078 | 1.007 | 0.981 | 1.092 | 0.754 | 1.363 | 0.345 |
| Group therapy | 0.869 | 0.495 | 1.281 | 0.107 | 0.891 | 0.529 | 0.833 | 0.320 | 1.082 | 0.789 | 1.103 | 0.774 |
| Individual therapy | 0.886 | 0.648 | 1.355 | 0.143 | 1.210 | 0.388 | 1.422 | 0.356 | 1.386 | 0.356 | 1.471 | 0.339 |
| Relapse prevention | 1.045 | 0.829 | 1.256 | 0.124 | 1.685 | 0.004 | 1.516 | 0.009 | 2.370 | 0.003 | 1.774 | 0.019 |
| Outpatient substance abuse services | 1.444 | 0.001 | 1.024 | 0.852 | 1.208 | 0.375 | 1.735 | 0.005 | 1.424 | 0.083 | 1.079 | 0.689 |
| Uses sliding fee scale | 0.868 | 0.306 | 0.695 | 0.001 | 0.629 | 0.000 | 0.782 | 0.076 | 0.558 | 0.003 | 0.475 | 0.000 |
| Accepts Medicare | 1.421 | 0.019 | 1.720 | 0.000 | 1.481 | 0.009 | 1.641 | 0.000 | 1.918 | 0.000 | 1.778 | 0.000 |
| Accepts Medicaid | 1.273 | 0.147 | 1.148 | 0.339 | 1.506 | 0.001 | 1.528 | 0.002 | 0.915 | 0.633 | 0.619 | 0.004 |
| Contracts w/MCOs | 1.283 | 0.022 | 1.488 | 0.000 | 1.740 | 0.002 | 1.295 | 0.114 | 1.903 | 0.000 | 1.957 | 0.001 |
| % clients treated for alcohol abuse only | 1.002 | 0.454 | 1.005 | 0.059 | 0.999 | 0.670 | 1.009 | 0.009 | 1.000 | 0.940 | 0.990 | 0.042 |
| Accredited by JCAHO | 1.764 | 0.000 | 1.604 | 0.000 | 1.520 | 0.018 | 2.430 | 0.000 | 2.112 | 0.000 | 2.783 | 0.000 |
| Licensed by State Mental Health Department | 1.104 | 0.429 | 1.171 | 0.122 | 1.080 | 0.513 | 0.779 | 0.065 | 1.132 | 0.412 | 1.642 | 0.049 |
| Licensed by State Public Health Department | 1.286 | 0.012 | 1.036 | 0.735 | 1.323 | 0.054 | 1.333 | 0.027 | 1.240 | 0.187 | 1.592 | 0.003 |
| Licensed by hospital licensing authority | 1.712 | 0.000 | 2.328 | 0.000 | 2.100 | 0.000 | 1.317 | 0.260 | 1.933 | 0.005 | 1.818 | 0.023 |
| Midwest | 0.777 | 0.282 | 1.227 | 0.525 | 1.810 | 0.083 | 1.058 | 0.904 | 1.752 | 0.262 | 1.019 | 0.969 |
| South | 0.392 | 0.000 | 1.022 | 0.947 | 1.926 | 0.051 | 1.372 | 0.487 | 1.377 | 0.438 | 1.112 | 0.810 |
| West | 0.606 | 0.071 | 1.999 | 0.014 | 1.328 | 0.385 | 1.542 | 0.189 | 3.278 | 0.002 | 0.733 | 0.477 |
| Primary focus: mental health services | 1.355 | 0.128 | 1.781 | 0.001 | 1.087 | 0.731 | 2.153 | 0.001 | 1.076 | 0.771 | 0.435 | 0.012 |
| Primary focus: substance abuse/mental health mix | 1.763 | 0.000 | 2.001 | 0.000 | 1.108 | 0.419 | 1.940 | 0.001 | 1.523 | 0.035 | 0.515 | 0.009 |
| Primary focus: general health care | 1.518 | 0.354 | 1.099 | 0.771 | 0.813 | 0.616 | 3.617 | 0.005 | 2.617 | 0.015 | 1.339 | 0.417 |
| Primary focus: other | 0.418 | 0.228 | 0.599 | 0.489 | 0.345 | 0.267 | 0.457 | 0.460 | 1.881 | 0.246 | 0.000 | 0.000 |
| For-profit | 0.891 | 0.424 | 1.057 | 0.793 | 0.914 | 0.674 | 0.931 | 0.739 | 0.889 | 0.652 | 2.021 | 0.019 |
| Nonprofit | 0.588 | 0.000 | 0.869 | 0.256 | 0.553 | 0.002 | 0.527 | 0.000 | 0.598 | 0.031 | 0.639 | 0.063 |
| Medicaid state preferred drug list | 1.500 | 0.076 | 0.991 | 0.965 | 0.535 | 0.001 | 1.473 | 0.126 | 0.729 | 0.225 | 0.676 | 0.181 |
| Medicaid policy: generics required | 0.315 | 0.000 | 1.271 | 0.297 | 0.307 | 0.010 | 0.644 | 0.429 | 0.762 | 0.440 | 0.319 | 0.003 |
| Medicaid policy: lower generic copays | 1.275 | 0.105 | 1.185 | 0.311 | 1.078 | 0.755 | 1.042 | 0.871 | 0.710 | 0.263 | 1.105 | 0.793 |
| Medicaid policy: generics on PDL/formulary | 1.259 | 0.093 | 0.948 | 0.766 | 1.458 | 0.027 | 0.957 | 0.845 | 1.229 | 0.429 | 1.034 | 0.904 |
| % state budget spent on prevention, treatment, research | 2.156 | 0.001 | 1.112 | 0.546 | 1.404 | 0.174 | 1.321 | 0.390 | 1.122 | 0.651 | 1.284 | 0.349 |
| Number of drug-free coaltion grantees | 1.001 | 0.927 | 0.994 | 0.579 | 1.023 | 0.107 | 1.026 | 0.110 | 1.006 | 0.759 | 1.041 | 0.016 |
| Number of clinical trials network organizations | 1.071 | 0.002 | 1.018 | 0.336 | 0.990 | 0.714 | 1.008 | 0.838 | 0.989 | 0.767 | 0.992 | 0.828 |
| Alcohol tax collections for treatment | 1.102 | 0.621 | 0.712 | 0.129 | 2.214 | 0.000 | 0.948 | 0.857 | 0.495 | 0.010 | 1.268 | 0.465 |
| Health insurers must offer coverage | 1.033 | 0.868 | 1.322 | 0.120 | 1.087 | 0.706 | 0.839 | 0.535 | 0.854 | 0.685 | 0.924 | 0.753 |
| Cannot impose greater copayments | 1.677 | 0.000 | 0.822 | 0.190 | 0.898 | 0.533 | 1.242 | 0.315 | 1.124 | 0.621 | 0.737 | 0.152 |
| Health insurers exempt from some/all parity provisions | 0.360 | 0.023 | 0.876 | 0.634 | 0.311 | 0.034 | 0.588 | 0.305 | 0.326 | 0.176 | 0.348 | 0.211 |
| Insurers prohibited from denying payment of insurance benefits | 1.215 | 0.325 | 0.750 | 0.125 | 1.053 | 0.829 | 1.019 | 0.945 | 0.587 | 0.154 | 0.837 | 0.613 |
| Screening and Brief Intervention Grant | 0.795 | 0.127 | 0.612 | 0.005 | 0.967 | 0.884 | 0.677 | 0.073 | 0.432 | 0.023 | 0.439 | 0.021 |
| State agency director appointed by governor | 0.547 | 0.051 | 1.208 | 0.352 | 0.774 | 0.454 | 0.687 | 0.235 | 0.551 | 0.184 | 0.855 | 0.699 |
| Citizen ideology index | 0.970 | 0.000 | 0.994 | 0.481 | 1.005 | 0.616 | 1.013 | 0.413 | 1.025 | 0.010 | 0.990 | 0.391 |
| % of facilities per county adopting the pharmacotherapy | 1.041 | 0.000 | 1.045 | 0.000 | 1.080 | 0.000 | 1.064 | 0.000 | 1.058 | 0.000 | 1.098 | 0.000 |
| Years Offered Disulfiram | Years Offered Naltrexone | Years Offered Buprenorphine | Years Offered Disulfiram | Years Offered Naltrexone | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Facility and State Characteristics (Effects Reported in Odds Ratios) | Offered 3 Years versus 0 Years | Offered 4 Years versus 0 Years | ||||||||
| Comprehensive assessment | 1.162 | 0.628 | 0.823 | 0.495 | 10.456 | 0.013 | 1.368 | 0.377 | 1.970 | 0.088 |
| Group therapy | 1.090 | 0.684 | 2.411 | 0.035 | 0.914 | 0.756 | 2.063 | 0.042 | 1.419 | 0.200 |
| Individual therapy | 1.151 | 0.621 | 1.183 | 0.635 | 1.831 | 0.111 | 1.295 | 0.427 | 0.881 | 0.717 |
| Relapse prevention | 0.945 | 0.779 | 1.110 | 0.731 | 1.508 | 0.240 | 1.693 | 0.004 | 2.334 | 0.001 |
| Outpatient substance abuse services | 1.643 | 0.012 | 1.543 | 0.017 | 1.931 | 0.001 | 3.004 | 0.000 | 1.681 | 0.003 |
| Uses sliding fee scale | 0.748 | 0.059 | 0.555 | 0.006 | 0.504 | 0.000 | 0.601 | 0.002 | 0.561 | 0.002 |
| Accepts Medicare | 2.419 | 0.000 | 1.948 | 0.003 | 1.886 | 0.000 | 2.529 | 0.000 | 2.967 | 0.000 |
| Accepts Medicaid | 1.011 | 0.966 | 0.828 | 0.320 | 0.700 | 0.091 | 0.721 | 0.094 | 0.479 | 0.001 |
| Contracts w/MCOs | 1.696 | 0.001 | 2.214 | 0.000 | 2.227 | 0.001 | 1.375 | 0.077 | 1.869 | 0.000 |
| % clients treated for alcohol abuse only | 1.008 | 0.065 | 1.009 | 0.029 | 0.992 | 0.138 | 1.007 | 0.029 | 1.007 | 0.029 |
| Accredited by JCAHO | 3.584 | 0.000 | 4.756 | 0.000 | 2.942 | 0.000 | 3.994 | 0.000 | 3.921 | 0.000 |
| Licensed by State Mental Health Department | 1.096 | 0.511 | 1.164 | 0.397 | 0.924 | 0.690 | 0.824 | 0.179 | 0.838 | 0.290 |
| Licensed by State Public Health Department | 0.877 | 0.392 | 0.950 | 0.796 | 1.239 | 0.411 | 0.943 | 0.665 | 0.860 | 0.542 |
| Licensed by hospital licensing authority | 1.548 | 0.074 | 1.773 | 0.020 | 2.214 | 0.000 | 2.082 | 0.000 | 2.340 | 0.000 |
| Midwest | 0.839 | 0.585 | 0.616 | 0.211 | 0.506 | 0.148 | 0.572 | 0.061 | 0.478 | 0.028 |
| South | 0.887 | 0.759 | 0.823 | 0.610 | 1.665 | 0.321 | 0.838 | 0.532 | 0.698 | 0.265 |
| West | 1.905 | 0.047 | 2.282 | 0.027 | 1.707 | 0.245 | 2.572 | 0.000 | 2.206 | 0.008 |
| Primary focus: mental health services | 1.318 | 0.254 | 1.263 | 0.457 | 0.769 | 0.469 | 0.947 | 0.858 | 0.916 | 0.797 |
| Primary focus: substance abuse/mental health mix | 1.713 | 0.012 | 1.767 | 0.001 | 0.874 | 0.551 | 1.165 | 0.438 | 1.316 | 0.289 |
| Primary focus: general health care | 2.193 | 0.114 | 1.213 | 0.696 | 2.748 | 0.129 | 2.961 | 0.004 | 2.860 | 0.003 |
| Primary focus: other | 0.963 | 0.966 | 0.000 | 0.000 | 1.495 | 0.433 | 0.466 | 0.212 | 0.475 | 0.502 |
| For-profit | 0.801 | 0.483 | 0.895 | 0.762 | 2.855 | 0.001 | 0.398 | 0.000 | 0.436 | 0.001 |
| Nonprofit | 0.495 | 0.002 | 0.554 | 0.066 | 1.391 | 0.320 | 0.245 | 0.000 | 0.286 | 0.000 |
| Medicaid state preferred drug list | 0.846 | 0.465 | 0.703 | 0.103 | 0.535 | 0.048 | 0.614 | 0.022 | 0.539 | 0.009 |
| Medicaid policy: generics required | 0.781 | 0.646 | 1.071 | 0.809 | 0.089 | 0.000 | 0.594 | 0.276 | 2.639 | 0.092 |
| Medicaid policy: lower generic copays | 1.193 | 0.338 | 1.101 | 0.639 | 1.707 | 0.017 | 1.240 | 0.296 | 1.194 | 0.364 |
| Medicaid policy: generics on PDL/formulary | 1.279 | 0.256 | 0.904 | 0.628 | 1.887 | 0.010 | 0.944 | 0.725 | 0.646 | 0.021 |
| % state budget spent on prevention, treatment, research | 1.114 | 0.732 | 1.353 | 0.185 | 1.223 | 0.581 | 1.544 | 0.087 | 0.903 | 0.731 |
| Number of drug-free coaltion grantees | 1.005 | 0.816 | 1.027 | 0.111 | 1.023 | 0.111 | 1.016 | 0.321 | 1.051 | 0.002 |
| Number of clinical trials network organizations | 0.941 | 0.090 | 0.967 | 0.362 | 0.949 | 0.097 | 1.017 | 0.546 | 0.931 | 0.053 |
| Alcohol tax collections for treatment | 0.487 | 0.035 | 0.268 | 0.000 | 1.746 | 0.037 | 0.370 | 0.001 | 0.235 | 0.000 |
| Health insurers must offer coverage | 0.789 | 0.469 | 2.442 | 0.003 | 2.813 | 0.000 | 1.421 | 0.079 | 2.285 | 0.003 |
| Cannot impose greater copayments | 1.133 | 0.523 | 0.633 | 0.005 | 0.644 | 0.015 | 0.759 | 0.031 | 0.420 | 0.000 |
| Health insurers exempt from some/all parity provisions | 1.144 | 0.819 | 1.004 | 0.991 | 0.136 | 0.000 | 0.986 | 0.980 | 2.589 | 0.053 |
| Insurers prohibited from denying payment of insurance benefits | 1.686 | 0.090 | 0.850 | 0.683 | 1.464 | 0.309 | 0.897 | 0.618 | 1.449 | 0.170 |
| Screening and brief intervention grant | 1.006 | 0.983 | 0.586 | 0.002 | 0.698 | 0.074 | 0.400 | 0.000 | 0.411 | 0.000 |
| State agency director appointed by governor | 1.225 | 0.671 | 1.105 | 0.762 | 0.916 | 0.765 | 0.785 | 0.542 | 2.013 | 0.095 |
| Citizen ideology index | 1.014 | 0.252 | 0.979 | 0.010 | 0.985 | 0.275 | 0.994 | 0.558 | 0.984 | 0.223 |
| % of facilities per county adopting the pharmacotherapy | 1.068 | 0.000 | 1.067 | 0.000 | 1.111 | 0.000 | 1.078 | 0.000 | 1.073 | 0.000 |
Statistically significant coefficient estimates (at α = 0.05) are in bold.
Other facility-level factors that influence adoption of these three addiction medications include the availability of relapse prevention counseling and outpatient substance abuse services, which appear to be used in combination with or support of these medications, as commonly recommended by medical professionals. The highest odds ratio observed among these factors suggests that the odds of offering disulfiram for all 4 years are 200 percent higher if the facility is also offering outpatient substance abuse services. Facility-level treatment financing arrangements are also important predictors for all three medications. Consistent with prior research on participation in managed care arrangements and naltrexone adoption (Fuller et al. 2005), facilities that accept Medicare and contract with managed care organizations have significantly higher odds (up to 197 and 123 percent greater, respectively) of offering all three of these medical treatments. However, if they use a sliding fee scale for determining costs, the odds of adoption and continuing to offer the medications are about 30–52 percent lower.
One facility-level finding that differs across these medications is the role of organizational form, that is private for-profit or nonprofit facilities versus public organizations (i.e., federal, local, county, or community-operated facilities that make up the reference category) in addiction treatment. Past research has produced mixed findings, with some studies suggesting positive roles of for-profit organizations in adoption of these medications (Knudsen, Ducharme, and Roman 2006; Roman, Ducharme, and Knudsen 2006; Oser and Roman 2007), and other studies pointing to more limited payment arrangements and/or service offerings in for-profit facilities (Friedmann, Alexander, and D’Aunno 1999; Wheeler and Nahra 2000; Heinrich and Lynn 2002). The results in Table 3 show that for-profit treatment facilities are significantly less likely (compared with public facilities, the reference category) to offer disulfiram or naltrexone, while they are significantly more likely to adopt buprenorphine. During this study time period, the use of buprenorphine by medical professionals for treating opioid dependence had just been approved by the FDA (in 2002). One possible explanation of the differences in findings for naltrexone and disulfiram versus buprenorphine might relate to the timing of research investigations relative to the approval of these medical treatments. For example, prior to states taking policy actions to make these treatments more affordable (e.g., requiring generics), these treatments might be more likely to be offered in private for-profit treatment facilities that receive more of their revenues from patient fees and private insurance. Oser and Roman (2007) also suggest that for-profit facilities are more likely to possess the “financial slack” to absorb the start-up costs of early pharmacological adoption, in addition to the human resources required to pursue innovation and experimentation.
Findings on State Policies and Other State-Level Factors
The results in Table 3 indicate that facilities in states with Medicaid PDLs are less likely to adopt disulfiram, naltrexone, and buprenorphine and have significantly lower odds of doing so for multiple years (e.g., as much as 46 percent lower for buprenorphine and naltrexone). Table 3 also shows that state Medicaid policies have the strongest effects on the adoption and continued offering of buprenorphine over this period. While lowering generic co-pays and including generics on state PDLs/formularies significantly increase the odds of facilities offering buprenorphine over 3 years (by 71–89 percent), requiring generics significantly lowers the odds of adoption by more than 90 percent. We speculate that these relationships are stronger for buprenorphine (compared with disulfiram and naltrexone) over this period (2003–2005) because it had just received FDA approval for expanded use in addiction treatment.
In addition to state Medicaid policies, state policies requiring health insurance parity for alcohol-related treatment are also significantly related to facilities’ adoption and continued offering of these medications. Particularly for adoption of buprenorphine and naltrexone, state policies that require insurers to provide coverage for alcohol-related treatment significantly increase the odds of adoption and continued offering of these treatments over 3 to 4 years (by as much as 181 percent). On the contrary, state policies that restrict facilities from imposing greater copayments or coinsurance on alcohol-related treatment significantly reduce the odds that facilities offer buprenorphine and naltrexone, again particularly over multiple years.10 Exempting health insurers from some or all of the parity provisions is also negatively related (and statistically significantly, primarily for buprenorphine) to facilities’ adoption and continued offering of these medications.
Another interesting but mixed finding on state policies affecting treatment financing is the relationship between state alcohol tax collections dedicated for treatment and the adoption of these three medications. Facilities in states with alcohol tax collections dedicated for treatment have up to 121 percent higher odds of adopting buprenorphine (the direction of the relationship expected); however, the odds of them adopting disulfiram or naltrexone are reduced by about half if the state dedicates some portion of tax revenues to treatment. States that dedicate tax collections for treatment are also significantly more likely to restrict facilities from imposing greater copayments and to require generics, but they are less likely to allow lower copays for generics, suggesting that they may shift a larger share of the burden for making treatment affordable to the facilities.
Table 3 also shows statistically significant relationships among interorganizational, networking, and other external/environmental factors that theory suggests may be potentially important in explaining the adoption and diffusion of substance abuse treatment innovations. For example, as discussed, “early knowing” and exposure to innovations are expected to increase diffusion through both direct and indirect ties with other organizations. The number of Drug-Free Coalition grantees in the states increases the odds of adoption of all three medications; this variable is statistically significant for naltrexone and buprenorphine, with approximately 2 percent greater odds of adoption for each additional grantee. One might find it surprising to see some negative relationships between the number of Clinical Trials Network members and recipients of SAMHSA Screening and Brief Intervention grantees in the states and facilities’ adoption and continued offering of these medications. However, a comparison of their locations with the maps in Figures 1 and 2 showing geographical clustering of adoption suggests that some of these grants and networking efforts were being targeted to counties and (large) states where adoption and diffusion rates were relatively low, such as Texas, Illinois, Ohio, and Pennsylvania. As the allocation of Screening and Brief Intervention grants is first observed in our data in 2004, we do not expect to find effects of these interventions over the study period.
The distribution and density of treatment facilities offering disulfiram, naltrexone, and buprenorphine were included to capture the role of conformity pressures or the proximity of leading/legitimizing behavior by facilities in the adoption and diffusion of these medications. These variables were among the most influential and precisely estimated predictors of adoption over time. For each additional percentage of facilities in a given county that are offering addiction medications, the odds of a given facility adopting them are 4.4–11 percent higher. Indeed, the pattern of effects suggests that proximity of other adopters is even more important in increasing the odds that facilities continue to offer these medications in additional years. One can also “eyeball” these patterns in Figures 1 and 2, which show “darkening” (i.e., increased adoptions over the years) in the shaded areas that indicate clustering among facilities (and possible diffusion of these medications).
Conclusions
Our study findings confirm that policy makers and treatment facility managers have policy and administrative levers that they can use to increase the availability of evidence-based addiction medications and support their continued use by substance abuse treatment facilities. The most influential factors at both facility-and state-levels corroborate the tenets and implications of diffusion theory, pointing to the importance of awareness and exposure to the adoption behavior of others and to the dissemination of information about the feasibility and effectiveness of innovations in addiction treatment. We found clear evidence of the significance of geographical clustering of facility adoptions and their influence on continued offering of these medications over time, and the results also bear out the significant role that licensing and accreditation organizations such as JCAHO and hospital licensing authorities serve in legitimizing adoption of addiction medications. In addition, the significant, positive association of Drug-Free Coalition grants with facilities’ adoption of these medications is also encouraging and suggests their likely effectiveness in supporting and strengthening collaborations among communities, governments, and private organizations in addressing substance abuse problems.
The study findings also suggest that actions by state policy makers to make these medications more affordable to deliver as well as for patients to access are important for adoption and diffusion. We found that states that lower generic copays and include generics on their PDLs may increase the odds that facilities adopt and continue offering these treatments. If states require health insurers to provide coverage for alcohol-related treatments, the odds of facility adoption of addiction medications are also significantly higher, as long as they do not exempt insurers from some or all of the parity provisions. Furthermore, facilities that contract with managed care organizations are more likely to offer these medications, possibly reflecting both diffusion through exposure/collaboration and important roles that these organizations play in managing treatment costs.
Finally, about one third of the facilities stopped offering these addiction medications in a given year, even though state policies seemed to be moving in the right direction of making them more affordable and accessible. This suggests that states and organizations attempting to encourage use of these treatments need to not only promote their adoption but also their continued availability once they are offered. The factors discussed above—licensing and accreditation and proximity to other adopters that support legitimization, the role of financing arrangements in increasing affordability, and the availability of relapse prevention counseling and related therapies—were observed to be even more influential in the offering of these medications over multiple years than on their adoption for 1 year. These factors also appeared to be potentially more important for more recently approved treatments (e.g., buprenorphine), compared with a medication such as disulfiram that had long been in use and had more stable rates of new adoptions and continued availability.
This study is one of the first to examine and compare the adoption, continued offering, and possible diffusion of different medications over time that have been approved for addiction treatment. By linking facility-and state-level data and constructing GIS measures relevant to three different medications at different stages of adoption and diffusion, this study was able to explore how these factors might operate differently at different time points since their approval and initial adoption. However, there were also limitations in using these data. Access to data that allowed for the linking of N-SSATS treatment facilities over time was restricted to the years 2002–2005, and data were not available for some state-level measures every year. In addition, the adoption and diffusion of buprenorphine may be markedly different today compared with the timeframe of this study, although this does not imply that one cannot still learn from this period about adoption and diffusion patterns. Finally, the N-SSATS data do not include individual client information, which precludes the observation of client use of these addiction medications.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We are grateful for the funding support provided for this research by an Institute for Clinical and Translational Research (ICTR) Type 2 Translational Research Health Policy Assistantship Award and Regina Laughlin Scholar funds at the University of Wisconsin-Madison.
Disclosures
None.
Disclaimers
None.
Notes
In this study, we heretofore use “addiction medications” as a shortened form for “addiction medications used in treating alcohol and opioid use disorders,” which are also referred to in the literature as pharmaceutical agents or pharmacotherapies.
The specific N-SSATS questionnaire item that is used to construct the measures of addiction medication adoption (for each year in our sample) asks: Which of the following services are provided by this facility at this location? Under the “pharmacotherapies” subheading, the respondent can check any of the following: Antabuse, Naltrexone, or Buprenorphine (Subutex, Suboxone).
Detailed information on all data sources and measures is available from the authors upon request.
In a few cases for variables derived from the Kaiser Family Foundation surveys (in 2003 and 2005), we inferred 2005 values based on 2003 data. For cases in which a state participated in the 2003 survey but not in 2005, we assumed that a state would not reverse policies that lower the cost of Medicaid prescription benefits (as this was the trend observed for all other states with complete data). However, if a state did not have the cost-saving policy in 2003, we did not make any assumptions about their 2005 policies.
We suspect that patterns in which facilities apparently adopted, stopped, and then continued offering treatment (or vice versa) more likely reflect problems with consistency in respondents; this affected only about 2 percent of facilities for each pharmaceutical agent.
We also include a widely used state citizen ideology index developed by political scientists to measure the ideological “center of gravity” of a state government’s elected institutions.
To create these maps, we calculated (by year) the percentage of facilities per county that offered each addiction medication. We merged these percentages, by state and county FIPS code, to the 2009 county TIGER/Line® Shapefiles from the U.S. Census Bureau. We then mapped the percentages for each year and each addiction medication separately, using GRASS GIS Version 6.4.0svn and Quantum GIS Version 1.0. In addition, we used the 2009 state TIGER/Line® Shapefiles to outline state borders.
The green-to-blue shading may be viewed in electronic copies of this publication; the printed journal version shows the coloration in grayscale, with darker shading suggesting higher rates of addiction medication adoption.
In the ordered logit model, we included the values of explanatory variables for 2005, as facility-level characteristics were relatively unchanged over the study period, and 2005 was more likely to capture the direction in which policies were evolving. The ordered logit and multilevel model results, as well as additional details of the estimation, are available from the authors upon request.
The results suggest that the odds of adoption of disulfiram for just 1 (vs. 0) years are higher for facilities in states that restrict facilities from imposing greater copayments or coinsurance on alcohol-related treatment; however, this relationship is reversed for facilities offering disulfiram all 4 years (with the odds 24 percent lower for facilities in states that impose this restriction). For a medication such as this that has long been available, adoption for just 1 year over the 4-year period of this study might reflect a late or trial adopter that is unsuccessful in continuing to offer the medication.
For brevity, we do not show the graphic for naltrexone in 2002.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Author Matrix.
References
- Backer TE. Assessing and Enhancing Readiness for Change: Implications for Technology Transfer. In: Backer TE, David SL, Soucy G, editors. Reviewing the Behavioral Science Knowledge Base on Technology Transfer (NIDA Research Monograph 155, NIH Publication No. 95 – 4035) Rockville, MD: National Institute on Drug Abuse; 1995. pp. 169–85. edited by. [Google Scholar]
- Berry FS, Berry WD. State Lottery Adoptions as Policy Innovations: An Event History Analysis. American Political Science Review. 1990;84:395–415. [Google Scholar]
- Berry FS, Berry WD. Tax Innovation in the States: Capitalizing on Political Opportunity. American Journal of Political Science. 1992;36:715–42. [Google Scholar]
- Borins S. Innovating with Integrity: How Local Heroes Are Transforming American Government. Washington, DC: Georgetown University Press; 1998. [Google Scholar]
- Brown BS, Flynn PM. The Federal Role in Drug Abuse Technology Transfer: A History and Perspective. Journal of Substance Abuse Treatment. 2002;22(4):245–57. doi: 10.1016/s0740-5472(02)00228-3. [DOI] [PubMed] [Google Scholar]
- Damanpour F. Organizational Innovation: A Meta-Analysis of Effects of Determinants and Moderators. Academy of Management Journal. 1991;34:555–90. [Google Scholar]
- Drake R, Essock SM, Shaner A, Carey KB, Minkoff K, Kola L, Lynde D, Osher FC, Clark RE, Richards L. Implementing Dual Diagnosis Services for Clients with Severe Mental Illness. Psychiatric Services. 2001;52:469–76. doi: 10.1176/appi.ps.52.4.469. [DOI] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM, Johnson JA. Innovation Adoption in Substance Abuse Treatment: Exposure, Trialability, and the Clinical Trials Network. Journal of Substance Abuse Treatment. 2007;32(4):321–9. doi: 10.1016/j.jsat.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Alexander JA, D’Aunno TA. Organizational Correlates of Access to Primary Care and Mental Health Services in Drug Abuse Treatment Units. Journal of Substance Abuse Treatment. 1999;16:71–80. doi: 10.1016/s0740-5472(98)00018-x. [DOI] [PubMed] [Google Scholar]
- Fuller BE, Rieckmann T, McCarty D, Smith KW, Levine H. Adoption of Naltrexone to Treat Alcohol Dependence. Journal of Substance Abuse Treatment. 2005;28(3):273–80. doi: 10.1016/j.jsat.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Goldman HH, Ganju V, Drake R, Gorman P, Hogan M, Hyde PS, Morgan O. Policy Implications for Implementing Evidence-Based Practices. Psychiatric Services. 2001;52:1591–7. doi: 10.1176/appi.ps.52.12.1591. [DOI] [PubMed] [Google Scholar]
- Harris KM, Thomas C. Naltrexone and Pharmacy Benefit Management. Journal of Addictive Diseases. 2004;23(4):11–29. doi: 10.1300/J069v23n04_02. [DOI] [PubMed] [Google Scholar]
- Heinrich CJ, Fournier E. Dimensions of Publicness and Performance in Substance Abuse Treatment Organizations. Journal of Policy Analysis and Management. 2004;23(1):49–70. doi: 10.1002/pam.10178. [DOI] [PubMed] [Google Scholar]
- Heinrich CJ, Hill CJ. The Role of State Policies in the Adoption of Naltrexone for Substance Abuse Treatment. Health Services Research. 2008;43(3):951–70. doi: 10.1111/j.1475-6773.2007.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich CJ, Lynn LE. Improving the Organization, Management, and Outcomes of Substance Abuse Treatment Programs. The American Journal of Drug and Alcohol Abuse. 2002;28(4):601–22. doi: 10.1081/ada-120015871. [DOI] [PubMed] [Google Scholar]
- Jensen JL. Policy Diffusion through Institutional Legitimation: State Lotteries. Journal of Public Administration Research and Theory. 2003;13(4):521–42. [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM. Early Adoption of Buprenorphine in Substance Abuse Treatment Centers: Data from the Private and Public Sectors. Journal of Substance Abuse Treatment. 2006;30(4):363–73. doi: 10.1016/j.jsat.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM. Modeling the Use of Innovations in Private Treatment Organizations: The Role of Absorptive Capacity. Journal of Substance Abuse Treatment. 2004;26:51–9. doi: 10.1016/s0740-5472(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM. Financial Factors and the Implementation of Medications for Treating Opioid Use Disorders. Journal of Addiction Medicine. 2012;6:280–6. doi: 10.1097/ADM.0b013e318262a97a. [accessed on August 23, 2012]. Available at http://ctndisseminationlibrary.org/display/870.htm (in press) (4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM, Link TJ. Buprenorphine Diffusion: The Attitudes of Substance Abuse Treatment Counselors. Journal of Substance Abuse Treatment. 2005a;29:95–106. doi: 10.1016/j.jsat.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM, Ducharme LD, Johnson AJ. Organizational Predictors of Pharmacological Innovation Adoption: The Case of Disulfiram. Journal of Drug Issues. 2005b;35:553–68. [Google Scholar]
- Kovas A, McFarland B, McCarty D, Boverman J, Thayer J. Buprenorphine for Acute Heroin Detoxification: Diffusion of Research into Practice. Journal of Substance Abuse Treatment. 2007;32(2):199–206. doi: 10.1016/j.jsat.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Mooney CZ. Modeling Regional Effects on State Policy Diffusion. Political Research Quarterly. 2001;54(1):103–24. [Google Scholar]
- Oser CB, Roman RM. Organizational-Level Predictors of Adoption across Time: Naltrexone in Private Substance-use Disorders Treatment Centers. Journal of Studies on Alcohol. 2007;68(6):852–61. doi: 10.15288/jsad.2007.68.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann T, Kovas AE, Rutkowski BA. Adoption of Medications in Substance Abuse Treatment: Priorities and Strategies of Single State Authorities. Journal of Psychoactive Drugs. 2010;42(suppl 6):227–38. doi: 10.1080/02791072.2010.10400546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EM. Diffusion of Innovations. 5th edition. New York: Free Press; 2003. [Google Scholar]
- Roman PM, Ducharme LJ, Knudsen HK. Patterns of Organization and Management in Private and Public Substance Abuse Treatment Programs. Journal of Substance Abuse Treatment. 2006;31(3):235–43. doi: 10.1016/j.jsat.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Roman PM, Johnson JA. Adoption and Implementation of New Technologies in Substance Abuse Treatment. Journal of Substance Abuse Treatment. 2002;22:211–8. doi: 10.1016/s0740-5472(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Weisner C. Developments in Alcoholism Treatment. In: Galanter M, editor. Recent Developments in Alcoholism, Volume 11: Ten Years of Progress. New York: Plenum Press; 1993. pp. 369–96. edited by. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Rieckmann T, Abraham A, Molfenter T, Capoccia V, Roman P, Gustafson DH, McCarty D. Advancing Recovery: Implementing Evidence-Based Treatment for Substance Use Disorders at the Systems Level. Journal of Studies on Alcohol and Drugs. 2012;73(3):413–22. doi: 10.15288/jsad.2012.73.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD. A Conceptual Framework for Transferring Research to Practice. Journal of Substance Abuse Treatment. 2002;22:171–82. doi: 10.1016/s0740-5472(02)00231-3. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA. Linking the Elements of Change: Program and Client Responses to Innovation. Journal of Substance Abuse Treatment. 2007;33(2):201–9. doi: 10.1016/j.jsat.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, McCarty D. Adoption of Drug Abuse Treatment Technology in Specialty and Primary Care Settings. In: Harwood HJ, Myers TG, editors. New Treatments for Addiction: Behavioral, Ethical, Legal, and Social Questions. Washington, DC: The National Academies Press; 2004. pp. 140–72. edited by. [PubMed] [Google Scholar]
- Walker J. The Diffusion of Innovations among the American States. American Political Science Review. 1969;63:880–99. [Google Scholar]
- Wheeler JRC, Nahra TA. Private and Public Ownership in Behavioral Health Care: Do We Have a Two-Tiered System? Administration and Policy in Mental Health. 2000;27(4):197–209. doi: 10.1023/a:1021357318246. [DOI] [PubMed] [Google Scholar]
- Yi H, Chen CM, Yoon Y, Hilton ME. Effect of Health Insurance Parity Laws on Healthcare Coverage for Alcohol-Related Hospitalizations in the United States. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health; 2007. Working paper. [Google Scholar]
- Zarkin GA, Galinis DN, French MT, Fountain DL, Ingram PW, Guyett JA. Financing Strategies for Drug Abuse Treatment Programs. Journal of Substance Abuse Treatment. 1995;12:385–99. doi: 10.1016/0740-5472(95)02012-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Matrix.


