Abstract
ObjectiveTo describe trends in the use of percutaneous coronary intervention (PCI) following the COURAGE trial, which found that medical therapy is as effective as PCI for patients with stable angina.
Data SourcesWe used the National Hospital Discharge Survey; inpatient and outpatient discharge data from Florida, Maryland, and New Jersey; and the English Hospital Episode Statistics database.
Study DesignWe report trends in PCI volume by diagnosis (stable angina vs. unstable angina or AMI) before and after publication of the COURAGE trial.
Principal FindingsThe number of PCIs in patients without a diagnosis of AMI or unstable angina in Florida, Maryland, and New Jersey declined from 48,000 in 2006 to 40,000 in 2008 (−17 percent). There was no change in the number of PCIs in patients with a diagnosis of AMI. We observed similar patterns in U.S. community hospitals. PCI volume did not decline in England.
ConclusionsPCI volume declined after publication of the COURAGE trial. The experience of the COURAGE trial suggests that comparative effectiveness research can lead to cost-saving changes in medical practice patterns. However, there are many patients with stable coronary disease who continue to receive PCI post-COURAGE.
Keywords: Medical decision making, access, demand, utilization of services, technology adoption, diffusion, use
When a major study finds that a widely used medical treatment is no better than a less expensive alternative, do physicians stop using it? The premise behind comparative effectiveness research is that expensive treatments often diffuse into clinical practice without evidence that they are better than existing therapies (Chandra, Jena, and Skinner 2011). Policy makers hope that comparative effectiveness studies will identify ineffective but costly treatments, leading to cost savings. However, it remains unclear how studies that report “negative” results influence medical treatment patterns. The same factors that promote rapid adoption of new medical technologies—fee-for-service reimbursement, third-party payment, etc. (Emanuel and Fuchs 2008)—may retard the abandonment of widely used technologies found to be ineffective.
Percutaneous coronary intervention (PCI) is an invasive procedure to open clogged coronary arteries. Medicare spent over $5 billion on PCIs in 2005 (Committee on Finance, United States Senate 2010). In 1999 the Department of Veterans Affairs initiated a multimillion dollar study, the COURAGE trial, to compare PCI and medical therapy versus a regimen of medical therapy alone in patients with stable angina. The study, which was published in April 2007 in the New England Journal of Medicine (Boden et al. 2007), found that the treatments were equivalent in terms of survival time and heart attack risk.
Articles in the Wall St. Journal (Weinstein 2010) and the Washington Post (Brown 2012) used the COURAGE trial to illustrate the challenges facing policy makers who want to rein in costs using comparative effectiveness research. However, there has been little research on the impact of the COURAGE trial on practice patterns. In this study, we assess trends in the use of PCI by indication following publication of the COURAGE trial.
Technological abandonment
Many technologies diffuse into clinical practice without evidence that they are effective. David Eddy (1993) summarized the prevailing mindset with respect to new technologies as: “When in doubt, do it.” Subjecting established medical practices to trials can identify treatments that are no better than less expensive, less invasive alternatives. Prasad, Cifu, and Ioannidis (2012) reviewed all studies published in the New England Journal of Medicine in 2009 and found that reversals—studies reporting negative findings for established medical practices—are common, constituting nearly half of studies that evaluated established practices. Presumably, the number of reversals will increase as the newly formed Patient-Centered Outcomes Research Institute begins to fund comparative effectiveness studies.
Many observers are pessimistic about the potential impact of studies reporting negative results. Once established, treatments may be difficult to dislodge. The same factors that promote rapid diffusion of new health care technologies—fee-for-service reimbursement, third-party payment, and the technological imperative in modern medicine (Phelps 1992; Emanuel and Fuchs 2008)—may retard the abandonment of widely used technologies found to be ineffective. Treatment decisions may be subject to inertia, and, in some cases, entire specialties are defined by the delivery of a specific technology (Schroeder and Showstack 1979). Specialists have a “pro-intervention bias” (Timbie et al. 2012) and may ignore “inconveniently negative” results (Lenzer 2012).
A handful of studies have examined the impact of comparative effectiveness research studies reporting negative results. Examples of technologies where the use of the treatment continued unabated after the publication of negative results include percutaneous coronary angioplasty (PCI) in patients with occluded infarct-related arteries identified more than 24 hours postmyocardial infarction (Deyell et al. 2011), calcium channel blockers, and angiotensin-converting enzyme inhibitors as first-line treatments for patients with hypertension (Stafford et al. 2006), radiotherapy in older women with smaller, early-stage breast tumors (Soulos et al. 2012), and directional coronary atherectomy (Omoigui et al. 1998). These cases stand in stark contrast to instances where positive results for new technologies have led to rapid adoption, in some cases even before the results have been published (Gross et al. 2000; Giordano et al. 2006).
In some cases negative results influence practice patterns. Trials reporting negative results for intermittent positive pressure breathing therapy (Duffy and Farley 1992), high-dose chemotherapy/hematopoietic cell transplants for women with breast cancer (Howard et al. 2011), PCI for patients with stable angina (Howard and Shen 2012), and arthroscopic surgery for osteoarthritis of the knee (Howard, Brophy, and Howell 2012) have led to reductions in the use of these procedures.
The COURAGE Trial
Chronic stable angina is characterized by chest pain during exertion caused by a narrowing of the coronary arteries. Treatment options include revascularization via PCI or a low-cost regimen of medical therapy and lifestyle modification. PCI entails cardiac catheterization, use of balloon angioplasty to mechanically open the artery, and, in most cases, placement of a stent to maintain blood flow. The Department of Veterans Affairs–sponsored Clinical Outcomes Using Revascularization and Aggressive Drug Evaluation (COURAGE) trial randomized 2,287 patients with stable angina to receive optimal medical therapy alone or PCI plus medical therapy between 1999 and 2004. The main finding from the COURAGE trial was that there was no difference between treatment arms in the incidence of mortality or acute myocardial infarction (AMI) (Boden et al. 2007). The results “shook the world of cardiology” (Weinstein 2010).
A subsequent analysis found that although patients in the PCI arm experienced earlier resolution of symptoms, quality of life was no different after 3 years (Weintraub et al. 2008a). Lifetime costs for stable ischemic heart disease patients treated with PCI are over $9,000 higher than for patients treated with optimal medical therapy alone (Weintraub et al. 2008b).
Like all trials, the COURAGE trial had limitations, and cardiologists disagree on the relevance of the COURAGE trial to clinical practice. Some cardiologists criticized the trial on the grounds that the inclusion criteria were too restrictive, rates of compliance with medical therapy were much higher than is typically observed in routine practice, the trial was underpowered, the trial was performed before the widespread diffusion of drug-eluting stents, which are associated with better outcomes, and cardiologists should have implanted more stents in patients randomized to PCI with multivessel disease (Kereiakes et al. 2007). Other cardiologists believe that the trial has important and wide-ranging implications for practice.
Prominent cardiologists perceive that fee-for-service reimbursement is a major obstacle to implementing the COURAGE trial results. Peterson and Rumsfeld (2008) write: “[The COURAGE trial] underscores a major challenge to clinicians—how to successfully execute a strategy of optimal medical therapy in a health care system that provides strong financial incentives for PCI but few rewards for careful management of medications.” The principal investigator of the COURAGE trial, Dr. William Boden, echoed these sentiments (Weinstein 2010): “What’s going to continue to drive practice is reimbursement.” We are unaware of changes to insurers’ coverage polices around 2007, COURAGE related or otherwise, that would have materially affected PCI rates.
Several studies have examined the impact of the COURAGE trial on practice patterns. Ahmed et al. (2011), using data from 10 hospitals in Maine, New Hampshire, and Vermont, found that there was an approximately 25 percent decline in PCI volume among patients with stable angina following publication of the COURAGE trial. The hospitals are all nonprofit and participate in a voluntary cardiac disease registry. Borden et al. (2011) found that there was only a small (1.2 percentage point) increase in the proportion of patients with stable coronary disease undergoing PCI who received optimal medical therapy prior to PCI. They did not assess trends in the volume of PCIs among patients with stable coronary disease. Although both Ahmed et al. and Borden et al. provide evidence on the impact of the COURAGE trial, neither reports trends in the use of PCI in a large, representative sample of hospitals.
Methods
Overview
We compare trends in the use of PCI among patients with diagnoses similar to those of patients in the COURAGE trial with trends in the use of PCI with diagnoses that would have made them ineligible for participation in COURAGE. We use three datasets. Note that only one of the four datasets we use to identify the impact of the COURAGE trial is a sample. The other datasets capture the universe of discharges in their respective regions or health systems, and so estimates of the number of PCIs in each year are not subject to sampling variation.
Data Sources
National Hospital Discharge Survey (NHDS)
The NHDS is a random sample of hospital discharges in U.S. community hospitals. The sample size ranges between 120,000 and 300,000 discharges annually.
We identified patients undergoing PCI using International Classification of Diseases, Volume 9 (ICD-9), procedure codes 36.01, 36.02, 36.05, 36.06, 36.07, and 36.09. We grouped patients into two mutually exclusive categories. The “Other Diagnoses, Elective” group includes patients without a diagnosis of AMI (ICD-9 code 410.X) where the admission type was recorded as “Elective.” The NHDS, unlike the other databases we use, includes a variable indicating whether the admission was “Elective,” “Urgent,” or an “Emergency.” Criteria follow inpatient billing criteria and are described in Buie et al. (2010). The “AMI and Other Diagnoses, Non-Elective” group includes all patients with a diagnosis of AMI and patients with other diagnoses with an “urgent” or “emergency” admission. Procedure counts were weighted to produce nationally representative estimates.
Florida, Maryland, and New Jersey
We tracked trends in PCI volume in Florida, Maryland, and New Jersey using the State Inpatient Discharge and State Ambulatory Surgery databases. These databases capture 100 percent or close to 100 percent of hospital discharges and hospital-based outpatient surgical procedures, depending on the state.
We identified patients undergoing PCI in the State Inpatient Discharge databases using the ICD-9 procedure codes listed above. We also used Current Procedural Terminology codes 92973, 92980, 92981, 92982, 92983, 92984, 92985, 92996, G0290, G0291, and S2220 to identify patients undergoing PCI in the State Ambulatory Surgery database. We also specifically identified patients who received drug-eluting stents using ICD-9 procedure code 36.07.
We grouped patients into three categories: (1) stable angina (records excluding ICD-9 codes 410.X [AMI] and 411.1 [unstable angina]); (2) unstable angina (records with ICD-9 code 411.1); and (3) AMI (records with ICD-9 code 410.X). Patients in the first group include those with a diagnosis of stable angina or with a general ischemic heart disease diagnosis code, but no diagnosis of AMI or unstable angina. Pre-COURAGE, patients with stable angina accounted for one third of all PCI procedures (Peterson and Rumsfeld 2008), and it is likely that a large share of patients in the stable angina group would have satisfied the COURAGE inclusion criteria.
English Hospitals
The Hospital Episode Statistics is a census of discharges from English National Health Service hospitals. We identified PCIs using ICD-10 codes K49.X and K75.X. We grouped patients based on diagnosis using codes I21.X (AMI) and I20.0 (unstable angina). English cardiologists are salaried. Hospitals are paid under fee-for-service–like reimbursement scheme, “Payment by Results.”
Analysis
We describe trends in PCI volume by indication and test whether the proportion of patients undergoing PCI with stable angina (or, alternatively, patients without a diagnosis of unstable angina or AMI) has changed over time. The rationale for this analysis is as follows.
Let Nt be the number of patients with stable angina in period t, nt be the number who receive a PCI in period t, and αt be a parameter describing treatment practices such that nt = αtNt. Our hypothesis is that the treatment threshold for patients with stable angina is different pre-and post-COURAGE: αPRE > αPOST.
If we observed all patients with stable angina, Nt, including those who did not receive treatment, we could estimate αPRE and αPOST directly. As an alternative, we make use of the information contained in PCI volume for patients with unstable angina or AMI. The equation definining the relationship between the number of patients with these conditions and the number receiving PCI is mt = γMt, which is analogous to nt = αtNt. Also, assume that Nt = δMt, which describes the relationship between the total number of patients with stable angina and the number with unstable angina or AMI.
The number of PCIs in patients with stable angina as a fraction of total PCIs is 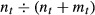 . By substituting nt = αNt, mt = γMt, and then mt = γMt, we have:
. By substituting nt = αNt, mt = γMt, and then mt = γMt, we have:
Testing θPRE = θPOST amounts to a test of αPRE = αPOST under the assumptions that the relationships between the number of patients with unstable angina or AMI and PCI use (γ) and the number of patients with unstable angina or AMI and stable angina (δ) have remained stable over time. These assumptions are not literally true. The prevalence of coronary artery disease has declined over time (Centers for Disease Control and Prevention [CDC] 2011) due to changes in risk factors such as cholesterol levels, smoking rates, and systolic blood pressure levels (Ford et al. 2007; Wijeysundera et al. 2010). The introduction of drug-eluting stents and other new technologies has altered physicians’ approaches for treating patients with unstable coronary artery disease (Epstein et al. 2011). However, none of these changes is occuring so rapidly as to impart substantial bias over the short time frame over which we are examining trends in PCI use.
We test θPRE = θPOST using a t-test for proportions. Note that this analysis accounts for secular trends in the use of PCI; it does not assume that Nt and Mt are constant over time.
Results
Collectively, the datasets include observations for 652,000 PCIs. There was little change in the demographic characteristics of patients undergoing PCI (see Appendix Table).
Figure 1 presents nationally representative trends in quarterly PCI volume from the NHDS. The NHDS included observations for 46,760 PCIs over the period 2001–2008. Trends are presented relative to volume in the first quarter of 2001 (= 1 on the y-axis) to facilitate comparison between groups.
Figure 1.
Quarterly PCI Volume by Indication, U.S. Hospitals
The vertical dashed line indicates the publication date of the COURAGE trial: April 12, 2007. The markers on either side of the dashed line indicate relative PCI volume in the first and second quarters of 2007. The graph shows that PCI volume declined between the first and second quarters. It does not indicate whether the decline began before or after April 12.
PCI volume in the Other Diagnoses, Elective group, increased in 2001 and 2002, plateaued between 2003 and 2006, and declined rapidly following publication of the COURAGE trial. There was also a decrease in PCI volume in late 2007 among patients in the AMI and Other Diagnosis, Nonelective group.
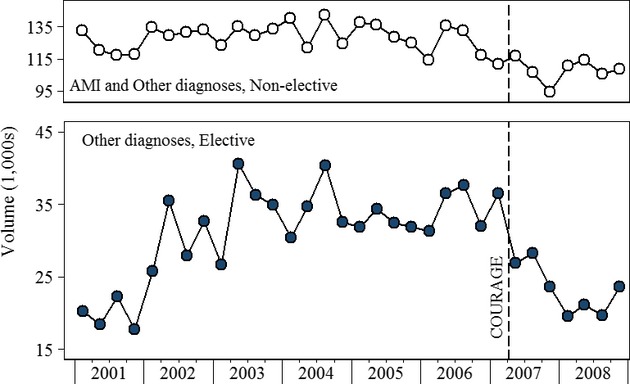
Figure 2 presents PCI volume trends from the State Inpatient Discharge and State Ambulatory Surgery databases for Florida, Maryland, and New Jersey. There are observations for 361,364 PCIs. Of these, 219,169 were performed in Florida, 51,435 in Maryland, and 91,993 in New Jersey. Trends are presented relative to volume in the first quarter of 2008 (= 1 on the y-axis). PCI volume in all three groups declined immediately following publication of the COURAGE trial. PCI volume in AMI patients rebounded to pre-COURAGE levels, whereas volume in patients with diagnoses of unstable angina or stable angina and other diagnoses remained below 2006 levels.
Figure 2.
Quarterly PCI Volume by Indication, United States (FL, MD, NJ)
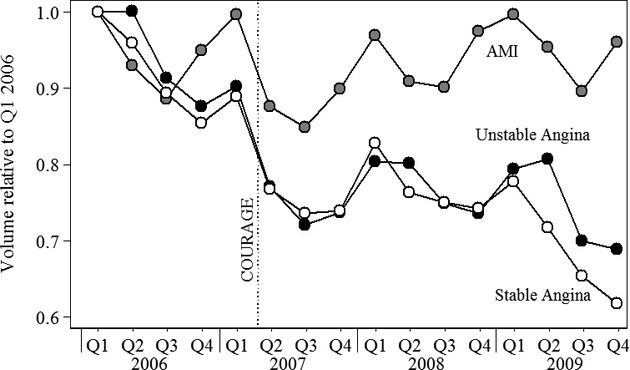
A September 2006 persentation at an international cardiology conference (Camenzind, Steg, and Wijns 2006) raised concerns about the safety of drug-eluting stents. Although cardiologists could have substituted bare metal stents, and subsequent studies reaffirmed the benefits of drug-eluting stents (Malenka et al. 2008; James et al. 2009), the findings may have temporarily depressed demand for PCI. To help disentangle the impact of the stent study and the COURAGE trial, Figure 3 reports trends in PCI volume (all diagnoses) and the percentage of PCIs in which patients received a drug-eluting stent. PCI volume is displayed relative to levels in the first quarter of 2006 so that PCI and drug-eluting stent trends are displayed on a common scale. We focus on the period 2006–2007 to isolate the impact of each study. Use of drug-eluting stents, depicted by the dashed line with hollow triangles, declined between the third and fourth quarters of 2006, coincident with the release of the drug-eluting stent abstract. Use continued to decline through 2007, but the rate of the decline had already started to slow by the time the COURAGE trial was published. Overall PCI volume declined prior to the release of the drug-eluting stent abstract but did not decline again until the COURAGE trial was published.
Figure 3.
Use of DES and PCI Volume Relative to Q1 2006 by Indication, United States (FL, MD, NJ)
Figure 4 displays trends in PCI volume in English hospitals. The Hospital Episode Statistics database included observations for 243,202 PCIs over the period 2006 through the first quarter of 2009. There was a slight drop in PCI volume among patients with stable angina/other diagnoses following publication of the COURAGE trial; it does not appear that the trial had a long-lasting effect on volume.
Figure 4.
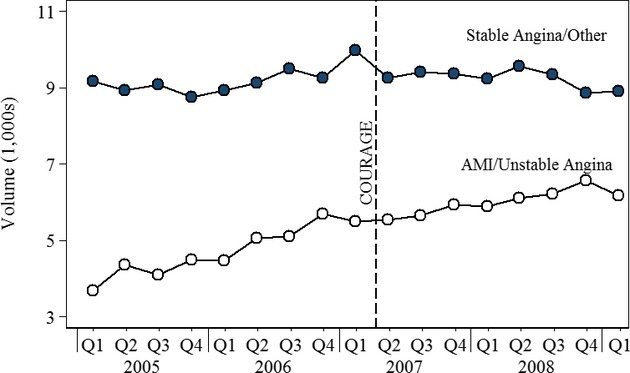
Quarterly PCI Volume by Indication, England
Table 1 presents relative trends in PCI volume by indication. According to the NHDS, the number of PCIs in patients without a diagnosis of AMI or unstable angina and whose procedures were deemed elective declined from 137,000 in 2006 to 84,000 in 2008 (−39 percent). The number of PCIs in patients with a diagnosis of AMI or whose procedures were deemed nonelective declined from 500,000 in 2006 to 439,000 in 2008 (−12 percent).
Table 1.
Change in PCIs by Indication, 2006–2008
| PCIs | |||
|---|---|---|---|
| No. (1,000s) [Rate per 100 K persons age ≥45] | |||
| Elective | Nonelective | Proportion | |
| United States: NHDS | |||
| 2006 | 137 [116] | 500 [420] | 0.22 |
| 2008 | 84 [70] | 439 [369] | 0.16* |
| Change | −54 (−39%) | −61 (−12%) | |
| Stable Angina | AMI | Proportion | |
|---|---|---|---|
| FL, MD, and NJ: SID and SASD | |||
| 2006 | 48 [178] | 30 [112] | 0.61 |
| 2008 | 40 [147] | 30 [112] | 0.57* |
| Change | −8 (−17%) | 0 (0%) | |
| England hospital episode statistics | |||
| 2006 | 37 [171] | 14 [63] | 0.73 |
| 2008 | 37 [172] | 18 [82] | 0.68* |
| Change | 0 (1%) | 4 (31%) | |
The difference in proportions between 2006 and 2008 is significant at the 0.001 level.
AMI, acute myocardial infarction; NHDS, national hospital discharge survey; PCI, percutaneous coronary intervention; SASD, state ambulatory surgery data; SID, state inpatient discharge data.
For the purposes of assessing the impact of the COURAGE trial in the other datasets, we compare trends among patients with stable angina/other diagnoses and AMI. According to the State Inpatient Discharge and State Ambulatory Surgery databases, the number of PCIs in patients without a diagnosis of AMI or unstable angina in Florida, Maryland, and New Jersey declined from 48,000 in 2006 to 40,000 in 2008 (−17 percent). The number of PCIs in patients with a diagnosis of AMI did not decline.
PCI volume in England among patients with stable angina/other diagnoses increased slightly, from 36.8 to 37.0 thousand. Procedure volume among patients with a diagnosis of AMI increased from 14,000 to 18,000 (4 percent).
The last column of Table 1 presents the proportion of PCIs performed in patients with stable angina/other diagnoses in 2006 and 2008. In each case, the decline in the proportion was significant at the 0.001 percent level. The decline in the proportion in England reflects the increase in the use of PCIs in patients with AMI rather than a decline in patients with stable angina/other diagnoses.
Discussion
The number of PCIs performed in the United States declined after 2006. Our best estimate, based on combined inpatient and outpatient data, is that PCI use declined by 17 percent in the United States among patients without diagnoses of AMI or unstable angina. Seasonality in PCI use, secular trends in the number of patients with ischemic heart disease, and the release of other studies on the effectiveness of PCI (e.g., Camenzind, Steg, and Wijns 2006) make it difficult to precisely identify the impact of the COURAGE trial on PCI use. However, several factors suggest that COURAGE contributed to declining PCI use. First, there was a large decrease in use immediately following publication of the COURAGE trial on April 12, 2007. Second, use of PCI for angina declined while use for AMI remained at pre-COURAGE levels.
We were surprised that use of PCI for patients with a diagnosis of unstable angina declined post-COURAGE. Patients with unstable angina would not have been candidates for inclusion in COURAGE. We speculate that either some cardiologists may have extrapolated the results of the COURAGE trial to other patient groups or, more likely, diagnostic coding on claims was insufficiently specific to distinguish between stable and unstable angina.
Our inability to observe patients with stable angina who do not receive PCI precludes use of regression models to assess the statistical significance of changes in PCI volume or measurement of trends in the use of alternatives to PCI. Use of claims data could potentially circumvent this problem, but the alternatives to PCI are so widely used that we doubt it would be possible to identify patients who considered but did not receive PCI post-COURAGE. Our statistical analysis assumes that publication of the COURAGE trial did not affect use of PCI among patients with unstable angina or AMI. This is a strong assumption, given the trendlines in Figures 1 and 2, but, to the extent that COURAGE depressed PCI use in this group, the analysis would be biased against finding a significant result because we assume these declines reflect a secular trend.
Stable angina is rarely recorded as a diagnosis in inpatient discharge data. We tried to identify patients for whom the COURAGE results were relevant by identifying patients without a diagnosis of AMI or unstable angina or, in the NHDS, patients whose procedure was classified as “elective.” Patients who met these criteria were included in the Stable Angina category. The proportion of patients in the category varied across datasets, reflecting differences in geographic coverage and care setting (inpatient only vs. inpatient and outpatient). The Stable Angina category includes a mix of patients, only some of whom would have met the COURAGE inclusion criteria. Pre-COURAGE, patients with stable angina accounted for one third of all PCI procedures (Peterson and Rumsfeld 2008). On the basis of these data, we suspect that the COURAGE trial is relevant for many, though not all, of the patients in our Stable Angina group. Our results overstate the proportion of patients with stable angina but, because of misclassification, understate the impact of the COURAGE trial on practice patterns.
The share of PCIs performed on an outpatient basis in Florida, Maryland, and New Jersey was 1.9 percent in 2006, 4.3 percent in 2007, 7.9 percent in 2008, and 7.9 percent in 2009. Excluding patients with AMI or unstable angina, the share increased from 3.4 to 24.1 percent over this period. The NHDS includes inpatient PCIs only, and so these results should be viewed with caution. However, it is unlikely that the sharp decline in PCI volume observed in 2007 can be fully explained by the shift in care settings. To the best of our knowledge, PCI was not performed on an outpatient basis in England during the study period.
PCI rates did not decline in England following COURAGE. Pre-COURAGE, PCI rates were lower in England due to differences in the prevalence of heart disease (Banks et al. 2006) and treatment patterns (Mark et al. 1994; Adams et al. 1998; Yusuf et al. 1998; TECH Research Network 2001). However, over half the patients undergoing PCI in the United Kingdom receive the procedure for stable angina and other forms of stable coronary disease (NHS Information Center 2007), suggesting that there are unexploited opportunities to substitute medical therapy for PCI. The impact of COURAGE on PCI volume in England may have been dampened by capacity constraints. If demand exceeds capacity, then a shift from PCI to medical therapy would show up as a decline in waiting times rather than a change in observed procedure volume.
Prominent cardiologists were pessimistic about the potential impact of the COURAGE trial in the United States given the incentives facing cardiologists. Other cardiologists argued that the trial lacked external generalizability and relevance in an era of widespread use of drug-eluting stents. The experience of the COURAGE trial highlights the difficulty of designing trials that will influence clinical practice in the event they find a negative result, especially in fields where technology and practice are constantly evolving. This issue has bedeviled trials of PCI. Jones (as quoted in Park 2013) notes:
As long as you continue to innovate in a way that, at face value, looks to be an improvement, the believers can always step out from under the weight of negative clinical experience by saying that the research necessarily applies to an earlier state of medical technology.
Another trial testing PCI versus medical therapy alone, The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches, is ongoing and will address some of the shortcomings of the COURAGE trial.
Comparative effectiveness trials such as COURAGE have important implications for health care spending. Lifetime costs for stable ischemic heart disease patients treated with PCI are over $9,000 higher than for patients treated with optimal medical therapy alone (Weintraub et al. 2008b). If medical practice patterns respond asymetrically to positive and negative evidence—physicians adopt new treatments based on “positive” results but do not abandon existing ones following “negative” studies—then comparative effectiveness research is unlikely to reduce spending and may very well lead to increased costs.
We find that use of PCI declined following publication of the COURAGE trial. The rate of decline was largest among the diagnostic groups that include the types of patients who were enrolled in the COURAGE trial. The experience of the COURAGE trial suggests that comparative effectiveness research can lead to cost-saving changes in medical practice patterns. However, our results and audit studies (Chan et al. 2011) indicate that there may be additional opportunities to substitute medical therapy for PCI.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Author Matrix.
Trends in PCI Volume by Dataset and Diagnosis.
References
- Adams PC, Skinner JS, Cohen M, McBride R, Fuster V. Acute Coronary Syndromes in the United States and United Kingdom: A Comparison of Approaches. Clinical Cardiology. 1998;21(5):348–52. doi: 10.1002/clc.4960210510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed B, Dauerman HL, Piper WD, Robb JF, Verlee MP, Ryan TJ, Jr, Goldberg D, Boss RA, Jr, Phillips WJ, Fedele F, Butzel D, Malenka DJ Northern New England Cardiovascular Disease Study. Recent Changes in Practice of Elective Percutaneous Coronary Intervention for Stable Angina. Circulation: Cardiovascular Quality and Outcomes. 2011;3:300–5. doi: 10.1161/CIRCOUTCOMES.110.957175. [DOI] [PubMed] [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith JP. Disease and Disadvantage in the United States and in England. Journal of the American Medical Association. 2006;295(17):2037–45. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. New England Journal of Medicine. 2007;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and Intensity of Medical Therapy in Patients Undergoing Percutaneous Coronary Intervention. Journal of the American Medical Association. 2011;305:1882–9. doi: 10.1001/jama.2011.601. [DOI] [PubMed] [Google Scholar]
- Brown D. Why Do Cardiologists Often Pass Up Safe, Low-Tech Treatments for Chest Pain. Washington Post. 2012. February 6, 2012.
- Buie VC, Owings MF, DeFrances CJ, Golosinskiy A. National Hospital Discharge Survey: 2006 Summary. Vital and Health Statistics. 2010;13(168) ). [accessed on June 19, 2013]. Available at: http://www.cdc.gov/nchs/data/series/sr_13/sr13_168.pdf.” National Center for Health Statistics. [PubMed] [Google Scholar]
- Camenzind E, Steg PG, Wijns W. 2006. Safety of Drug-Eluting Stents: Insights from Meta Analysis.” Presented at Hotline Session I, World Congress of Cardiology 2006, Barcelona, September 2–5, 2006. Abstract [accessed on March 1, 2013]. Available at: http://www.escardio.org/congresses/World_Congress_Cardiology_2006/congressreports/Pages/707009-Camenzind.aspx.
- Centers for Disease Control and Prevention (CDC) Prevalence of Coronary Heart Disease–United States, 2006–2010. Morbitity and Mortality Weekly Report. 2011;60(40):1377–81. [PubMed] [Google Scholar]
- Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, Brindis RG, Spertus JA. Appropriateness of Percutaneous Coronary Intervention. Journal of the American Medical Association. 2011;306(1):53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Jena AB, Skinner JS. The Pragmatist’s Guide to Comparative Effectiveness Research. Journal of Economic Perspectives. 2011;25(2):27–46. doi: 10.1257/jep.25.2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Finance, United States Senate. Staff Report on Cardiac Stent Usage at St. Joseph Medical Center. Washington, DC: U. S. Government Printing Office; 2010. [Google Scholar]
- Deyell MW, Buller CE, Miller LH, Wang TY, Dai D, Lamas GA, Srinivas VS, Hochman JS. Impact of National Clinical Guideline Recommendations for Revascularization of Persistently Occluded Infarct-Related Arteries on Clinical Practice in the United States. Archives of Internal Medicine. 2011;171(18):1636–43. doi: 10.1001/archinternmed.2011.315. JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SQ, Farley DE. The Protracted Demise of Medical Technology: The Case of Intermittent Positive Pressure Breathing. Medical Care. 1992;30(8):718–36. doi: 10.1097/00005650-199208000-00004. [DOI] [PubMed] [Google Scholar]
- Eddy DM. Clinical Decision Making: From Theory to Practice. Journal of the American Medical Association. 1993;270(4):520–6. ”. Three battles to watch in the 1990s. [PubMed] [Google Scholar]
- Emanuel EJ, Fuchs VR. The Perfect Storm of Overutilization. Journal of the American Medical Association. 2008;299(23):2789–91. doi: 10.1001/jama.299.23.2789. [DOI] [PubMed] [Google Scholar]
- Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary Revascularization Trends in the United States 2001–2008. Journal of the American Medical Association. 2011;305(17):1769–76. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the Decrease in U.S. Deaths from Coronary Disease, 1980–2000. New England Journal of Medicine. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- Fuchs VR. The Growing Demand for Medical Care. National Bureau of Economic Research, Inc: NBER Books; 1968. [Google Scholar]
- Fuchs VR. The Health Economy. Cambridge: Harvard University Press; 1986. [Google Scholar]
- Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Freeman J, Goodwin JS. Impact of a Scientific Presentation on Community Treatment Patterns for Primary Breast Cancer. Journal of the National Cancer Institute. 2006;98(6):382–8. doi: 10.1093/jnci/djj090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CP, Steiner CA, Bass EB, Powe NR. Relation between Prepublication Release of Clinical Trial Results and the Practice of Carotid Endarterectomy. Journal of the American Medical Association. 2000;284(22):2886–93. doi: 10.1001/jama.284.22.2886. [DOI] [PubMed] [Google Scholar]
- Howard DH, Brophy R, Howell S. Negative Results from Trials of Knee Surgery for Osteoarthritis Led to Coverage Changes and Declines in Procedure Rates. Health Affairs. 2012;31(10):2242–9. doi: 10.1377/hlthaff.2012.0644. [DOI] [PubMed] [Google Scholar]
- Howard DH, Kenline C, Hassebroek A, Horowitz MM, Parsons SK, Rizzo JD, Majhail NS. Abandonment of High Dose Chemotherapy/Hematopoietic Cell Transplants for Breast Cancer. Health Services Research. 2011;46(6):1762–77. doi: 10.1111/j.1475-6773.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DH, Shen YC. Comparative Effectiveness Research, Technological Abandonment, and Health Care Spending. Advances in Health Economics and Health Services Research. 2012;23:103–12. doi: 10.1108/s0731-2199(2012)0000023007. [DOI] [PubMed] [Google Scholar]
- James SK, Stenestrand U, Lindback J, Carlsson J, Scherstén F, Nilsson T, Wallentin L, Lagerqvist B SCAAR Study Group. Long-Term Safety and Efficacy of Drug-Eluting versus Bare-Metal Stents in Sweden. New England Journal of Medicine. 2009;360:1933–45. doi: 10.1056/NEJMoa0809902. and. [DOI] [PubMed] [Google Scholar]
- Kereiakes DJ, Teirstein PS, Sarembock IJ, Holmes DR, Jr, Krucoff MW, O’Neill WW, Waksman R, Williams DO, Popma JJ, Buchbinder M, Mehran R, Meredith IT, Moses JW, Stone GW. The Truth and Consequences of the COURAGE Trial. Journal of the American College Cardiology. 2007;50(16):1598–603. doi: 10.1016/j.jacc.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Lenzer J. Study Questions Hypertension Tack. Minneapolis Star Tribune. 2012. August 24, 2012.
- Malenka DJ, Kaplan AV, Lucas FL, Sharp SM, Skinner JS. Outcomes Following Coronary Stenting in the Era of Bare-Metal vs the Era of Drug-Eluting Stents. Journal of the American Medical Association. 2008;299:2868–76. doi: 10.1001/jama.299.24.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark DB, Naylo CD, Hlatky MA, Califf RM, Topol EJ, Granger CB, Knight JD, Nelson CL, Lee KL, Clapp-Channing NE, Sutherland W, Pilote L, Armstrong PW. Use of Medical Resources and Quality of Life after Acute Myocardial Infarction in Canada and the United States. New England Journal of Medicine. 1994;331(17):1130–5. doi: 10.1056/NEJM199410273311706. [DOI] [PubMed] [Google Scholar]
- NHS Information Center. London, UK: The NHS Information Centre; 2007. National Coronary Angioplasty Audit 2007. [Google Scholar]
- Omoigui NA, Silver MJ, Rybicki LA, Rosenthal M, Berdan LG, Pieper K, King SV, Califf RM, Topol EJ. Influence of a Randomized Clinical Trial on Practice by Participating Investigators: Lessons from the Coronary Angioplasty versus Excisional Atherectomy Trial (CAVEAT) Journal of the American College of Cardiology. 1998;31(2):265–72. doi: 10.1016/s0735-1097(97)00498-1. [DOI] [PubMed] [Google Scholar]
- Park A. Harvard Magazine. 2013. A Cardiac Conundrum. March–April 2013. [Google Scholar]
- Peterson ED, Rumsfeld JS. Finding the Courage to Reconsider Medical Therapy for Stable Angina. New England Journal of Medicine. 2008;359(7):751–3. doi: 10.1056/NEJMe0804662. [DOI] [PubMed] [Google Scholar]
- Phelps CE. Diffusion of Information in Medical Care. Journal of Economic Perspectives. 1992;6(3):23–42. doi: 10.1257/jep.6.3.23. [DOI] [PubMed] [Google Scholar]
- Prasad V, Cifu A, Loannidis JP. Reversals of Established Medical Practices: Evidence to Abandon Ship. Journal of the American Medical Association. 2012;307(1):37–8. doi: 10.1001/jama.2011.1960. 2012. [DOI] [PubMed] [Google Scholar]
- Schroeder SA, Showstack JA. In: Medical Technology: The Culprit behind Health Care Costs? Altman SH, Blendon R, editors. Washington, DC: U.S. Department of Health and Human Services; 1979. : DHEW publication 79(3216) [Google Scholar]
- Soulos PR, Yu JB, Roberts KB, Raldow AC, Herrin J, Long JB, Gross CP. Assessing the Impact of a Cooperative Group Trial on Breast Cancer Care in the Medicare Population. Journal of Clinical Oncology. 2012;30(14):1601–7. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford RS, Monti V, Furberg CD, Ma J. Long-Term and Short-Term Changes in Antihypertensive Prescribing by Office-Based Physicians in the United States. Hypertension. 2006;48(2):213–8. doi: 10.1161/01.HYP.0000229653.73128.b6. [DOI] [PubMed] [Google Scholar]
- TECH Research Network. Technological Change around the World: Evidence from Heart Attack Care. Health Affairs. 2001;20(3):25–42. doi: 10.1377/hlthaff.20.3.25. [DOI] [PubMed] [Google Scholar]
- Timbie JW, Fox DS, Schneider K, Van Busum EC. Five Reasons that Many Comparative Effectiveness Studies Fail to Change Patient Care and Clinical Practice. Health Affairs. 2012;31(10):2168–75. doi: 10.1377/hlthaff.2012.0150. [DOI] [PubMed] [Google Scholar]
- Weinstein KJ. 2010. A Simple Health-Care Fix Fizzles Out Wall Street Journal February 11, 2010.
- Weintraub WS, Boden WE, Zhang Z, Kolm P, Zhang Z, Spertus JA, Hartigan P, Veledar E, Jurkovitz C, Bowen J, Maron DJ, O’Rourke R, Dada M, Teo KK, Goeree R, Barnett PG. Cost-Effectiveness of Percutaneous Coronary Intervention in Optimally Treated Stable Coronary Patients. Circulation: Cardiovascular Quality and Outcomes. 2008a;1:12–20. doi: 10.1161/CIRCOUTCOMES.108.798462. [DOI] [PubMed] [Google Scholar]
- Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O’Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on Quality of Life in Patients with Stable Coronary Disease. New England Journal of Medicine. 2008b;359(7):677–87. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- Wijeysundera HC, Machado M, Farahati F, Wang X, Witteman W, Tu G, van der Velde JV, Lee DS, Goodman SG, Petrella R, Flaherty MO, Krahn M, Capewell S. Association of Temporal Trends in Risk Factors and Treatment Uptake with Coronary Heart Disease Mortality, 1994–2005. Journal of American Medical Association. 2010;303:1841–7. doi: 10.1001/jama.2010.580. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Flather M, Pogue J, Hunt D, Varigos J, Piegas L, Avezum A, Anderson J, Keltai M, Budaj A, Fox K, Ceremuzynski L. Variations between Countries in Invasive Cardiac Procedures and Outcomes in Patients with Suspected Unstable Angina or Myocardial Infarction without Initial ST Elevation. Lancet. 1998;352:507–14. doi: 10.1016/s0140-6736(97)11162-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Matrix.
Trends in PCI Volume by Dataset and Diagnosis.


