Abstract
Abstract Cross-talk between organelles and plasma membrane Ca2+ channels is essential for modulation of the cytosolic Ca2+ ([Ca2+]C) signals, but such modulation may differ among cells. In chromaffin cells Ca2+ entry through voltage-operated channels induces calcium release from the endoplasmic reticulum (ER) that amplifies the signal. [Ca2+]C microdomains as high as 20–50 μm are sensed by subplasmalemmal mitochondria, which accumulate large amounts of Ca2+ through the mitochondrial Ca2+ uniporter (MCU). Mitochondria confine the high-Ca2+ microdomains (HCMDs) to beneath the plasma membrane, where exocytosis of secretory vesicles happens. Cell core [Ca2+]C is much smaller (1–2 μm). By acting as a Ca2+ sink, mitochondria stabilise the HCMD in space and time. In non-excitable HEK293 cells, activation of store-operated Ca2+ entry, triggered by ER Ca2+ emptying, also generated subplasmalemmal HCMDs, but, in this case, most of the Ca2+ was taken up by the ER rather than by mitochondria. The smaller size of the [Ca2+]C peak in this case (about 2 μm) may contribute to this outcome, as the sarco-endoplasmic reticulum Ca2+ ATPase has much higher Ca2+ affinity than MCU. It is also possible that the relative positioning of organelles, channels and effectors, as well as cytoskeleton and accessory proteins plays an important role.
Key points
Cross-talk between organelles and plasma membrane Ca2+ channels modulates cytosolic Ca2+ signals in different ways.
In chromaffin cells Ca2+ entry through voltage-operated channels is amplified by Ca2+ release from the endoplasmic reticulum (ER) and generates subplasmalemmal high Ca2+ microdomains (HCMDs) as high as 20–50 μm, which trigger exocytosis. Subplasmalemmal mitochondria take up Ca2+ from HCMDs and avoid progression of the Ca2+ wave towards the cell core.
In non-excitable HEK293 cells activation of store-operated Ca2+ entry triggered by ER Ca2+ emptying also generates subplasmalemmal HCMDs of about 2 μm. In this case most of the Ca2+ is taken up by the ER rather than by mitochondria. This outcome may be explained because sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) has much higher Ca2+ affinity than mitochondria. The relative positioning of organelles, channels and accessory proteins may also play a role.
Introduction
Cell activation is often triggered by an increase of the cytosolic Ca2+ concentration ([Ca2+]c). At rest, [Ca2+]c is kept low (∼10−7 m) by the operation of the Ca2+-dependent ATPases, which pump Ca2+ away from the cytosol, to the extracellular medium and to the intracellular Ca2+ stores (ICSs) (Alvarez et al. 2012; Berridge et al. 1999; Clapham, 2003; Berridge, 2006). During cell activation Ca2+ channels located either at the plasma membrane or at endomembranes open, and Ca2+ flows into the cytosol. In excitable cells Ca2+ dynamics is directed by plasma membrane voltage-operated Ca2+ channels (VOCCs), but ICSs can cooperate to amplify the [Ca2+]C signal. In non-excitable cells Ca2+ release from ICSs is often the trigger of activation, but the [Ca2+]C signal is amplified by Ca2+ entry, which is activated by ICS emptying [store-operated calcium entry (SOCE)] (Clapham, 2003; Cahalan, 2009). Therefore, cross-talk between plasma membrane and organelles is essential for Ca2+ homeostasis (Rizzuto et al. 1993; Alvarez et al. 2012; Berridge et al. 1999; Singaravelu et al. 2006; Rizzuto et al. 2008). We shall refer here to the role of endoplasmic reticulum (ER) and mitochondria. ER acts as normally filled ICSs and can release Ca2+ to the cytosol to generate or reinforce [Ca2+]c signals. Mitochondria acts as a normally empty ICS, but can accumulate large amounts of Ca2+ when [Ca2+]c increases above 10−6 m. Mitochondrial Ca2+ accumulation is usually transient, following [Ca2+]c peaks generated during cell activation (Alonso et al. 1999; Rizzuto et al. 2008). The increase of mitochondrial [Ca2+] ([Ca2+]M) stimulates respiration, thus contributing to adjust ATP synthesis to the increased energy demand, and Ca2+ overload produces mitochondrial damage and may trigger apoptotic mechanisms (Alonso et al. 1999; Duchen et al. 2010; Spat et al. 2009; Rizzuto et al. 2008).
Recent research on Ca2+ homeostasis has evolved from global (cellular) to local (subcellular) signalling, produced by opening of a few Ca2+ channels located near the effector of a physiological action (Alvarez et al. 2012; Garcia et al. 2008; Clapham, 2003; Berridge, 2006). In this way, Ca2+ control of different cellular processes is made possible by coincidental compartmentalization of the pertinent Ca2+ microdomain with its physiological target. Control of cell secretion illustrates this spatiotemporal specificity (Garcia et al. 2008, 2012; Parekh, 2009). The affinity of the secretory machinery for Ca2+ is low (10−5 to 10−4 m) and, because of physical constraints imposed by diffusion and intracellular Ca2+ buffering, these concentrations can only be reached in the vicinity of Ca2+ channels (Garcia et al. 2008). Generation of high [Ca2+]c microdomains (HCMDs) near the exocytic machinery allows secretion to proceed without disturbance of other Ca2+-triggered functions, which have a different subcellular location. Certain geometric or structural arrangements may favour the generation of HCMDs. This is the case for synaptic bulbs in neurones or the particular arrangement of T tubules and terminal cisternae in muscle. On the other hand, the signal generated by Ca2+ release from ICSs by SOCE (Clapham, 2003; Cahalan, 2009) requires reinforcement via SOCE at the immunological synapse for normal lymphocyte function (Oh-hora & Rao, 1995).
Measuring [Ca2+] inside organelles
Selective [Ca2+] measurements at subcellular locations often requires the use of targeted protein probes. As discussed in detail elsewhere (Alonso et al. 1999), aequorin (AEQ) probes are very well suited for measurement of Ca2+ inside cytoplasmic organelles and/or in specific subcellular microdomains. AEQ emits photoluminescence in response to Ca2+ according to the reaction:
AEQ has three active Ca2+ binding sites and hence gain of the light output/[Ca2+] relation is very steep, making it ideal for detection and measurement of Ca2+ microdomains (Alonso et al. 1999). Other advantages include a dynamic range much larger than other Ca2+ probes, no need of illumination to excite light emission and the possibility of being engineered to modify Ca2+ affinity and/or to target different locations. Fusion with green fluorescent protein (GFP) or other fluorescent proteins improves stability and allows easy tracing of distribution by fluorescence microscopy (Alonso et al. 1999). Figure 1 shows some of the available variants, which allow extending the range of [Ca2+] measurements from under 10−7 to over 10−3 m. Constructs with viral vectors allow transfection in vitro, ex vivo or in vivo (Alonso et al. 1998). Because of the small light output, imaging is difficult, but possible (see sample movies with [Ca2+]M oscillations in Supplemental material; Villalobos et al. 2011, 2010; Nunez et al. 2000).
Figure 1.
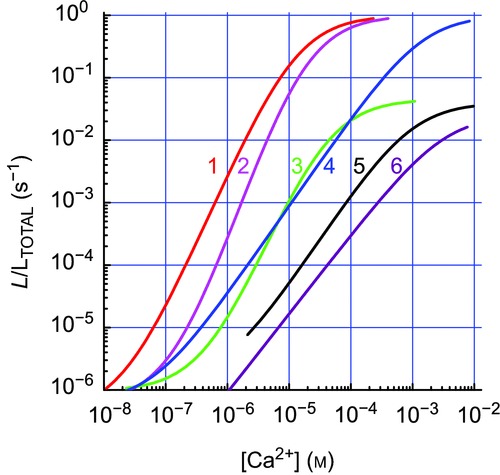
Different AEQ/coelenterazine pairs cover a broad Ca2+ concentration range with a high gain1, native AEQ + native coelenterazine at 37°C (red); 2, same at 22°C (pink); 3, native AEQ + coelenterazine at 22°C (green); 4, low Ca2+ affinity mutated AEQ at 22°C + native colenterazine (blue) (Montero et al. 2010); 5, GFP fusion to mutated low affinity AEQ + colenterazine at 22°C (black) (Manjarres et al. 2009); 6, low Ca2+ affinity mutated AEQ + colenterazine at 22°C (purple). Light emissions (ordinate) are quantified as rates of photoluminescence emission/total c.p.s remaining at each time and divided by the integration period (L/LTOTAL in s-1).
Functional triads and their role in control of secretion in adrenal chromaffin cells
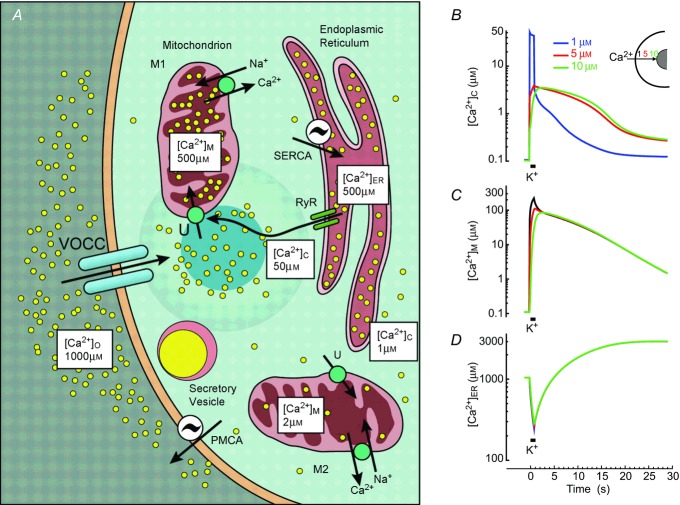
Depolarization of chromaffin cells triggers a series of effects that end to catecholamine secretion. The principal player is a triad made up of plasma membrane VOCCs, subplasmalemmal mitochondria and ER (Fig. 2; Montero et al. 2010; Garcia et al. 2008, 2012). The joint action of these three elements generates HCMDs besides the plasma membrane that are adequate for triggering exocytosis of secretory vesicles, but that do not propagate to the cell core, and therefore do not interfere with other Ca2+-dependent processes. The sequence of events is as follows: Ca2+ entering through VOCCs is taken up by a pool of mitochondria close to the plasma membrane (M1 in Fig. 2) and this stops progression of the Ca2+ wave towards the cell core. This is revealed by mitochondrial AEQ decay, which detects two very different rates of consumption that must correspond to two different mitochondrial pools, M1 and M2. These two pools take up Ca2+ at very different velocities, 2000 and 12 μmol l−1 cells s-1, respectively (Montero et al. 2010; Villalobos et al. 2010). These rates are the ones reached at about 30 and 2 μm [Ca2+]C, respectively, which should correspond to the Ca2+ concentrations attained at the subplasmalemmal region, where exocytosis takes place, and at the cell core (Fig. 2A). The increase of [Ca2+]M would keep respiration increased, thus providing the basis for subcellular tuning of mitochondrial function to match the local energy needs. A similar compartmentalization of Ca2+ signalling by mitochondrial function has been suggested in pituitary cells (Villalobos et al. 2011), pancreatic acinar cells (Park et al. 2013; Walsh et al. 2001; Petersen, 2011), beta cells (Quesada et al. 2001) and sympathetic neurons (Nunez et al. 2000).
Figure 2.
Functional triads shape Ca2+ stimuli and regulate secretion in adrenal chromaffin cells A, schematic representation of the contribution of VOCCs, mitochondria and ER. B–D, simulations of [Ca2+]C (B), [Ca2+]M (C) and [Ca2+]ER (D) changes during stimulation of a chromaffin cell by a depolarizing 1 s high K+ pulse. For details see Villalobos et al. (2010). The [Ca2+] near the mouth of the channel (blue) and 5 (red) and 10 μm away (green) are shown. A, reproduced with permission from García et al. (2008), Physiol Rev 86, 1093–1131.
Calcium-induced calcium release (CICR) amplifies the Ca2+ signals generated by Ca2+ entry through VOCCs (Alonso et al. 1998). Measurements of [Ca2+]ER during stimulation with high K+ show net decreases of 60–100 μm (10–15% of the total content) with each stimulating pulse. This is an averaged value that may consist of strong liberation in certain places partly compensated for by strong uptake in others. CICR was sensitized by low concentrations of caffeine or by increasing the load of Ca2+ stored inside the ER, and it was blocked by ryanodine (Alonso et al. 1998). The mitochondrial AEQ pool sensitive to CICR overlaps with the pool reached by plasma membrane depolarization (Montero et al. 2010; Villalobos et al. 2010), suggesting physical co-localization of VOCCs and the ryanodine receptors (RyRs). Recent studies on the subcellular organization of the secretory machinery suggest that it accumulates at preferential sites that co-localize with the Ca2+ channel clusters. The cortical F-actin cytoskeleton may play a role in the association (Torregrosa-Hetland et al. 2012, 2008).
Therefore, the local [Ca2+]C hotspots result from the joint action of the functional triad elements: the calcium channel acts as the trigger, the RyR, located at strategic places, is the signal amplifier, and the cortical mitochondria act as a contention barrier that avoids propagation of the high Ca2+ tide to the cell core, where such a huge signal is not required. Using the kinetic parameters for transport of Ca2+ in every organelle (Villalobos et al. 2010), we have modelled concentrations at different cell locations, near the cytosolic opening of the channel pore (0 μm) and 5 and 10 μm away, in cytosol, mitochondria and ER (Fig. 2B–D). [Ca2+]C reaches a very high level at the mouth of the channel (Fig. 2B; note log scale) and a much smaller one at the core, 5 or 10 μm away. Mitochondria take up most of the Ca2+ load and [Ca2+]M increases quickly to very high levels (Fig. 2C). Much of the Ca2+ that enters mitochondria at the subplasmalemmal locations may diffuse through the mitochondrial matrix to other cell locations and be eventually extruded from mitochondria closer to the cell core. [Ca2+]ER first decreases by release during CICR, and then increases due to Ca2+ uptake through the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) (Fig. 2D). Note that once VOCCs close, [Ca2+]C decreases quickly because of Ca2+ pumping from the cytosol both to the ER (through SERCA) and out of the cells (through plasma membrane Ca2+ ATPase, PMCA) (Fig. 2A). It takes several seconds to clear out the Ca2+ load that entered through VOCCs. This is due to the fact that most of the load is inside mitochondria by that time, and has to exit from the matrix to the cytosol (through the mitochondrial Na+/Ca2+ exchanger) before being available to PMCA and SERCA (Villalobos et al. 2010). Because of the action of PMCA, the subplasmalemmal [Ca2+]C reaches, during the post-stimulation period, lower levels than the core [Ca2+]C (Fig. 2B). This faster decrease may be important for termination of the secretory process by the end of the stimulus.
Ca2+ released from the ER through inositol- trisphosphate receptors (IP3Rs) is closely coupled to mitochondrial uptake through the mitochondrial calcium uniporter (MCU; Rizzuto et al. 2012). It has been discovered recently that dynamin-related mitofusins are responsible for the tethering mechanism between both organelles, thereby ensuring rapid and high-fidelity Ca2+ signalling between them (Parekh, 2008). This ER–mitochondria cross-talk has been studied extensively in several tissues (Csordas et al. 2009; Rizzuto & Pozzan, 2012; Csordas et al. 2007; Chen et al. 2006; Rizzuto et al. 2008). It is relevant here to ask whether this cross-talk implies, in chromaffin cells, the same mitochondrial subpopulation as the one involved in the functional triads described above. It seems that the response to histamine, which is mediated by IP3Rs, includes a pool of mitochondria that amounts to about 30% and responds with [Ca2+]M increases larger than 1 μm, while the rest respond with smaller changes. These mitochondria may overlap with the triads, as previous stimulation with caffeine occludes the response to histamine. However, only 2–3% of these mitochondria are able to generate [Ca2+]M transients comparable in size to those observed after high K+ or caffeine stimulation (Montero et al. 2008), suggesting that coupling of mitochondria to IP3Rs is poorer than to RyRs. The situation is complicated further by the fact that the responses to histamine are not homogeneous, but much larger in adrenergic than in noradrenergic cells (Nunez et al. 2002).
Chromaffin cells have a plasmalemmal Na+/Ca2+ exchanger (NCX) which may mediate either Ca2+ entry or Ca2+ extrusion, depending on the relative Na+ and Ca2+ concentrations and membrane potential. NCX, activity of which is highly dependent on temperature, may contribute to shape the [Ca2+]C transients and the exocytic responses elicited by Ca2+ entry through VOCCs. NCX blockade potentiates the secretion elicited by caffeine or histamine, whereas it either did not modify or antagonized the secretion elicited by high K+ pulses (Padin et al. 2007).
Privileged coupling between SOCE and SERCA in non-excitable cells
Figure 3 illustrates measurements of coupling between plasma membrane Ca2+ entry (through SOC) and calcium uptake into the nucleus, the cytosol (same values as for nucleus; not shown for clarity), mitochondria or ER of HEK293 cells. The slope of the signal strength/flow relationship was much larger for ER (about 30 times larger, note double log scale) and approached the excitation–response coupling efficiency found in muscle or in synaptic transmission (Manjarres et al. 2012; Alonso et al. 1998). This is not surprising given the close relationships between Orai-1, the plasma membrane Ca2+ channel of SOC, STIM1, the ER Ca2+ sensor of SOCE and activator of Orai1, and SERCA, which is the third element of SOCE (Sampieri et al. 1998; Manjarres et al. 2011). As a consequence, Ca2+ entering through SOC goes almost directly to the ER and mitochondrial uptake is very small (Manjarres et al. 2011; Alonso et al. 1998). This contrasts with the behaviour of chromaffin cells, where most of the calcium is taken up by mitochondria rather than ER (see above). These differences are in part explained by the size and location of Ca2+ microdomains. Figure 4 compares the kinetics of Ca2+ accumulation by ER and mitochondria, which is very similar in both models, the excitable chromaffin cells and the non-excitable HEK293 cells. At [Ca2+]C concentrations near 10−7 m, found at rest (box 1 in Fig. 4), ER takes up Ca2+ much faster than mitochondria. When [Ca2+]C reaches 10−6 m, as in HCMDs associated with SOCs, the calcium uptake is still faster (about 10 times as much) in the ER than in mitochondria (box 2 in Fig. 4). When [Ca2+]C exceeds 10−4 m, as in the HCMDs generated by VOCC activation plus CICR amplification in chromaffin cells, then transport into mitochondria through the low-affinity/high-capacity MCU dominates (3 in Fig. 4) and most of the Ca2+ is taken up by mitochondria.
Figure 3.
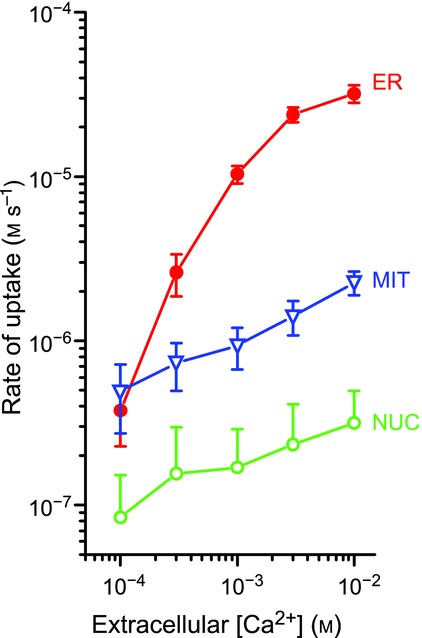
Coupling of Ca2+ entry through SOCs to calcium uptake by different organelles in HEK293 cells Measurements were performed using aequorins targeted to ER, mitochondria (MIT) and nucleus (NUC). SOC was activated by Ca2+ emptying of the ER by treatment with 10 μm tert-butyl hydroquinone (TBH), and Ca2+ entry was started by washing TBH and adding different extracellular Ca2+ concentrations (0.1 to 10 mm), as shown. The rate of entry should increase linearly with [Ca2+] (abscissa). Reproduced with permission from Alonso et al. (1998), Mol Cell Endocrinol 353, 37–44.
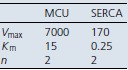
Figure 4.
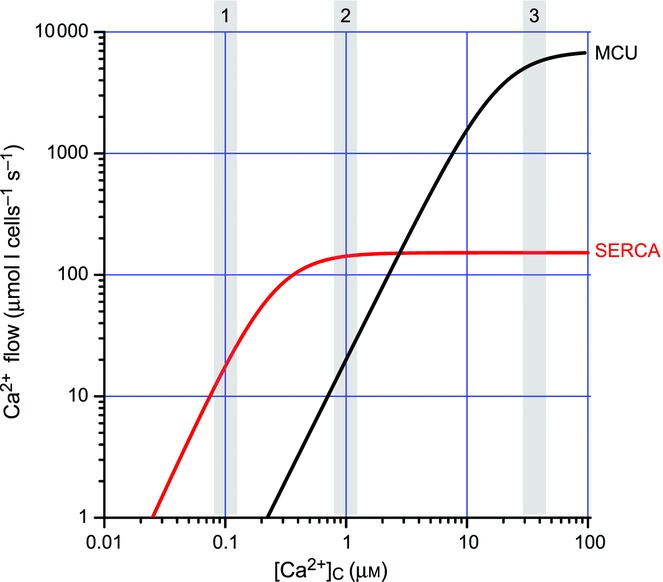
Comparison of intracellular calcium stores refilling at different [Ca2+]C The curves shown have been generated with the equation:
and are approximately valid for both chromaffin cells and HEK293 cells. The values used for Vmax and Km are given in the table below. The shaded boxes correspond to [Ca2+]C values at rest (box 1) and small (box 2) and large (box 3) high Ca2+ microdomains. MCU, mitochondrial calcium uniporter.
 |
Relative positioning of the effector could also be relevant. We have mentioned above arguments for the close proximity, both physical and functional, of SOCs and SERCA (Manjarres et al. 2011; Alonso et al. 1998). By contrast, it has been reported that mitochondria are seldom located close to the plasma membrane or Orai1 channels (Korzeniowski et al. 2011; Csordas et al. 2007; Singaravelu et al. 2006). It has been proposed that the cytoskeleton and microtubule end tracking protein EB1 could play a role in regulating association and dissociation of the SOCE components and other protein complexes (Sampieri et al. 1998; Vaca, 2013). On the other hand, nuclear factor of activated T-cells (nFAT) migration to nucleus is exquisitely sensitive to [Ca2+]C microdomains generated near SOCs (Kar et al. 2006, 2012), and selective cross-talk with cAMP signalling (Willoughby, 2009) and with RyRs (Thakur et al. 2011) has been suggested. In the case of chromaffin cells, association among VOCCs, mitochondria and the exocytic machinery has been repeatedly reported (Montero et al. 2010; Villalobos et al. 2010; Garcia et al. 2008; Torregrosa-Hetland et al. 2012, 2008; Garcia et al. 2012), and communication between L-type VOCCs and cAMP response element-binding protein (CREB) regulation of excitation transcription coupling via a Ca2+/calmodulin-dependent protein kinase II acting near the plasma membrane channel has been brilliantly demonstrated (Wheeler et al. 2002).
Acknowledgments
The technical assistance by Ms Miriam D. García Cubillas and Mr Jesús Fernández is gratefully acknowledged.
Glossary
- [Ca2+]C
[Ca2+]M, [Ca2+]ER, Ca2+ concentration in cytosol, mitochondria and ER, repscetively
- AEQ
aequorin
- CICR
Ca2+-induced Ca2+ release
- CREB
cAMP response element-binding protein
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- HCMDs
high Ca2+ microdomains
- ICS
intracellular calcium store
- MCU
mitochondrial Ca2+ uniporter
- NCX
Na+/Ca2+ exchanger
- nFAT
nuclear factor of activated T-cells
- VOCCs
voltage-operated Ca2+ channels
- PMCA
plasma membrane Ca2+ ATPase
- RyR
ryanodine receptor
- SERCA
sarco-endoplasmic reticulum Ca2+ ATPase
- SOCs
store-operated channels
- SOCE
store operated Ca2+ entry.
Competing interests
None.
Funding
This work was supported by grants from the EU ERA-Net programme, the Spanish Ministerio de Economia y Competitividad (SAF2008-03175-E, BFU2010-17379) and the Instituto de Salud Carlos III (RD06/0010/0000).
References
- Alonso MT, Barrero MJ, Carnicero E, Montero M, Garcia-Sancho J, Alvarez J. Functional measurements of [Ca2+] in the endoplasmic reticulum using a herpes virus to deliver targeted aequorin. Cell Calcium. 24:87–96. doi: 10.1016/s0143-4160(98)90076-8. [DOI] [PubMed] [Google Scholar]
- Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, Garcia AG, Garcia-Sancho J, Montero M, Alvarez J. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J Cell Biol. 144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MT, Manjarres IM, Garcia-Sancho J. Privileged coupling between Ca2+ entry through plasma membrane store-operated Ca2+ channels and the endoplasmic reticulum Ca2+ pump. Mol Cell Endocrinol. 1998;353:37–44. doi: 10.1016/j.mce.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Alonso MT, Villalobos C, Chamero P, Alvarez J, Garcia-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 1999;40:513–525. doi: 10.1016/j.ceca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Montero M, Garcia-Sancho J. Subcellular Ca2+ dynamics. News Physiol Sci. 2012;14:161–168. [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 1999;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signalling. Cell. 2003;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2009;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. Imaging interorganelle contacts and local calcium dynamics at the ER–mitochondrial interface. Mol Cell. 2007;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, 2nd, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res. 2006;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2010;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Sancho J, de Diego AM, Garcia AG. Mitochondria and chromaffin cell function. Pflugers Arch. 2012;464:33–41. doi: 10.1007/s00424-012-1074-2. [DOI] [PubMed] [Google Scholar]
- Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signalling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2008;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- Garcia AG, Padin F, Fernandez-Morales JC, Maroto M, Garcia-Sancho J. Cytosolic organelles shape calcium signals and exo-endocytotic responses of chromaffin cells. Cell Calcium. 2012;51:309–320. doi: 10.1016/j.ceca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2006;286:14795–14803. doi: 10.1074/jbc.M111.220582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. CRAC channels drive digital activation and provide analog control and synergy to Ca2+-dependent gene regulation. Curr Biol. 2012;22:242–247. doi: 10.1016/j.cub.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Korzeniowski MK, Szanda G, Balla T, Spat A. Store-operated Ca2+ influx and subplasmalemmal mitochondria. Cell Calcium. 2011;46:49–55. doi: 10.1016/j.ceca.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjarres IM, Alonso MT, Garcia-Sancho J. Calcium entry-calcium refilling (CECR) coupling between store-operated Ca2+ entry and sarco/endoplasmic reticulum Ca2+-ATPase. Cell Calcium. 2012;49:153–161. doi: 10.1016/j.ceca.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Manjarres IM, Chamero P, Domingo B, Molina F, Llopis J, Alonso MT, Garcia-Sancho J. Red and green aequorins for simultaneous monitoring of Ca2+ signals from two different organelles. Pflugers Arch. 2009;455:961–970. doi: 10.1007/s00424-007-0349-5. [DOI] [PubMed] [Google Scholar]
- Manjarres IM, Rodriguez-Garcia A, Alonso MT, Garcia-Sancho J. The sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) is the third element in capacitative calcium entry. Cell Calcium. 2011;47:412–418. doi: 10.1016/j.ceca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Montero M, Alonso MT, Albillos A, Cuchillo-Ibanez I, Olivares R, Villalobos C, Alvarez J. Effect of inositol 1,4,5-trisphosphate receptor stimulation on mitochondrial [Ca2+] and secretion in chromaffin cells. Biochem J. 2008;365:451–459. doi: 10.1042/BJ20011722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Alonso MT, Carnicero E, Cuchillo-Ibanez I, Albillos A, Garcia AG, Garcia-Sancho J, Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol. 2010;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- Nunez L, De La Fuente MT, Garcia AG, Garcia-Sancho J. Differential Ca2+ responses of adrenergic and noradrenergic chromaffin cells to various secretagogues. Am J Physiol. 2002;269:C1540–1546. doi: 10.1152/ajpcell.1995.269.6.C1540. [DOI] [PubMed] [Google Scholar]
- Nunez L, Senovilla L, Sanz-Blasco S, Chamero P, Alonso MT, Villalobos C, Garcia-Sancho J. Bioluminescence imaging of mitochondrial Ca2+ dynamics in soma and neurites of individual adult mouse sympathetic neurons. J Physiol. 2000;580:385–395. doi: 10.1113/jphysiol.2006.126524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-hora M, Rao A. Calcium signalling in lymphocytes. Curr Opin Immunol. 1995;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padin JF, Fernandez-Morales JC, Olivares R, Vestring S, Arranz-Tagarro JA, Calvo-Gallardo E, de Pascual R, Gandia L, Garcia AG. The plasmalemmal sodium–calcium exchanger shapes the calcium and exocytotic signals of chromaffin cells at physiological temperature. Am J Physiol Cell Physiol. 2007 doi: 10.1152/ajpcell.00016.2013. doi: 10.1152/ajpcell.00016.2013. [DOI] [PubMed] [Google Scholar]
- Parekh A. Calcium signalling: mitofusins promote interorganellar crosstalk. Curr Biol. 2008;19:R200–203. doi: 10.1016/j.cub.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. 2009;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2013;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH. Specific mitochondrial functions in separate sub-cellular domains of pancreatic acinar cells. Pflugers Arch. 2011;464:77–87. doi: 10.1007/s00424-012-1099-6. [DOI] [PubMed] [Google Scholar]
- Quesada I, Villalobos C, Nunez L, Chamero P, Alonso MT, Nadal A, Garcia-Sancho J. Glucose induces synchronous mitochondrial calcium oscillations in intact pancreatic islets. Cell Calcium. 2001;43:39–47. doi: 10.1016/j.ceca.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 2012;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2008;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1993;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2012;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Sampieri A, Zepeda A, Asanov A, Vaca L. Visualizing the store-operated channel complex assembly in real time: identification of SERCA2 as a new member. Cell Calcium. 1998;45:439–446. doi: 10.1016/j.ceca.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Singaravelu K, Nelson C, Bakowski D, de Brito OM, Ng SW, Di Capite J, Powell T, Scorrano L, Parekh AB. Mitofusin 2 regulates STIM1 migration from the Ca2+ store to the plasma membrane in cells with depolarized mitochondria. J Biol Chem. 2006;286:12189–12201. doi: 10.1074/jbc.M110.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spat A, Szanda G, Csordas G, Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2009;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur P, Dadsetan S, Fomina AF. Bidirectional coupling between ryanodine receptors and Ca2+ release-activated Ca2+ (CRAC) channel machinery sustains store-operated Ca2+ entry in human T lymphocytes. J Biol Chem. 2011;287:37233–37244. doi: 10.1074/jbc.M112.398974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrosa-Hetland CJ, Villanueva J, Garcia-Martinez V, Exposito-Romero G, Frances Mdel M, Gutierrez LM. Cortical F-actin affects the localization and dynamics of SNAP-25 membrane clusters in chromaffin cells. Int J Biochem Cell Biol. 2008;45:583–592. doi: 10.1016/j.biocel.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Torregrosa-Hetland CJ, Villanueva J, Giner D, Lopez-Font I, Nadal A, Quesada I, Viniegra S, Exposito-Romero G, Gil A, Gonzalez-Velez V, Segura J, Gutierrez LM. The F-actin cortical network is a major factor influencing the organization of the secretory machinery in chromaffin cells. J Cell Sci. 2012;124:727–734. doi: 10.1242/jcs.078600. [DOI] [PubMed] [Google Scholar]
- Vaca L. SOCIC: the store-operated calcium influx complex. Cell Calcium. 2013;47:199–209. doi: 10.1016/j.ceca.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Nunez L, Chamero P, Alonso MT, Garcia-Sancho J. Mitochondrial [Ca2+] oscillations driven by local high [Ca2+] domains generated by spontaneous electric activity. J Biol Chem. 2011;276:40293–40297. doi: 10.1074/jbc.C100465200. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Nunez L, Montero M, Garcia AG, Alonso MT, Chamero P, Alvarez J, Garcia-Sancho J. Redistribution of Ca2+ among cytosol and organella during stimulation of bovine chromaffin cells. FASEB J. 2010;16:343–353. doi: 10.1096/fj.01-0630com. [DOI] [PubMed] [Google Scholar]
- Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A. Modulation of calcium signalling by mitochondria. Biochim Biophys Acta. 2001;1787:1374–1382. doi: 10.1016/j.bbabio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation–transcription coupling. 2002. 183:849–863. doi: 10.1083/jcb.200805048. J Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D. Organization of cAMP signalling microdomains for optimal regulation by Ca2+ entry. Biochem Soc Trans. 2008;40:246–250. doi: 10.1042/BST20110613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.