Abstract
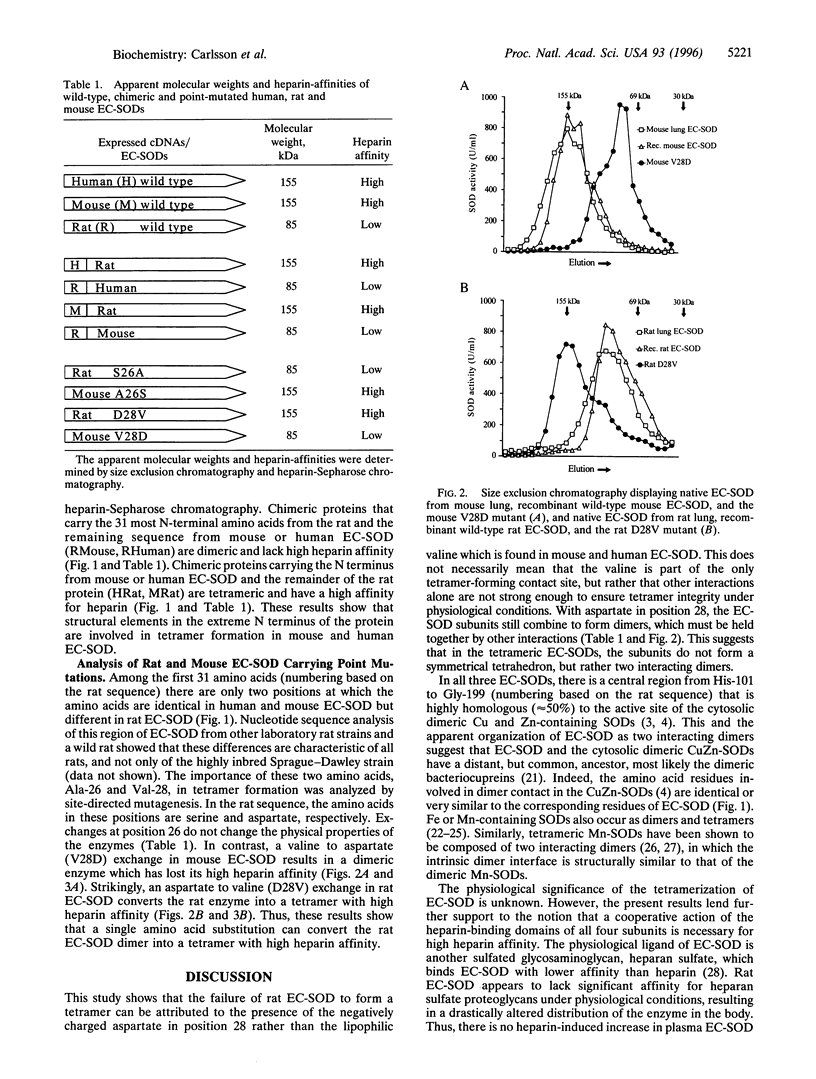
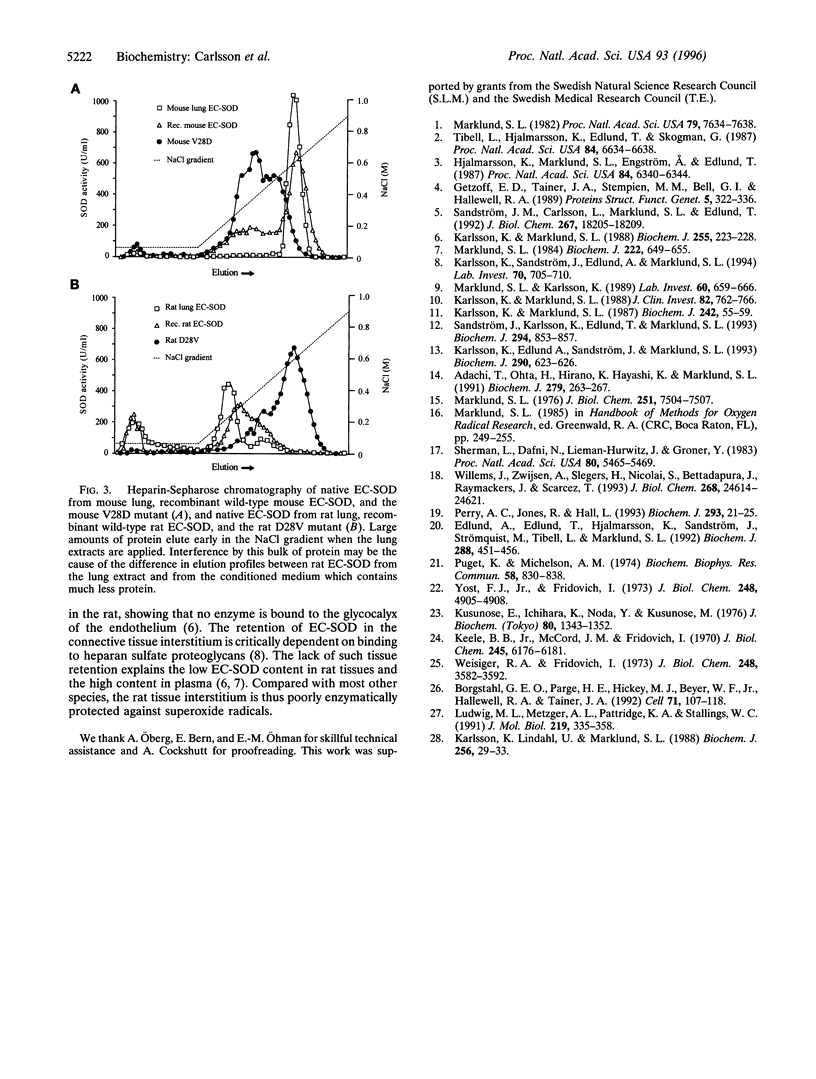
Extracellular superoxide dismutase (EC-SOD) is a secreted Cu and Zn-containing glycoprotein. While EC-SOD from most mammals is tetrameric and has a high affinity for heparin and heparan sulfate, rat EC-SOD has a low affinity for heparin, does not bind to heparan sulfate in vivo, and is apparently dimeric. To examine the molecular basis of the deviant physical properties of rat EC-SOD, the cDNAs of the rat and mouse EC-SODs were isolated and the deduced amino acid sequences were compared with that of human EC-SOD. Comparison of the sequences offered no obvious explanation of the differences. Analysis of a series of chimeric and point mutated EC-SODs showed that the N-terminal region contributes to the oligomeric state of the EC-SODs, and that a single amino acid, a valine (human amino acid position 24), is essential for the tetramerization. This residue is replaced by an aspartate in the rat. Rat EC-SOD carrying an Asp --> Val mutation is tetrameric and has a high heparin affinity, while mouse EC-SOD with a Val --> Asp mutation is dimeric and has lost its high heparin affinity. Thus, the rat EC-SOD dimer is converted to a tetramer by the exchange of a single amino acid. Furthermore, the cooperative action of four heparin-binding domains is necessary for high heparin affinity. These results also suggest that tetrameric EC-SODs are not symmetrical tetrahedrons, but composed of two interacting dimers, further supporting an evolutionary relationship with the dimeric cytosolic Cu and Zn-containing SODs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Ohta H., Hirano K., Hayashi K., Marklund S. L. Non-enzymic glycation of human extracellular superoxide dismutase. Biochem J. 1991 Oct 1;279(Pt 1):263–267. doi: 10.1042/bj2790263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgstahl G. E., Parge H. E., Hickey M. J., Beyer W. F., Jr, Hallewell R. A., Tainer J. A. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992 Oct 2;71(1):107–118. doi: 10.1016/0092-8674(92)90270-m. [DOI] [PubMed] [Google Scholar]
- Edlund A., Edlund T., Hjalmarsson K., Marklund S. L., Sandström J., Strömqvist M., Tibell L. A non-glycosylated extracellular superoxide dismutase variant. Biochem J. 1992 Dec 1;288(Pt 2):451–456. doi: 10.1042/bj2880451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Stempien M. M., Bell G. I., Hallewell R. A. Evolution of CuZn superoxide dismutase and the Greek key beta-barrel structural motif. Proteins. 1989;5(4):322–336. doi: 10.1002/prot.340050408. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Edlund A., Sandström J., Marklund S. L. Proteolytic modification of the heparin-binding affinity of extracellular superoxide dismutase. Biochem J. 1993 Mar 1;290(Pt 2):623–626. doi: 10.1042/bj2900623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Lindahl U., Marklund S. L. Binding of human extracellular superoxide dismutase C to sulphated glycosaminoglycans. Biochem J. 1988 Nov 15;256(1):29–33. doi: 10.1042/bj2560029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Binding of human extracellular-superoxide dismutase C to cultured cell lines and to blood cells. Lab Invest. 1989 May;60(5):659–666. [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988 Oct 1;255(1):223–228. [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Plasma clearance of human extracellular-superoxide dismutase C in rabbits. J Clin Invest. 1988 Sep;82(3):762–766. doi: 10.1172/JCI113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Sandström J., Edlund A., Marklund S. L. Turnover of extracellular-superoxide dismutase in tissues. Lab Invest. 1994 May;70(5):705–710. [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- Ludwig M. L., Metzger A. L., Pattridge K. A., Stallings W. C. Manganese superoxide dismutase from Thermus thermophilus. A structural model refined at 1.8 A resolution. J Mol Biol. 1991 May 20;219(2):335–358. doi: 10.1016/0022-2836(91)90569-r. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Perry A. C., Jones R., Hall L. Isolation and characterization of a rat cDNA clone encoding a secreted superoxide dismutase reveals the epididymis to be a major site of its expression. Biochem J. 1993 Jul 1;293(Pt 1):21–25. doi: 10.1042/bj2930021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puget K., Michelson A. M. Isolation of a new copper-containing superoxide dismutase bacteriocuprein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):830–838. doi: 10.1016/s0006-291x(74)80492-4. [DOI] [PubMed] [Google Scholar]
- Sandström J., Carlsson L., Marklund S. L., Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J Biol Chem. 1992 Sep 5;267(25):18205–18209. [PubMed] [Google Scholar]
- Sandström J., Karlsson K., Edlund T., Marklund S. L. Heparin-affinity patterns and composition of extracellular superoxide dismutase in human plasma and tissues. Biochem J. 1993 Sep 15;294(Pt 3):853–857. doi: 10.1042/bj2940853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Willems J., Zwijsen A., Slegers H., Nicolaï S., Bettadapura J., Raymackers J., Scarcez T. Purification and sequence of rat extracellular superoxide dismutase B secreted by C6 glioma. J Biol Chem. 1993 Nov 25;268(33):24614–24621. [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]



