Abstract
Background
Gangliogliomas (GGs) represent <1% of primary brain tumors in adults. Little is known regarding prognostic features, clinical characteristics, or the impact of treatment on patient outcomes.
Methods
Our neuro-oncology longitudinal database was screened for patients with GG from 1992 to 2012. Sixty-seven patients (age >18 y) were identified.
Results
Sixty-two patients presented with low-grade GG and 5 with anaplastic GG. The median age at diagnosis was 29 years. With a median follow-up of 4.7 years after the initial diagnosis, 23 patients had progressive disease. Range of time to progression was 0.2–20 years. Nine patients with low-grade GG progressed to a malignant tumor. The median overall survival (OS) for all patients was not reached. The 2-, 5-, and 10-year OS for patients with low-grade GG were 100%, 88% (95% confidence interval [CI]: 73%, 95%), and 84% (95% CI: 67%, 93%), respectively.
Factors identified by univariate analysis that were significantly associated with OS were age, KPS, extent of resection (EOR), and grade. Factors on univariate analysis that were significantly associated with progression-free survival were grade and EOR. On multicovariate Cox regression, lower tumor grade and younger age were significant factors for longer OS. EOR is a significant factor for progression-free survival.
Conclusions
While GG has excellent prognosis, malignant histologic grade, older age, and diagnosis with biopsy could indicate worse prognosis. The late nature and high rate of progression emphasize the importance of long-term follow-up. The role of chemotherapy and radiation therapy for incompletely resected low-grade GG remains unclear.
Keywords: adults, ganglioglioma, malignant transformation, progression, treatment
Ganglioglioma (GG) is a rare, slowly growing tumor composed of neoplastic mature ganglion cells in combination with glial cells, representing 0.4% of all CNS tumors and 1%–7.6% of all primary brain tumors.1–3 Data from large case series indicate that the incidence of GG is highest in children and young adults, with a slight male predominance.1–4 Most GGs correspond to World Health Organization grade I. Anaplastic GGs have been rarely described and are poorly characterized. The anaplastic component usually arises from the glial component of the tumor. These tumors occur throughout the CNS but are most common in the temporal and frontal lobes and therefore are commonly associated with seizures.5 GGs are the most common tumors associated with chronic temporal lobe epilepsy and have been reported in 15%–25% of patients undergoing surgery for epilepsy.2,5
Optimal treatment for low-grade GGs is considered to be complete resection, which can be curative.5,6 With partial resection, adjuvant or salvage radiation treatment is considered but remains controversial.6–8 Studies investigating treatment of high-grade GGs report a potential benefit of radiation in local control but not overall survival (OS).6,9 Reports of treatment with chemotherapy for GGs are scarce, and the impact of chemotherapy on these tumors is unknown.3,4 Many published studies combine the analysis of both pediatric and adult patient populations, when experience with other primary brain tumors would suggest that there may be differences in outcomes and responses to treatment. This report describes the MD Anderson Cancer Center experience with adult patients with GG and describes progression-free survival (PFS) and OS and the impact of surgery, radiation, and chemotherapy on outcome in these patients.
Materials and Methods
After securing institutional review board approval, we queried the Neuro-Oncology database at the University of Texas MD Anderson Cancer Center for patients who were diagnosed with GG from 1992 to 2012.
Patient's' demographic and clinical characteristics, treatments, and outcomes were reviewed. The clinical variables of interest included age at diagnosis, gender, race, KPS, seizure history, tumor location, histology, and extent of tumor resection. The extent of resection was assessed by the clinician's impression or postoperative imaging when available. Seizure outcomes were classified according to the Engel epilepsy surgery outcome scales.10,11 The clinical endpoints included OS and PFS, both time from first surgery.
Statistical Methods
Data were first summarized using standard descriptive statistics and frequency tabulation. Association between or among categorical variables was assessed by a chi-square test or Fisher's exact test when appropriate. Time to event endpoints, including OS and PFS, both from first surgery, was estimated using the Kaplan–Meier method and compared between/among patient groups by log-rank test. Patients were censored at the time of their last follow-up if no event was recorded. Univariate and multivariate Cox proportional hazard models were applied to assess the effect of covariates of interest on OS and PFS. All statistical analyses were carried out in SAS 9.2 and S-plus 8.0 (Tibco Software).
Results
Clinical Characteristics
A total of 67 adult patients (age >18) with GG were identified. Sixty-two patients presented with low-grade GG and 5 with an anaplastic GG. One of the 62 patients with low-grade GG had a nondiagnostic biopsy of a likely low-grade GG, as the patient developed an anaplastic GG 11 years later at that same location. The patient's characteristics at time of diagnosis are summarized in Table 1. The median age at diagnosis for patients with low-grade and high-grade GG was 27 years (18–65) and 39 years (25–57), respectively. The median KPS at presentation was 100 (70–100). Most patients presented with a seizure but had no prior seizure history.
Table 1.
Clinical characteristics of patients with GG
| Variable | Levels | All = 67 (100%) | Pathology at Presentation |
High Grade at Recurrence n = 8 | |
|---|---|---|---|---|---|
| Low Grade n = 62 (92.5%) | High Grade n = 5 (7.5%) | ||||
| Gender | Female | 29 (43%) | 26 (42%) | 3 (60%) | 2 (25%) |
| Male | 38 (57%) | 36 (58%) | 2 (40%) | 6 (75%) | |
| Race | Asian | 1 (1.5%) | 1 (20%) | ||
| Black | 5 (7.5%) | 5 (8%) | 1 (12%) | ||
| Hispanic | 6 (9%) | 6 (10%) | |||
| White | 55 (82%) | 51 (82%) | 4 (80%) | 7 (88%) | |
| Age | 18–39 | 54 (81%) | 51 (82%) | 3 (60%) | 3 (38%) |
| 40≤ | 13 (19%) | 11 (18%) | 2 (40%) | 5 (62%) | |
| KPS | 60–80 | 6 (9%) | 4 (6.5%) | 2 (40%) | 1 (12%) |
| 90–100 | 59 (88%) | 56 (90%) | 3 (60%) | 7 (88%) | |
| Unknown | 2 (3%) | 2 (3.5%) | |||
| Presenting symptom | Focal neurological deficit | 5 (7.5%) | 4 (6.5%) | 1 (20%) | 1 (11%) |
| Headache/pain | 15 (22%) | 15 (24%) | 1 (11%) | ||
| Seizure | 40 (60%) | 38 (61%) | 2 (40%) | 6 (67%) | |
| Prior seizure history | <6 mo | 8 (13%) | 7 (12%) | 1 (25%) | |
| >6 mo | 9 (15%) | 9 (16%) | 1 (12%) | ||
| None | 44 (72%) | 41 (72%) | 3 (75%) | 7 (88%) | |
| Unknown | 6 (.%) | 5 (.%) | 1(.%) | ||
| Tumor location | Infratentorium | 13 (19%) | 11 (18%) | 2 (40%) | |
| Supratentorium | 54 (81%) | 51 (82%) | 3 (60%) | 8 (100%) | |
Treatment Patterns
In our study, 52% of patients initially underwent gross total resection (GTR), 35% had a subtotal resection (STR), and 13% had a biopsy only. Eleven patients with STR or biopsy had a second surgery within 8 weeks to achieve a better resection or obtain additional tissue for diagnostic purposes, resulting in 61% GTR, 34% STR, and 5% biopsy (Table 2). This is the definition used in OS and PFS analyses. Fifteen patients received radiation at the time of diagnosis and an additional 7 patients received radiation after recurrence. Most received radiation for either a high-grade lesion (41%, 9 of 22) or an incomplete resection of a low-grade tumor (41%, 9 of 22). One patient received radiation therapy for a completely resected low-grade tumor, and 3 patients received radiation therapy due to a prior misdiagnosis of their low-grade GG as a glioblastoma, oligodendroglioma, or pilocytic astrocytoma.
Table 2.
Treatment patterns of patients with GG
| Treatment | All = 67 (100%) | Pathology at Presentation |
High Grade at Recurrence n = 8 | |
|---|---|---|---|---|
| Low Grade n = 62 (92.5%) | High Grade n = 5 (7.5%) | |||
| Resection | ||||
| Biopsy | 3 (5%) | 2 (3%) | 1 (20%) | |
| Subtotal | 23 (34%) | 19 (31%) | 4 (80%) | 5 (62%) |
| Total | 41 (61%) | 41 (66%) | 3 (38%) | |
| Radiation | ||||
| No | 45 (67%) | 44 (71%) | 2 (25%%) | |
| Yes | 22 (33%) | 18 (29%) | 4 (80%) | 6 (75%%) |
| Unknown | 1 (20%) | |||
| Chemotherapy | 13 (19%) | 5 (8%) | 3 (60%) | 6 (75%) |
*Resection refers to maximal resection done within 8 wk of the initial diagnosis.
Chemotherapy was given to 13 patients during the course of their disease (Table 2). While most patients received chemotherapy for either a new high-grade or recurrent low-grade tumor, 2 patients received chemotherapy after a pathological misdiagnosis of glioblastoma.
Of the patients diagnosed with anaplastic GG, 2 did not receive radiation therapy following diagnosis due to previous radiotherapy for a prior low-grade GG; these patients were treated with chemotherapy alone. The indication for the prior radiation treatment for these 2 patients was not clear.
Progression and Transformation to a Higher Grade
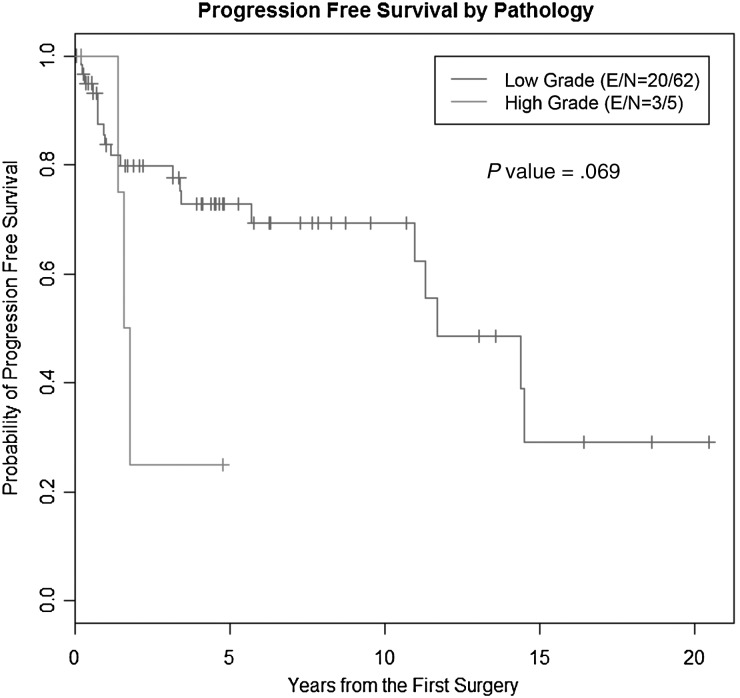
With a median follow-up of 4.7 years after the initial diagnosis of GG, 23 patients developed tumor progression. The range of time to progression was less than a month to 20 years after initial diagnosis. A quarter of the progressions occurred more than 10 years after diagnosis. The median PFS time in patients with low-grade tumors has not been reached yet. The 2-, 5-, and 10-year PFS rates for low-grade GG were 79.4% (95% confidence interval [CI]: 65.8%, 88%), 72.3% (95% CI: 57.5%, 82.7%), and 68.8% (95% CI: 53%, 80.3%), respectively; the median time to progression for patients with a high-grade tumor at diagnosis was 4.4 years (95% CI: 1.5, 5.9) and the 2-year PFS for high-grade GG was 25% (95% CI: 0.9%, 66.5%). The difference in PFS between patients with low-grade and high-grade tumors was not statistically significant (P = .07, log-rank test; Fig. 1).There was a difference in PFS between patients who underwent STR and GTR with 5-year PFS rates of 61.8% (95% CI: 37.9%, 78.8%) and 77.7% (95% CI: 58.6%, 88.8%); however, the difference was not significant (P = .18, log-rank test).
Fig. 1.
PFS in patients with GG according to grade at diagnosis. Kaplan–Meier analysis comparing low- versus high-grade GG at diagnosis. Abbreviations: E, number of events; N, number of patients.
Eight patients with low-grade tumor had malignant transformation from a low-grade GG to an anaplastic GG (2 of them transformed later to glioblastoma multiforme) and 1 patient transformed from grade III to grade IV tumor. Overall, 13 patients had high-grade tumors (primary or after recurrence), 5 at diagnosis and 8 after progression from a previously diagnosed low-grade lesion. Three patients progressed to glioblastoma. From the 3 patients who progressed to glioblastoma, 2 underwent GTR at time of initial diagnosis and 1 had a biopsy followed by resection, which may have resulted in a sampling error.
Radiotherapy and chemotherapy were not associated with an increased risk of malignant transformation. However, there was a strong association with increasing age and progressive disease, as the median PFS for patients younger than 40 was 14.4 years (95% CI: 11, NA) compared with median PFS of 3.4 years (95% CI: 0.9, NA) for patients older than 40 (P = .05).
Overall Survival Analysis
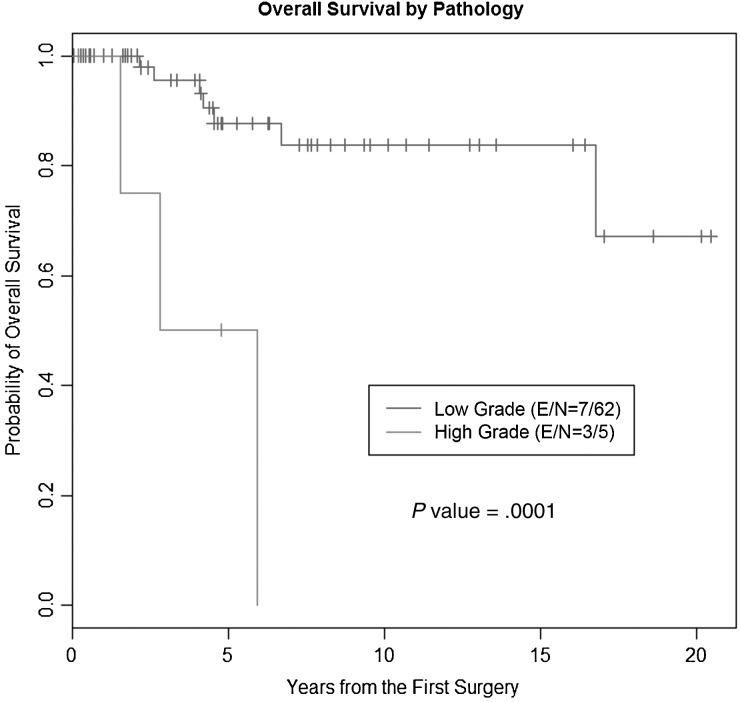
The median OS time for all patients was not reached with a median follow-up time of 4.7 years. The 2-, 5-, and 10-year OS rates for patients with low-grade GG were 100%, 87.5% (95% CI: 72.5%, 94.6%), and 83.7% (95% CI: 66.7%, 92.5%), respectively, and 1-, 2-, and 5-year OS rates for patients with a primary high-grade tumor were 100%, 75% (95% CI: 12.8%, 96.1%), and 50% (95% CI: 5.8%, 84.5%), respectively (P = .0001, log-rank test; Fig. 2).
Fig. 2.
OS in patients with GG according to grade at diagnosis. Kaplan–Meier analysis comparing low- versus high-grade GG at diagnosis. Abbreviations: E, number of events; N, number of patients.
Factors identified on univariate analysis that were significantly associated with OS were age at presentation, KPS at presentation, extent of resection, histologic grade (high vs low), and seizure control (Table 3). On multivariate Cox regression, lower tumor grade and younger age were significant factors for longer OS (Table 4). Extent of resection (biopsy vs total resection) is a significant factor for PFS.
Table 3.
Univariate analysis of OS and PFS
| Clinical Factors |
OS |
PFS |
|||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | ||
| Age | <40 vs ≥40 | 0.22 (0.006, 0.81) | .0233 | 0.39 (0.15, 1.05) | .06 |
| KPS | ≤80 vs ≥90 | 13.99 (2.96, 66.2) | .009 | NS | |
| Resection | Biopsy vs GTR | 7.38 (1.61, 33.76) | .01 | 3.46 (1.11, 10.76) | .03 |
| STR vs GTR | NS | NS | |||
| Pathology grade | Low grade vs high grade | 0.1 (0.02, 0.43) | .002 | 0.33 (0.09, 1.16) | .08 |
| Engel seizure scale | 1 vs 2–3 | 0.2 (0.04, 0.92) | .038 | NS | |
Table 4.
Multivariate analysis of OS and PFS
| Analysis of Maximum Likelihood Estimates | |||||||
|---|---|---|---|---|---|---|---|
| Parameter |
Parameter Estimate | Standard Error | P | Hazard Ratio | Hazard Ratio 95% CI | ||
| OS | |||||||
| Grade | Grade ≤II vs grade ≥III | −2.888 | 0.845 | .0006 | 0.056 | 0.011 | 0.292 |
| Age | <40 vs. ≥40 | −2.088 | 0.775 | .007 | 0.124 | 0.027 | 0.566 |
| PFS | |||||||
| Age | <40 vs ≥40 | −0.963 | 0.508 | .058 | 0.382 | 0.141 | 1.034 |
| Resection | Biopsy vs GTR | 1.977 | 0.812 | .015 | 7.222 | 1.47 | 35.496 |
| STR vs GTR | 0.544 | 0.440 | .217 | 1.723 | 0.727 | 4.084 | |
For the 62 patients with low-grade GG, radiation therapy at time of initial diagnosis did result in a statistically different OS. Those who received radiation did have a worse PFS (median PFS time for radiation vs no radiation: 1.3 y vs 14.5 y, P < .0001). To better account for the selection bias of the patients who received radiation therapy, a second analysis included only the 22 patients who underwent STR or biopsy. In this group, between the patients who received radiation (11 of 22) and those who did not (11 of 22), there was no statistical difference in OS or PFS.
In the 13 patients with anaplastic GGs there was no significant difference in OS for primary or secondary anaplastic GG. For the 3 patients with glioblastoma arising from a GG, median OS from time of diagnosis of glioblastoma multiforme was 1.5 years and median PFS was 1.1 years. Both of the patients who received only chemotherapy due to prior radiation did poorly, surviving only 0.9 and 1.6 years following diagnosis of anaplastic GG.
Eight of 43 patients treated for epilepsy were successfully weaned off of their antiepileptic medication; all of these patients had a grade I GG. Of these 8 patients, 7 had GTR.
Discussion
This retrospective review of 67 adult patients diagnosed with GG between 1992 and 2012 provides additional insight into the treatment patterns and outcome for this rare tumor diagnosis. The retrospective review corroborates the excellent prognosis of patients with low-grade GG who undergo GTR, as previously described in combined pediatric and adult patient populations.3,4,12 Additionally, our data identify several novel findings influencing survival of higher-grade GGs (anaplastic GGs and glioblastoma arising from GGs).13
In this study, histologic grade and extent of resection had clear prognostic significance in predicting PFS and OS in adult patients, as previously described.3,4,6 The improved outcome with GTR might indicate that tumors amenable to GTR might have a different underlying biological behavior than tumors that are not amenable to this surgical approach. Alternatively, the ability to resect the entire tumor may lessen the risk of recurrence and/or tumor dedifferentiation into a higher grade.
In our patients with low-grade GG who underwent biopsy or STR, there was no statistical benefit in median OS or median PFS in those patients receiving radiation therapy compared with those who did not receive radiation. Age, KPS, extent of resection, and seizure control were well balanced between groups. Previous studies analyzing the role of radiation therapy in GG patients have reached conflicting conclusions. A retrospective meta-analysis examining a nearly 30-year time spectrum of 402 patients reported that postoperative radiotherapy was beneficial in patients with GG who underwent STR but not in patients who had GTR.6 Outcomes from the patients in our series may differ because we did not include patients with diagnoses prior to 1992. Differences may result from technical advances in surgical techniques, as well as improvement in the accuracy of the pathological and radiographic diagnosis of GG. Finally, the difference in findings could be due to the relatively small size of our series compared with the meta-analysis.
Another retrospective study reported a trend in improved PFS in low-grade GGs in 88 pediatric and adult patients receiving STR and treated with radiation therapy.4 However, this study suffered from a small sample size in this subgroup of patients, and the patients who did not receive radiation therapy had an unusually short PFS of 1.1 years. The discrepancy in median PFS may be due to inclusion of higher-grade tumors due to sampling error or because pediatric GG may have a distinct behavior compared with adult GG. One additional case series that included 42 adults with supratentorial GGs found no correlation between adjuvant radiation and survival.12
The incidence of tumor progression in our study is similar to other studies, which had a range of 16%–35%, although much higher than the 3% reported by Luyken et al.3–6 The high incidence of progression in our series may be influenced by a referral bias, as uncomplicated GG patients may be less likely to be seen at our institution. With the observed rate of progression in combination with the late progression seen up to 20 years after diagnosis, patients with low-grade tumors should be carefully followed long term. There were late progressions seen even in patients receiving GTR, in contrast to a study finding no recurrences in those patients.14 As expected, PFS was shorter among patients with higher-grade tumors. In our patients with progression, there was a clear relationship with an older age of initial diagnosis. Patients with incomplete resections, higher-grade tumors, and older age warrant closer follow-up given their risk for progression.
High-grade GGs in this study have a survival pattern similar to that seen in other higher-grade gliomas, though the median survival in this group was longer than the median survivals of high-grade GG seen in prior studies.6,9 Treatment given to these patients was patterned after treatment used in other high-grade gliomas. A recently published Surveillance, Epidemiology and End Results database analysis of patients with anaplastic GG revealed that adjuvant radiotherapy did not influence overall survival.9 However, the 2 patients in our study who did not receive radiation therapy had poor survival, suggesting there may be a role for radiation therapy for these patients.
Due to lack of objective histologic criteria, it is difficult to diagnose this rare tumor. Retrospectively at least 3 patients with low-grade GG tumors may have received unnecessary adjuvant therapy based on different diagnoses. While no significant influence on outcome could be established due to the small number, those patients received therapy beyond standard of care and were potentially placed at risk of side effects from therapy.
Conclusions
Low-grade ganglioglioma is a tumor with excellent prognosis, even with a subtotal surgical resection. However, in our series, the frequency of progression emphasizes the importance of long-term follow-up in all patients. Ongoing molecular analysis of these tumors may identify tumors with a greater risk of recurrence. Benefit of radiation therapy in low-grade GG remains unclear. Definite conclusions regarding the use of chemotherapy and radiotherapy in this tumor remain difficult given its rarity.
Conflicts of interest. J.F.de-G. has advisory relationships with Genentech, VBL Therapeutics, Novartis, and Deciphera Therapeutics and received honoraria from Merck and research funding from Sanofi-Aventis, AstraZeneca, and EMD-Serono. None of the other authors declares a conflict of interest.
References
- 1.Becker A, Wiestler OD, Figarella-Branger D, Blumcke I. Ganglioglioma and Gangliocytoma. 4th edn. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 2.Zentner J, Hufnagel A, Wolf HK, et al. Surgical treatment of neoplasms associated with medically intractable epilepsy. Neurosurgery. 1997;41(2):378–386. doi: 10.1097/00006123-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Luyken C, Blumcke I, Fimmers R, Urbach H, Wiestler OD, Schramm J. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004;101(1):146–155. doi: 10.1002/cncr.20332. [DOI] [PubMed] [Google Scholar]
- 4.Compton JJ, Laack NN, Eckel LJ, Schomas DA, Giannini C, Meyer FB. Long-term outcomes for low-grade intracranial ganglioglioma: 30-year experience from the Mayo Clinic. J Neurosurg. 2012;117(5):825–830. doi: 10.3171/2012.7.JNS111260. [DOI] [PubMed] [Google Scholar]
- 5.Southwell DG, Garcia PA, Berger MS, Barbaro NM, Chang EF. Long-term seizure control outcomes after resection of gangliogliomas. Neurosurgery. 2012;70(6):1406–1413. doi: 10.1227/NEU.0b013e3182500a4c. discussion 1413–1414. [DOI] [PubMed] [Google Scholar]
- 6.Rades D, Zwick L, Leppert J, et al. The role of postoperative radiotherapy for the treatment of gangliogliomas. Cancer. 2010;116(2):432–442. doi: 10.1002/cncr.24716. [DOI] [PubMed] [Google Scholar]
- 7.Mehta MP. Neuro-oncology: gangliogliomas—what is the appropriate management strategy? Nat Rev Neurol. 2010;6(4):190–191. doi: 10.1038/nrneurol.2010.32. [DOI] [PubMed] [Google Scholar]
- 8.Rumana CS, Valadka AB. Radiation therapy and malignant degeneration of benign supratentorial gangliogliomas. Neurosurgery. 1998;42(5):1038–1043. doi: 10.1097/00006123-199805000-00049. [DOI] [PubMed] [Google Scholar]
- 9.Selvanathan SK, Hammouche S, Salminen HJ, Jenkinson MD. Outcome and prognostic features in anaplastic ganglioglioma: analysis of cases from the SEER database. J Neurooncol. 2011;105(3):539–545. doi: 10.1007/s11060-011-0615-4. [DOI] [PubMed] [Google Scholar]
- 10.Engel J, Van Ness P, Rasmussen T, Ojemann L. Outcome With Respect to Epileptic Seizures. 2nd edn. New York: Raven; 1993. [Google Scholar]
- 11.Wieser HG, Blume WT, Fish D, et al. ILAE commission report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282–286. [PubMed] [Google Scholar]
- 12.Rumana CS, Valadka AB, Contant CF. Prognostic factors in supratentorial ganglioglioma. Acta Neurochir (Wien) 1999;141(1):63–68. doi: 10.1007/s007010050267. discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 13.DeMarchi R, Abu-Abed S, Munoz D, Loch Macdonald R. Malignant ganglioglioma: case report and review of literature. J Neurooncol. 2011;101(2):311–318. doi: 10.1007/s11060-010-0248-z. [DOI] [PubMed] [Google Scholar]
- 14.Krouwer HG, Davis RL, McDermott MW, Hoshino T, Prados MD. Gangliogliomas: a clinicopathological study of 25 cases and review of the literature. J Neurooncol. 1993;17(2):139–154. doi: 10.1007/BF01050216. [DOI] [PubMed] [Google Scholar]