Abstract
Background
The functional diffusion map (fDM) has been suggested as a tool for early detection of tumor treatment efficacy. We aim to study 3 factors that could act as potential confounders in the fDM: areas of necrosis, tumor grade, and change in tumor size.
Methods
Thirty-four pediatric patients with brain tumors were enrolled in a retrospective study, approved by the local ethics committee, to examine the fDM. Tumors were selected to encompass a range of types and grades. A qualitative analysis was carried out to compare how fDM findings may be affected by each of the 3 confounders by comparing fDM findings to clinical image reports.
Results
Results show that the fDM in areas of necrosis do not discriminate between treatment response and tumor progression. Furthermore, tumor grade alters the behavior of the fDM: a decrease in apparent diffusion coefficient (ADC) is a sign of tumor progression in high-grade tumors and treatment response in low-grade tumors. Our results also suggest using only tumor area overlap between the 2 time points analyzed for the fDM in tumors of varying size.
Conclusions
Interpretation of fDM results needs to take into account the underlying biology of both tumor and healthy tissue. Careful interpretation of the results is required with due consideration to areas of necrosis, tumor grade, and change in tumor size.
Keywords: apparent diffusion coefficient, functional diffusion map, parametric response map, childhood tumors, diffusion-weighted magnetic resonance imaging
Brain tumors are the most common solid tumor in children and differ in biology and development from adult brain tumors. Few biological markers have been studied in childhood brain tumors compared with adults due to the relative rarity of the tumors. Limited literature studying childhood brain tumors, as compared to those in adults, thus exists; and these studies tend to include low patient numbers who receive diverse treatment regimens.1,2
Diffusion-weighted imaging (DWI) has been utilized as a tool for tumor diagnosis3 and prognosis4 in childhood brain tumors. By measuring the diffusion of water molecules in tissue and quantifying this in terms of the apparent diffusion coefficient (ADC), DWI is able to image cellularity as a consequence of water diffusion restriction in more densely packed tumors.5 The functional diffusion map (fDM), also known as the parametric response map, compares ADC changes over time. In the fDM, a voxel-wise comparison of pre- and posttreatment ADC maps is carried out in the tumor areas, and the difference in ADC is labeled in blue, green or red depending on whether a decrease, no change or an increase in ADC, respectively, was observed. In published studies, an increase in ADC is said to reflect a decrease in tumor cellularity and a good treatment response, whereas a decrease in ADC is said to reflect an increase in tumor cellularity and a poor treatment response. The fDM has thus been proposed as a tool to monitor early treatment response and efficacy in an attempt to identify patients who will benefit from further adjuvant therapy prior to a change in tumor size, which is the more conventional measure of tumor response.6–8
Our study examines 3 factors identified as possible confounders for the fDM in childhood brain tumors; and we hypothesize that necrosis, tumor grade, and change in tumor size need to be taken into consideration when interpreting fDM analysis in childhood brain tumors.
Firstly, tumor necrosis can be seen following both tumor growth (eg, as the tumor outgrows its blood supply) as well as a result of successful treatment. Limited literature exists linking areas of necrosis to survival; one study in patients with osteosarcoma showed no correlation between 90% necrosis and survival and suggested that more data were required to determine a possible correlation between 70% necrosis and survival.9 In another, earlier study in children with brain stem gliomas treated with radiotherapy, no association was found between the absence or presence of necrosis postradiation and outcome.10 Furthermore, the presence or absence of necrosis as a prognostic factor is dependent on the disease type in which it occurs. It is also being debated whether the inflammation in necrotic areas carries a good prognosis or not,11 as it is possible that a certain degree of necrosis may induce angiogenesis through the immune inflammatory cells that necrotic cell death promotes.12 We therefore aimed to study whether the fDM in necrotic areas discriminated between treatment outcome.
Secondly, tumor cellularity varies by tumor type and grade, and we applied the fDM technique to tumors of varying grades to establish its utility throughout a range of childhood tumors. Thirdly, some tumors change considerably in size after treatment. Since this may cause problems of registration, we aimed to identify whether the fDM can be successfully applied in these cases.
Materials and Methods
Patients
Thirty-four childhood brain-tumor patients (19 male, 15 female; aged 4 months to 16.5 years; mean 7.8 years), who had DWI as part of their clinical imaging between 2005 and 2012, were enrolled in a retrospective study. Ethical approval was given by the local ethics committee. Informed consent was not required for imaging data as this was obtained for clinical purposes. Informed consent was obtained in those cases were histology was used. All data were anonymized in accordance with the Data Protection Act.
Cases were selected so that fDM characteristics could be evaluated across a range of tumor types and grade. Cases were retained that had clinical DWI data available at 2 time points, with no surgery taking place between the 2 time points (which would obviate a meaningful assessment of the fDM) and with a significant tumor volume present in both images. Of the 34 patients, 18 were diagnosed with diffuse intrinsic pontine glioma (DIPG), 6 with optic pathway glioma (OPG), 4 with tuberous sclerosis and subependymal giant cell astrocytoma (SEGA), 3 with glioblastoma multiforme (GBM), and 3 with gliomatosis cerebri (GC). Histological diagnosis was confirmed in 4 SEGA patients, 3 GBM patients, 2 GC patients, and 1 OPG patient. In the remainder, the diagnosis was made on neuroradiological grounds with imaging discussed in a multidisciplinary team setting and accepted as a basis for treatment. The diagnostic histology was reviewed in 2 patients (1 GBM and 1 OPG) and compared with that from a patient showing normal-appearing white matter.
Image Acquisition
Imaging data were acquired on a 1.5T Siemens Magnetom Symphony MRI scanner, with a maximum magnetic field gradient strength of 30 mTm−1, and on a 1.5T Siemens Avanto scanner, with a maximum magnetic field gradient strength of 40 mTm−1. DWI data were obtained using a diffusion-sensitized single-shot echo planar imaging sequence (b = 0, 500, 1000 s mm−2). The clinical ADC, using all 3 b-values, was used in this analysis. Diffusion gradients were applied in 3 orthogonal directions, with an image matrix of 128 by 128 and field of view of 230 by 230 mm. On the Avanto scanner, 19 slices were acquired with a 5 mm thickness, 1.5 mm gap, and a total sequence time of 64 seconds, with TR = 2700 ms and TE = 96 ms. The Symphony protocol specifies 20 slices with a 5 mm thickness, 2.5 mm gap, and a total sequence time of 56 seconds, with TR = 3600 ms and TE = 107 ms.
fDM Analysis
Fig. 1 shows how the fDM is constructed. Prior to building the fDM, ADC maps at the time points analyzed were coregistered to the patient's T2-weighted image at diagnosis or postsurgery to exclude major changes in the tumor due to surgery. Where pretreatment images were not available, as some patients would have arrived at our institution with these images already having been taken, 2 posttreatment images were used. MATLAB (MathWorks) and SPM8 (Wellcome Trust Centre for Neuroimaging) were used for coregistration, applying the standard normalized mutual information and a trilinear interpolation algorithm. A visual inspection was carried out on all coregistered images to ensure successful registration.
Fig. 1.
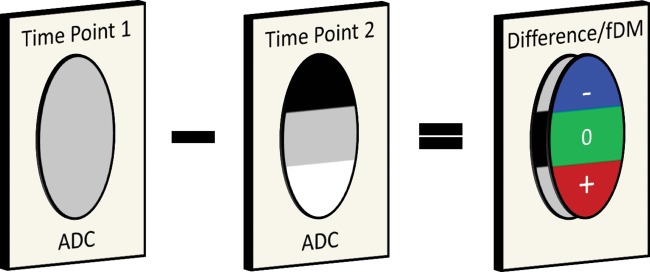
Construction of the fDM. The fDM is built by using tumor images at 2 time points. After registration of the 2 images, a difference image is calculated. A decrease in ADC is labeled in blue, an increase in ADC is labeled in red, and no change in ADC is labeled in green.
The fDM, implemented in MATLAB, was applied in tumor areas by specifying a region of interest (ROI) using FSLView (FMRIB). ROIs were defined across all tumor image slices by considering both ADC and T2-weighted images, including tumor regions and areas of necrosis but excluding peritumoral edema where possible. The tumor boundary was identified using both ADC and T2 weighted images. Areas of bright ADC outside the defined tumor boundary were considered to be peritumoral edema and were excluded. Infiltrative edema was included in the analysis as it cannot be easily differentiated from tumor using ADC and T2 weighted imaging.
A voxel-wise comparison was carried out, over the whole tumor, on the 2 time points being investigated. Specific thresholds were used to determine whether there was an increase, decrease, or no change in ADC when comparing ADC images. This study employed the suggested threshold of 0.40 × 10−3 mm2 s−1, the threshold indicating the highest sensitivity and specificity in the receiver operating characteristic (ROC) analysis.8 This means that a voxel with ADC increasing by more than this value was classified as increasing in ADC and displayed in red in the fDM. A voxel with ADC decreasing by more than this threshold was classified as decreasing in ADC and displayed in blue. Any voxels with an ADC change between these thresholds were classified as not changing in ADC and were displayed in green. fDM findings were compared with clinical imaging reports in order to identify whether the fDM correctly identified tumor response from tumor progression as reported by the clinical radiologist.
Areas of Necrosis
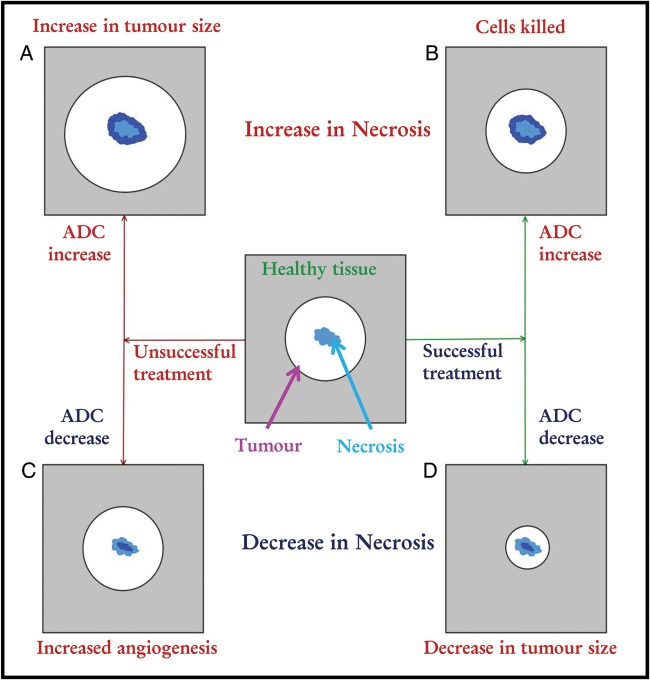
By showing the biological processes involved in the fDM, Moffat et al. briefly mentioned that necrotic or cystic regions can undergo a displacement of water resulting in a reduction in ADC as tumor cells move into the area.6 In addition to this, in theory, areas of necrosis can increase or decrease in size irrespective of treatment outcome. Fig. 2 shows a flow map for the different possible outcomes of treatment response in terms of necrotic areas within the tumor.
Fig. 2.
Theoretical change in areas of necrosis by treatment. Necrotic areas of a tumor can increase in size as a result of (A) tumor growth (causing increased hypoxic regions and hence necrosis) and (B) successful treatment (as cells are killed, tumor regions are replaced by areas of necrosis). Conversely, a reduction in size of necrotic regions can be due to (C) tumor growth through angiogenesis (making the tumor more vascular and hence more cellular in areas that would otherwise have been necrotic), and (D) tumor size reduction due to successful treatment (as the tumor shrinks in size, areas of necrosis may be replaced by glial cells).
The fDM was studied in areas of necrosis in patients with DIPG and with GC, as these were the cases that showed the most necrosis as compared with the other groups studied. Areas of necrosis were identified in each image as those voxels with an ADC value higher than 1.8 × 103 mm2 s−1 –, a value observed to make up most of the necrotic regions while excluding both normal and tumor tissue. The ROI was selected such that only tumor areas visible on both pre- and posttreatment images were included in the analysis. A comparison was made between fDM tumor treatment response classification when including and excluding necrosis to test the hypothesis that necrotic areas act as a confounder in the fDM.
Tumor Grade
Most fDM studies have been carried out on adults in high-grade tumors.13–15 Given that high- and low-grade tumors differ in ADC3, the corresponding fDM may also differ in terms of treatment response classification. Fig. 3 shows the theoretical changes that can occur to the ADC and the fDM when high- and low-grade tumors respond to treatment or show signs of progressive disease. The fDM in high-grade (GBM, WHO grade IV), mid-grade (DIPG, WHO grades II and III), and low-grade (OPG, WHO grade I) tumors were analyzed, and findings were compared with the outcome described in clinical reports obtained at the time of the follow-up (second) imaging session.
Fig. 3.
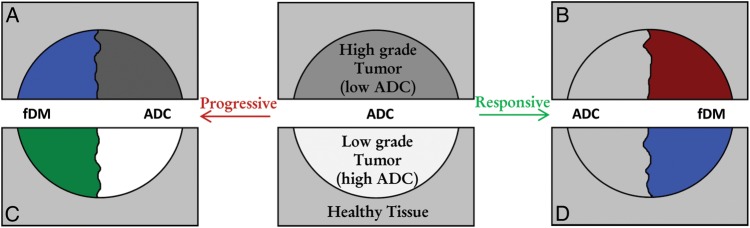
Theoretical changes in the fDM in tumors of varying grade. (Red represents an increase, blue a decrease, and green no change in ADC). The upper half of the image shows the theoretical change in ADC in high-grade tumors, which appear dark with low ADC values. (A) Progressive high-grade tumors will increase in cellularity and result in a lower and darker ADC value (blue in fDM). (B) Conversely, a high-grade tumor responding well to therapy will decrease in cellularity and increase in ADC to values more similar to those of healthy tissue (red in fDM). The lower half of the image shows the theoretical change in ADC in low-grade tumors, which appear bright with high ADC values. (C) In progressive disease, it is expected that the tumor will either grow or become necrotic. Hence, excluding areas of necrosis, it is not expected to change in ADC (green in fDM), which is also indicative of stable disease. (D) Low-grade tumors that respond to therapy are likely to be replaced by lower ADC healthy tissue, and hence, responsive low-grade tumors would decrease in ADC (blue in fDM).
Change in Tumor Size
SEGA patients were found to respond well to rapamycin,16 and the effect of a reduction in tumor size was addressed by analyzing the fDM in this patient group as there was a good response to treatment, with the tumor decreasing in size considerably in 3 of 4 cases. The images were analyzed using first a pretreatment mask and then the overlap between the pretreatment tumor areas and posttreatment tumor areas. fDM findings were again compared with clinical imaging reports at the time of the follow-up (second) image.
Statistical Analysis
In order to analyze the fDM in areas of necrosis and in tumors of varying grade, contingency tables were constructed (Tables 1 and 2). Fisher' exact test17 was applied to these tables using MATLAB and a predefined function.18 Contingency tables show how categorical variables are related to each other by representing the frequency distribution of the variables analyzed. Fisher' exact test allows for the analyses of such tables, particularly when the sample size is small.
Table 1.
The fDM in necrotic areas compared with clinical response and change in size in necrotic areas
| fDM in Necrotic Areas: |
Inc | NC | Dec | P value | |
|---|---|---|---|---|---|
| Size of necrotic area | Increase | 6 | |||
| No change | 3 | ||||
| Decrease | 4 | P < .001 | |||
| Clinical response | Response | 3 | 1 | 1 | |
| Stable | 2 | ||||
| Progression | 1 | 2 | 3 | P = .31 | |
Abbreviations: Dec, decrease in apparent diffusion coefficient; fDM, functional diffusion map; Inc, increase in apparent diffusion coefficient; NC, no change.
Table 2.
fDM changes by tumor grade and clinical response
| Tumor Grade: | High | Mid | Low | P value | ||
|---|---|---|---|---|---|---|
| fDM increase in ADC | Clinical Response | Response | 1 | |||
| Stable | ||||||
| Progression | 5 | 1 | P < .001 | |||
| fDM no change | Clinical Response | Response | 2 | |||
| Stable | 3 | 6 | ||||
| Progression | 1 | 3 | P = .04 | |||
| fDM decrease in ADC | Clinical Response | Response | 5 | 4 | ||
| Stable | ||||||
| Progression | 2 | P < .001 |
Abbreviations: ADC, apparent diffusion coefficient; fDM, functional diffusion map.
Results
Of the patients included in this study, none of the DIPG and OPG patients had partial or total resection of tumor. Two GBM patients, 1 GC patient, and all 4 SEGA patients had partial or total resection of tumor. The fDM was built in 11 patients pre- and post treatment, in 20 patients using 2 posttreatment images, and in 3 patients using 2 pretreatment images where a “watch and wait” or a palliative care protocol was employed. The time interval between the 2 images ranged from 2 weeks to 13 months.
Areas of Necrosis
In the DIPG patient group, the tumor area consisted, on average, of 11.2% necrosis, while the GC patient group had on average 31.6% necrosis measured by calculating the percentage of voxels with an ADC higher than 1.8 × 10−3 mm2 s−1 in the tumor ROI. Ten of 18 patients with DIPG and all 3 patients with GC showed areas of necrosis >5%. In all of these cases, an increase or decrease in ADC in areas of necrosis was related to an increase or decrease in size of necrotic regions, respectively, and was not related to the treatment response identified in clinical image reports. Table 1 shows a summary of the fDM in necrotic areas and how these related to the size of necrotic areas and clinical response. Fisher' exact test on this data confirmed the fDM in areas of necrosis to be related to the change in size of necrotic areas (P < .001) and not related to clinical response (P = .31). When classifying tumor response using the fDM, excluding areas of necrosis made a difference in 4 of 21 cases studied. Fig. 4 shows 2 examples of the fDM showing an increase in ADC in areas of necrosis, while clinical image reports identified stable disease in both cases.
Fig. 4.
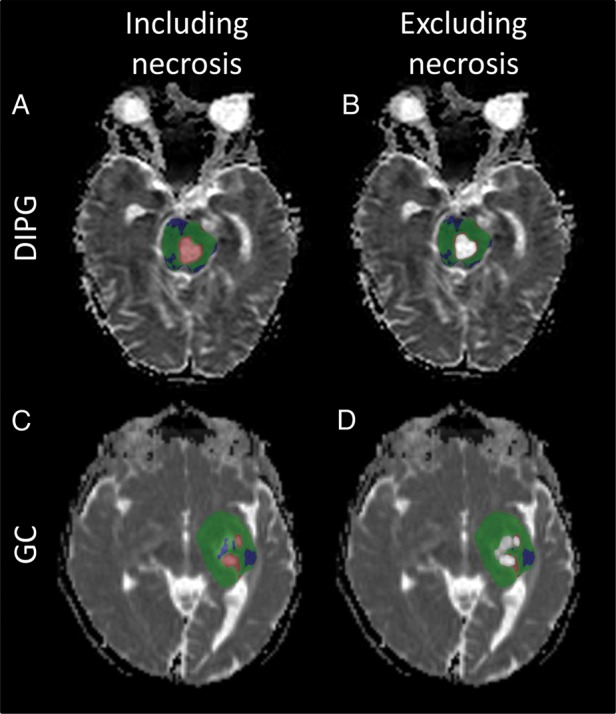
The fDM in areas of necrosis. The DIPG patient was treated with radiotherapy and chemotherapy, and the fDM was constructed from 1 post treatment image and a 3-month follow-up image. (A and B) The fDM in DIPG shows areas of increased ADC (red) (A) that are eliminated when excluding areas of necrosis (B). The GC patient was treated via surgery followed by chemotherapy, and the fDM was constructed from 1 image taken 3 months after start of chemotherapy and a 1-year follow-up image. (C and D) The fDM in GC showed areas of increased ADC in necrotic regions (C) , which were again eliminated when necrotic regions were excluded (D). Removal of the necrotic regions is concordant with no tumor response in 2 patients with no change in tumor size.
Tumor Grade
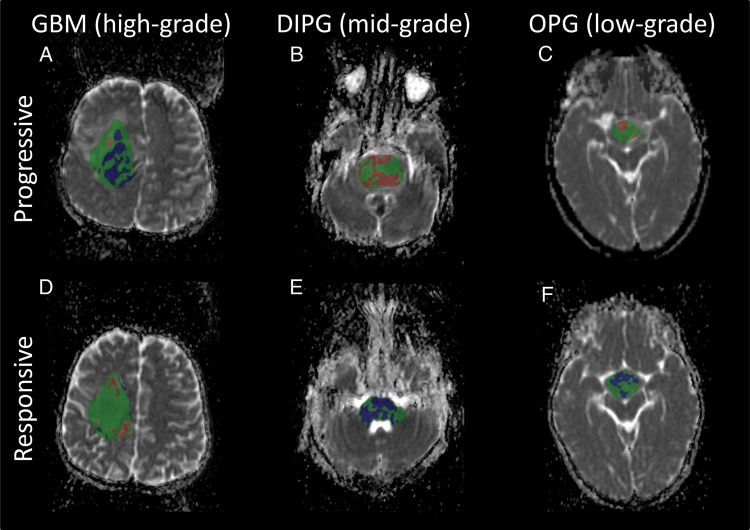
The fDM in GBM (high-grade) showed areas of decreased ADC at progression and areas of increased ADC with positive treatment response. Conversely, in DIPG (mid-grade) and OPG (low-grade), the fDM showed areas of increased ADC at progression and areas of decreased ADC at tumor treatment response (Fig. 5). In the lower-grade tumors, it was noted that an increase in ADC was mostly associated with an increase in necrotic/cystic components of the tumor.
Fig. 5.
The fDM in tumors of varying grade. A comparison is shown of the fDM in GBM (A and D), DIPG (B and E), and OPG (C and F) in areas of progression (top row) and treatment response (bottom row). (A) In high-grade tumors, a decrease in ADC (blue) was indicative of an increase in cellularity and progression. (E and F) In mid- and low-grade tumors, a decrease in ADC was indicative of positive treatment response as high ADC tumor was replaced by healthy tissue. (D) Similarly, an increase in ADC (red) was indicative of positive treatment response in high-grade tumors, and in the above cases (B and C), progression in mid- and low-grade tumors (B and C). Tumor progression and treatment response were defined by a radiologist at the time of second imaging.
Table 2 shows a summary of the changes in the fDM according to tumor grade and response. Fisher' exact test showed that there was a significant difference between tumor grade and clinical response for the increase and decrease in ADC (P < .001). The comparison between grade and clinical response when the tumor fDM did not change was only marginally significant (P = .04).
Of the 3 GBM patients, one showed small areas of increased ADC at response and large areas of decreased ADC at progression. A second patient showed small areas of decreased ADC at progression. In the third patient, negligible changes in ADC were observed at progression.
Of the 18 DIPG patients, 8 progressed at second imaging with 5 showing an increase in ADC and 3 showing minor to no changes in ADC; 7 responded to treatment, with 5 showing a decrease in ADC and 2 showing minor to no changes in ADC; 3 showed stable disease with minor changes in ADC.
Of the 6 OPG patients, 4 showed areas of decreased ADC at treatment response and minor to no change at stable disease, with one of these patients progressing at a later time point and showing increased ADC; 2 patients showed minor to no changes in ADC with stable disease.
Change in Tumor Size
Of the 4 SEGA patients analyzed, there was a large decrease in tumor size in 3 patients. When using pretreatment masks, the fDM showed a large area of decreased ADC in the 3 SEGA patients responding to treatment. These areas were excluded from the fDM when looking at only the overlap (Fig. 6). Outside of the tumor overlap areas, a decrease in ADC was observed when tumor areas were replaced by healthy tissue, and an increase in ADC was observed when tumor areas were replaced by areas of cerebrospinal fluid. In selecting only tumor overlap areas, a more accurate assessment of the fDM findings was therefore given, although results did not indicate that the fDM gave any more information than standard clinical imaging reports.
Fig. 6.
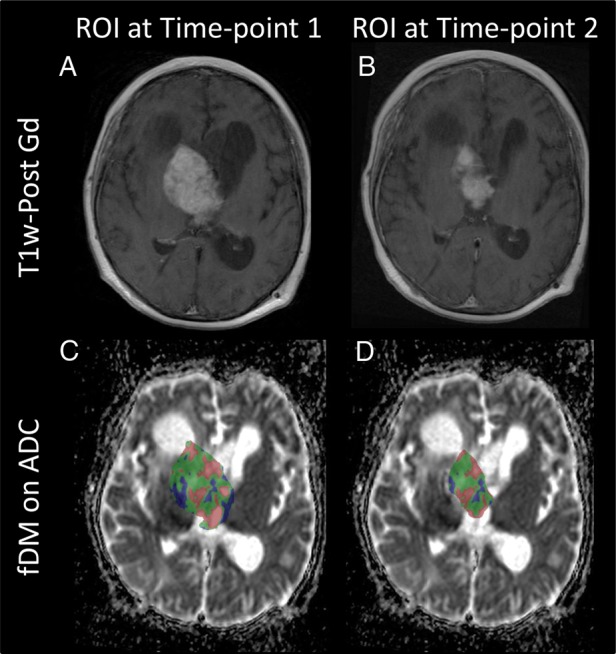
Change in tumor size. (A and C) A comparison of the fDM when using a pretreatment mask and (B and D) only the overlap between pre- and posttreatment images is shown in a SEGA patient. The top row shows T1-weighted postcontrast imaging. The bottom row shows the fDM in 1 case, showing (C) a mixture of areas of increased ADC (red) and decreased ADC (blue) when considering the pretreatment mask, and (D) mostly areas of increased ADC when considering the tumor overlap area.
Histology
A comparison of high-grade (GBM), low-grade (OPG), and normal-appearing white matter is shown in Fig. 7. The low-grade tumor had some areas of increased cellularity compared with normal-appearing white matter and some myxoid areas with a loose microcystic stroma. This may help explain the increased ADC observed in low-grade tumors. The high-grade tumor showed the highest cellularity; due to this restricted diffusion, these tumors appear dark in an ADC image.
Fig. 7.
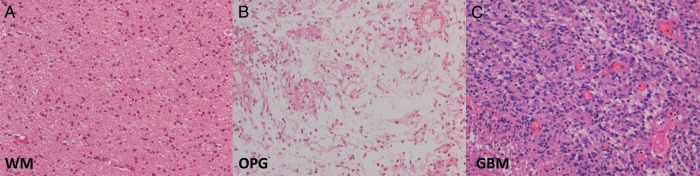
Histological comparison of low- and high-grade tumors. A comparison of a low-grade OPG (B), with a histological diagnosis of pilocytic astrocytoma (WHO grade I) and a high-grade GBM (C) are shown together with a comparative image from normal-appearing white matter (WM) (A). The low-grade tumor showed some areas with high cellularity (as compared with WM) as well as myxoid areas shown in B. The microcystic changes observed in the low-grade tumor could explain the increased ADC observed in these tumors when compared with normal-appearing white matter. The high-grade tumor (C) showed the highest cellularity, which restricts diffusion and explains why these tumors appear dark in ADC images. (All images are hematoxylin-eosin stained and at the same magnification [x20].)
Discussion
Determining treatment response early on in the treatment cycle is of vital importance for moving toward personalized medicine with the ability to alter doses or change therapy in those cases where the current treatment is seen to be ineffective. The fDM was previously shown to be an effective biomarker for detecting treatment response earlier than current standard techniques that consist of radiological assessment, in most cases at the end of therapy.13 However, in this analysis, a number of limitations have been identified and studied, which indicates the need to exercise caution when interpreting fDM results.
Areas of Necrosis
Necrotic areas of a tumor can increase in size both as a result of successful treatment (as cells are killed, tumor regions are replaced by areas of necrosis) and as a result of tumor growth (causing increased hypoxic regions and hence necrosis). Our results have shown the fDM does not give an accurate interpretation of treatment response in areas of necrosis. An increase/decrease in ADC in these areas was mostly related to an increase/decrease in necrotic areas rather than positive treatment response or progression. Eliminating areas of necrosis made a difference in treatment response classification in 20% of the cases studied. Given that such areas may act as a potential confounder in the fDM, we suggest excluding them from the fDM in order to accurately assess tumor response.
Tumor Grade
Our results have identified tumor grade as a potential confounder in the fDM, and we have shown the importance of taking this into consideration in analyzing the fDM. Previous publications have shown an increase in ADC in the fDM to be indicative of positive treatment response6 and a decrease in ADC to be indicative of tumor progression.15 This concept is underpinned by research that showed the ADC to be inversely correlated with cellularity.5 This was based on the assumption that the ADC is lower in tumor tissue than in surrounding healthy tissue due to the increased cellularity of tumors. While this is valid in high-grade tumors, tumor tissue can include microcystic areas or areas of infiltrative edema in lower-grade tumors, which drives the ADC up even though the cellularity of the tumor itself may be higher than that of surrounding tissue.3 Unless there is tumor progression from low- to high-grade, a decrease in ADC in low-grade tumors is therefore likely to be a sign of treatment response as the higher ADC tumor tissue is being replaced by healthy tissue, rather than the tumor progressing by becoming more cellular. Furthermore, an increase in ADC in low-grade tumors is more likely to be associated with an increase in necrotic regions and, as shown in this study, tumor response cannot be inferred from these areas.
Change in Tumor Size
Previous work has shown how image registration, particularly due to an increase in tumor size, may be a major limitation to the technique employed in the fDM, and methods of nonlinear registration may be beneficial.19 In the cases investigated in this study, there was a considerable decrease in tumor size. In tumor regions replaced by healthy tissue, a decrease in ADC was observed in the areas where there was a reduction in size back to healthy tissue and an increase in ADC where tumor areas were replaced by cerebrospinal fluid. In the leftover tumor volume, areas of decreased ADC were limited, and hence no inference could be made as regards treatment success or progression. Given the limited number of patients in this group, we cannot conclude whether reduction in size is a confounder in the fDM when using linear registration; however, a reduction in size is already an indicator of treatment success, and the fDM does not appear to give any further information in the cases we analyzed. That said, changes in tumor size need to be treated with caution, and careful visual inspection of registered images needs to be carried out in order to avoid problems of registration due to a change in tumor size.
Study Limitations
The main limitation of this study is that it was carried out on a group of childhood brain tumors with small numbers of similar tumors. However, different tumor types were specifically selected so that the fDM could be evaluated in pediatric tumors of differing grades. Case ascertainment was limited by the rarity of childhood brain tumors and the fact that patients often arrive at our institution with pretreatment imaging that did not include comparable DWI that would allow an fDM to be generated. Surgery took place immediately in some cases, and hence the fDM could only be applied to 2 posttreatment images. However, fDM results were compared with clinical imaging reports at time of follow-up (second) imaging, rather than final clinical outcome, to minimize this limitation. Further analyses are warranted on other tumor types and in larger numbers in order to evaluate more fully the effects of the confounders described here on the fDM.
Conclusion
In conclusion, our results have shown that, while the fDM may be a useful tool for determining tumor treatment response, careful interpretation needs to be carried out that considers the underlying biology of both tumor and healthy tissue in order to determine whether a tumor is responding positively to treatment or not. Areas of necrosis, tumor grade, and change in tumor size are all factors that need to be taken into account when carrying out fDM analyses.
Funding
This work was funded by Cancer Research UK, grant number C7809/A10342.
Acknowledgments
Thanks to Tina Banks at Great Ormond Street Hospital for Children, London, and Patrick Hales and Martin King at UCL Institute of Child Health, London, for their help in this study.
Conflict of interest statement. None declared.
References
- 1.Fleming AJ, Chi SN. Brain tumors in children. Curr Probl Pediatr Adolesc Health Care. 2012;42(4):80–103. doi: 10.1016/j.cppeds.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Jones C, Perryman L, Hargrave D. Paediatric and adult malignant glioma: close relatives or distant cousins? Nat Rev Clin Oncol. 2012;9(7):400–413. doi: 10.1038/nrclinonc.2012.87. [DOI] [PubMed] [Google Scholar]
- 3.Bull JG, Saunders DE, Clark CA. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur Radiol. 2012;22(2):447–457. doi: 10.1007/s00330-011-2255-7. [DOI] [PubMed] [Google Scholar]
- 4.Grech-Sollars M, Saunders DE, Phipps KP, Clayden JD, Clark CA. Survival analysis for apparent diffusion coefficient measures in children with embryonal brain tumours. Neuro Oncol. 2012;14(10):1285–1293. doi: 10.1093/neuonc/nos156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9(1):53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA. 2005;102(15):5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamstra DA, Chenevert TL, Moffat BA, et al. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA. 2005;102(46):16759–16764. doi: 10.1073/pnas.0508347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellingson BM, Malkin MG, Rand SD, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31(3):538–548. doi: 10.1002/jmri.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Ashana AO, Moretti VM, Lackman RD. The relation of tumour necrosis and survival in patients with osteosarcoma. Int Orthop. 2011;35(12):1847–1853. doi: 10.1007/s00264-011-1209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer. 1994;74(6):1827–1834. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13(24):7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Hamstra DA, Galbán CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26(20):3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbán CJ, Chenevert TL, Meyer CR, et al. Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res. 2011;17(14):4751–4760. doi: 10.1158/1078-0432.CCR-10-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellingson BM, Cloughesy TF, Zaw T, et al. Functional diffusion maps (fDMs) evaluated before and after radiochemotherapy predict progression-free and overall survival in newly diagnosed glioblastoma. Neuro Oncol. 2012;14(3):333–343. doi: 10.1093/neuonc/nor220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasin S, Harkness W, Cross J, Cohen N, Jacques T. Subependymal Giant Cell Astrocytoma (SEGA) cells respond to rapamycin in vitro. Neuropath Appl Neuro. 2010;0:41–42. [Google Scholar]
- 17.Ghent AW. A Method for Exact Testing of 2X2, 2X3, 3X3, and Other Contingency Tables, Employing Binomial Coefficients. Am Midl Nat. 1972;88(1):15–27. [Google Scholar]
- 18.Cardillo G. MyFisher33: a very compact routine for Fisher's exact test on 3×3 matrix. 2007 http://www.mathworks.co.uk/matlabcentral/fileexchange/15482-myfisher33. (last accessed 15 November 2013). [Google Scholar]
- 19.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Pope WB. Nonlinear registration of diffusion-weighted images improves clinical sensitivity of functional diffusion maps in recurrent glioblastoma treated with bevacizumab. Magn Reson Med. 2012;67(1):237–245. doi: 10.1002/mrm.23003. [DOI] [PubMed] [Google Scholar]