Abstract
Background
A predictive marker of bevacizumab activity is an unmet medical need. We evaluated the predictive value of selected circulating prebiomarkers involved in neoangiogenesis and invasion on patient outcome in recurrent high-grade glioma treated with bevacizumab.
Methods
Analyzed in plasma were a set of 11 prebiomakers of interest (vascular endothelial growth factor receptor [VEGF]; VEGF receptor 2; basic fibroblast growth factor; stromal cell derived factor 1; placenta growth factor; urokinase-type plasminogen activator; plasminogen activator inhibitor 1; matrix metalloproteinases 2, 7, and 9; and adrenomedulline), using ELISA, at baseline and 2 weeks after bevacizumab initiation in a prospective cohort of 26 patients (Cohort 1). Correlations were validated in a separate retrospective cohort (Cohort 2; n = 50) and tested in cohort patients treated with cytotoxic agents without bevacizumab (Cohort 3; n = 34). Dosages were correlated to objective response, progression-free survival (PFS), and overall survival (OS).
Results
In Cohort 1, high MMP2 baseline level was associated with a probability of objective response of 83.3% versus 15.4% for low MMP2 level (P = .001). In multivariate analysis, baseline level of MMP2 correlated with PFS (hazard ratio, 3.92; 95% confidence interval [CI]:1.46–10.52; P = .007) and OS (hazard ratio, 4.62; 95% CI: 1.58–13.53; P = .005), as decrease of VEGF (P = .038 for PFS and P = .013 for OS) and MMP9 (P = .016 for PFS and P = .025 for OS). In Cohort 2, MMP2, but not MMP9, confirmed its predictive significance. In Cohort 3, no association was found between MMP2, MMP9, and outcome.
Conclusion
In patients with recurrent high-grade glioma treated with bevacizumab, but not with cytotoxic agent, high MMP2 plasma levels are associated with prolonged tumor control and survival. MMP2 should be tested in randomized clinical trials that evaluate bevacizumab efficacy, and its biological role reassessed.
Keywords: bevacizumab, high grade glioma, matrix metalloproteinase 2, matrix metalloproteinase 9, predictive factor
Antiangiogenic agents that target the vascular endothelial growth factor (VEGF) pathway have been successfully developed and approved in the vast majority of cancers. As a consequence, an increasing use of antiangiogenic agents has been observed, leading to cost issues and reassessment of their benefit. However, activity on tumor response, and survival benefit of these agents, varies mostly among tumor types and with agents tested. Bevacizumab, a monoclonal antibody against VEGF, was the first antiangiogenic agent that has demonstrated a benefit on progression-free survival (PFS) with or without impact on survival, in patients with advanced and metastatic cancer.1
Glioblastoma multiforme (GBM) is a devastating disease, characterized by a highly angiogenic and invasive phenotype, suggesting a potential role for antiangiogenic strategies. Preclinical data as well as high levels of intratumoral VEGF expression have supported the evaluation of agents that target the VEGF pathway.2 Among them, bevacizumab has been recently approved by the FDA for patients with recurrent glioblastoma, based on a high response rate (RR) with prolonged PFS compared with historical controls3 and is under investigation in the first-line setting.4
Biomarkers able to predict response to antiangiogenic agents and particularly to bevacizumab are an unmet medical need for patients with cancer. The ideal biomarker should be easy to measure on multiple points upon treatment and standardized in their analysis.5 Baseline levels and/or variation of numerous intratumoral or circulating candidate prebiomarkers have been explored. However, to date their predictive significance has been generally weak and rarely confirmed among studies or compared with a cytotoxic treated population. In situ prebiomarkers such as VEGF, VEGF receptor (VEGFR) 2, and carbonic anhydrase 9, as well as plasma prebiomarkers such as VEGF, VEGFR1, intercellular adhesion molecule 1, and interleukin (IL)-6 and -8, have been reported to predict bevacizumab benefit, but this predictive value is generally weak and restricted to one end point (response, PFS, or overall survival [OS]).6 With other antiangiogenic agents such as cediranib in GBM and vandetanib in non–small-cell lung carcinoma, various prebiomarkers, including VEGF, VEGFR2, placenta growth factor (PlGF), basic fibroblast growth factor (bFGF), and matrix metalloproteinase (MMP) 2, present transition variations that have been related to either progression or survival.7,8 Given the remarkable but inconsistent activity of bevacizumab in GBM, we explored the value of selected plasma prebiomarkers to predict response and survival in patients treated with bevacizumab for recurrent high-grade glioma (HGG).
Materials and Methods
Patients
Initial cohort (Cohort 1)
Cohort 1 consisted of 26 patients prospectively included at Timone Hospital from July 2007 to January 2010 for the purpose of this study. Eligible patients included those aged 18 years or older with recurrent HGG treated with bevacizumab at least 3 months after the end of radiotherapy to avoid pseudoprogression phenomena. None of the patients had histological confirmation of recurrence, so that the histology reported is the initial documented histology. All patients were treated with the combination of bevacizumab 10 mg/kg and irinotecan 125 mg/m2 every 2 weeks. Plasma was collected before bevacizumab first dose administration, then at days 15 and 29, and then every month until progression. Only baseline and day 15 samples are reported in the present analysis. Characteristics of the 26 patients included are described in Table 1. At the time of last follow-up, 24 patients had died of disease. All patients provided written informed consent in accordance with institutional and national guidelines and the Declaration of Helsinki. This protocol was approved by an institutional review board.
Table 1.
Characteristics of patients' in Cohorts 1, 2, and 3
| Cohort 1 (n = 26) |
Cohort 2 (n = 50) |
Cohort 3 (n = 34) |
||||
|---|---|---|---|---|---|---|
| Age, y (range) | 56.1 (22.3–73.2) | 59.7 (18.3–76.7) | 57.7 (36.2–73.9) | |||
| Gender | 16 M/10 F | 34 M/16 F | 22 M/12 F | |||
| n Patients % | n Patients % | n Patients % | ||||
| Initial histology | ||||||
| Grade II | 1 | 3.8 | 5 | 10 | 0 | 0 |
| Anaplastic | 5 | 19.2 | 14 | 28 | 2 | 6 |
| Glioblastoma | 20 | 76.9 | 31 | 62 | 32 | 94 |
| IDH1 mutation | 3/18 | 16.7 | ||||
| Response | 12 | 48 | 18 | 36.7 | 1 | 3.2 |
| Complete | 3 | 12 | 1 | 2 | – | – |
| Partial | 9 | 36 | 17 | 34.7 | 1 | 3.2 |
| No response | 13 | 52 | 31 | 63.3 | 30 | 86.8 |
| Stable disease | 2 | 8 | 15 | 30.6 | 3 | 9.7 |
| Progression | 11 | 44 | 16 | 32.7 | 27 | 87.1 |
| Nonevaluable | 1 | 1 | 1 | |||
| Treatment lines* | ||||||
| 2 | 15 | 57.5 | 21 | 46 | 8 | 23.5 |
| 3 | 7 | 26.9 | 24 | 48 | 17 | 50 |
| 4 | 3 | 11.5 | 4 | 4 | 6 | 17.6 |
| ≥5 | 1 | 3.8 | 1 | 2 | 3 | 8.9 |
| KPS | ||||||
| 50 | 0 | 0 | 2 | 4 | 2 | 6.1 |
| 60 | 4 | 15.4 | 18 | 36 | 5 | 15.2 |
| 70 | 15 | 57.7 | 18 | 26 | 11 | 33.3 |
| ≥80 | 7 | 26.9 | 12 | 24 | 15 | 45.4 |
| OS (mo) | 8.7 | 7.1 | 6.1 | |||
| Responders | 13 | 14.6 | ||||
| Nonresponders | 4.5 | 5.8 | ||||
| PFS (mo) | 4.4 | 5.3 | 1.9 | |||
| Responders | 8.2 | 8 | ||||
| Nonresponders | 2.8 | 3 | ||||
*Before bevacizumab initiation.
Cohort 2
In view of the results observed in our initial cohort, we retrospectively identified in the plasma bank from our center a second cohort of 50 patients treated from August 2007 to March 2010 with bevacizumab and irinotecan for a recurrent HGG for whom plasma was available at baseline only. Treatment regimen, evaluation, and follow-up were similar to those for Cohort 1. Patients included presented similar characteristics, although 40% exhibited a KPS ≤60 (vs 15.4% in Cohort 1) and were more heavily pretreated (Table 1). At the time of last follow-up, all patients had died of disease.
Cohort 3
Because of the lack of appropriate material in our serum bank, patients of Cohort 3 were recruited from the plasma banks of Avicenne Hospital (Bobigny, France) and Pitié-Salpétrière Hospital (Paris, France). Eligible patients included those aged 18 years or older with recurrent HGG treated with cytotoxic (mainly alkylating) agents or immunotherapy at recurrence, excluding antiangiogenic agents such as bevacizumab at any time during the course of their disease (Table 1). Plasma was collected before the first administration of treatment.
Clinical Follow-up
Clinical follow-up of patients from Cohort 1 and Cohort 2 was performed every 4 weeks and MRI every 8 weeks by a senior consultant (M.B., M.M., O.C.). Disease evaluation was performed according to Macdonald criteria' adapted to take into account infiltrative progression on fluid attenuated inversion recovery if applicable. All responses had to be confirmed on subsequent MRI 2 months later. At the end of the study, response and date of progression observed in Cohort 1 and Cohort 2 were retrospectively reviewed (by E.M., O.C.) based on Revised Assessment in Neuro-Oncology criteria9 and mostly did not differ from the initial evaluation.
Plasma Marker Assay
Plasma samples were collected before cycle 1 and for Cohort 1 only, 2 weeks after first administration of bevacizumab. Peripheral blood was drawn into a citrated Vacutainer tube, centrifuged within 30 min of collection, and then stored at −80°C (Assistance Publique–Hôpitaux de Marseille Tumor Bank, AR 2008/70, certification iso-9001). Samples were analyzed for levels of VEGF, VEGFR2, PlGF, bFGF, stromal cell derived factor 1α (SDF1α), urokinase plasminogen activator (uPA), plasminogen activator inhibitor 1 (PAI1), and MMP2, MMP7, and MMP9 using commercially available ELISA kits (R&D Systems). The level of adrenomedullin was determined using radioimmunoassay as previously described.10 Samples were run in duplicate, and the average was recorded. Plasma samples from 16 healthy volunteers were analyzed for the presence of MMPs as an internal control. For kinetic prebiomarker analysis, we considered increase and decrease categories in case of absolute increase or decrease of marker plasma level at day 15 compared with baseline.
Statistical Analysis
Categorical variables were presented as frequencies and percentages, continuous variables as median and range. OS was defined as the time from first bevacizumab administration to death from any cause, censored at the date of last contact. PFS was defined as the time from first bevacizumab administration to documented progression or death, censored at the date of the last documented disease evaluation. The Kaplan–Meier method was used to estimate survival distributions. Log-rank tests were used for univariate comparisons. Cox proportional hazards models were used for multivariate analyses and to estimate hazard ratios in survival regression models. Considering survival associated with responses (Table 1), responses were dichotomized into those from responders and from nonresponders (stable disease or progression). Subjects were divided into 2 groups based on their baseline biomarker levels using the median value as the cutoff. Within each of the biomarker groupings, a Mann–Whitney U-test was used to compare quantitative values; qualitative values were analyzed by a Fisher exact test and a χ2 test. Correlations were analyzed using the Spearman test. All reported P values are 2-sided, and P < .05 was considered statistically significant. Sensitivity and specificity of MMP2 cutoff in the determination of response was performed using receiver operating characteristic (ROC) curve analysis. Survival status was updated in December 2012. All statistical analyses were performed by PASW Statistics software v17.
Results
Initial Cohort
In the original patient data set (n = 26), median PFS was 4.4 months (95% confidence interval [CI]: 2.1–5.5) with a median OS of 8.7 months (95% CI: 5.3–11.7). Of 25 patients evaluable for response, 12 (48%) exhibited an objective response, while 13 (52%) were stable or progressive. Median OS values were 13 months for responders (95% CI: 5.8–20.1) and 4.5 months for nonresponders (95% CI: 2.7–6.2) (P < .001; Table 1).
Association of prebiomarkers and bevacizumab treatment outcome was first analyzed for baseline levels. In univariate analysis, a strong correlation with objective response was observed for MMP2 and MMP9 levels, PFS, and OS. Among the 12 patients with high MMP2 level (≥227.5 ng/mL, the median value), 10 responses (83.3%) were observed, while in the 13 patients with low MMP2 level, only 2 patients (15.4%) experienced a response (P = .001; Table 2). This correlation remains significant if the value of the MMP2 level was considered a continuous variable (P = .005). Inversely, a low MMP9 level (<235 ng/mL, the median value) was associated with higher probability of response (P = .041). However, considering MMP9 as a continuous variable, correlation between MMP9 and response was not confirmed (P = .094). ROC curve analysis was performed in order to evaluate the performance of plasma MMP2 levels in discriminating between responders and nonresponders (Fig. 1). Plasma MMP2 level had a high discrimination value with an area under the curve of 0.827 (95% CI: 0.624–0.947; P = .002). With a cutoff value of 227.5, the sensitivity was 83.3% (95% CI: 50.9–97.1) and the specificity was 84.6% (95% CI: 53.7–97.3).
Table 2.
Response rates according to patient cohorts and plasmatic MMP2 level
| Cohort 1 |
Cohort 2 |
|||||
|---|---|---|---|---|---|---|
| Responders | Nonresponders | Response Rate | Responders | Nonresponders | Response Rate | |
| MMP2 < median Cohort 1 | 2 | 11 | 15.4% | 6 | 28 | 17.6% |
| MMP2 > Median Cohort 1 | 10 | 2 | 83.3% | 12 | 3 | 80% |
| P value | .001 | <.001 | ||||
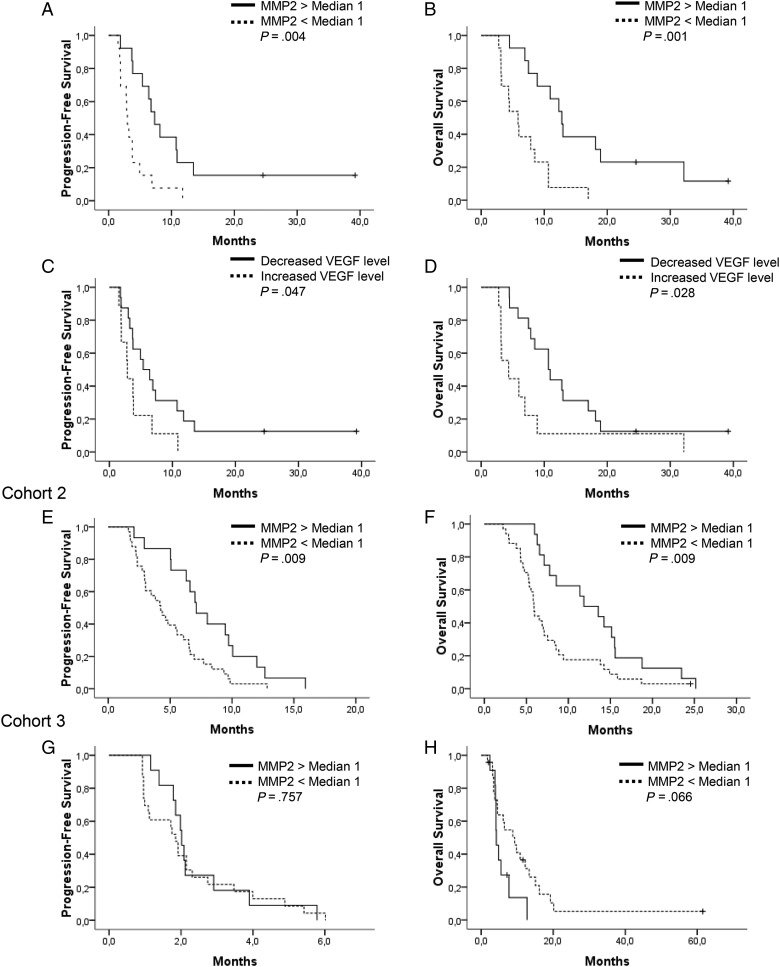
Fig. 1.
Survival analyses. (A and B) PFS and OS of Cohort 1 according to plasmatic MMP2 level. (C and D) PFS and OS of Cohort 1 according to initial change of plasmatic VEGF level. (E and F) PFS and OS of Cohort 2 according to plasmatic MMP2 level, dichotomized by Cohort 1 MMP2 median. (G and H) PFS and OS of Cohort 3 according to plasmatic MMP2 level, dichotomized by Cohort 1 MMP2 median.
In univariate analysis, MMP2 significantly impacted both PFS (P = .004) and OS (P = .001), as did MMP9 (P = .007 for PFS; P = .015 for OS). Patients with an initial high level of MMP2 had a median PFS of 7.3 months (95% CI: 5.2–9.4) and a median OS of 12.8 months (95% CI: 10.4–15.2) compared with a median PFS of 3.0 months (95% CI: 2.5–3.5) and a median OS of 5.9 months (95% CI: 4.0–7.8) in case of low MMP2 level (Fig. 1). Urokinase plasminogen activator and SDF1 were correlated with only OS and PFS, respectively (Table 3). Other factors, including age, KPS, histology, and number of previous treatment lines at the time of bevacizumab initiation, had no significant impact on outcome in Cohort 1. In a multivariate Cox regression model that included baseline prebiomarker levels and potential prognostic factors (age, KPS), MMP2 and MMP9 remained significant for PFS (P = .007 for MMP2; P = .016 for MMP9) and for OS (P = .005 for MMP2; P = .025 for MMP9). MMP2 and MMP9 were not correlated with steroid intake (analyzed for 22/26 patients), isocitrate dehydrogenase (IDH)1 status (analyzed for 16/26 patients), or tumor size (analyzed for 16/26 patients) for those whose data were available (Table 2).
Table 3.
Univariate and multivariate analyses of PFS and OS of Cohort 1 according to median plasmatic biomarkers
| Plasmatic Biomarkers | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate |
Univariate | Multivariate |
|||
| P value | P value | Hazard Ratio | P value | P value | Hazard Ratio | |
| VEGF | .255 | .263 | ||||
| VEGF evolution | .047 | .033* | 2.822 (1.088–7.321) | .028 | .021* | 3.170 (1.193–8.422) |
| VEGFR2 | .789 | .6 | ||||
| VEGFR2 evolution | .191 | .447 | ||||
| PlGF | .195 | .475 | ||||
| bFGF | .841 | .904 | ||||
| FGF evolution | .692 | .543 | ||||
| SDF1 | .046 | .068* | 2.267 (0.942–5.456) | .101 | ||
| SDF1 evolution | .951 | .966 | ||||
| uPA | .063 | .016 | .004* | 4.289 (1.598–11.516) | ||
| uPA evolution | .612 | .982 | ||||
| PAI1 | .408 | .389 | ||||
| PAI1 evolution | .627 | .565 | ||||
| MMP2 | .004 | .007* | 3.925 (1.465–10.517) | .001 | .005* | 4.618 (1.577–13.527) |
| MMP2 evolution | .672 | .621 | ||||
| MMP7 | .121 | .259 | ||||
| MMP7 evolution | .754 | .493 | ||||
| MMP9 | .007 | .016* | 4.290 (1.306–14.084) | .015 | .025* | 3.487 (1.170–10.390) |
| MMP9 evolution | .303 | .42 | ||||
| AM | .429 | .401 | ||||
| AM evolution | .748 | .763 | ||||
| Age | .373 | NS | .368 | NS | ||
| KPS | .949 | NS | .600 | NS | ||
| IDH1 mutation | .488 | .452 | ||||
Abbreviations: AM, adrenomedullin; NS, nonsignificant.
*Adjusted by age and KPS.
Prebiomarker kinetics after the first bevacizumab administration were characterized by an increase of PlGF levels in all patients tested, while other markers exhibited a heterogeneous variation at day 15 (Table 1). In univariate analysis, only initial kinetics of VEGF were correlated with outcome, for both PFS (P = .047) and OS (P = .021) (Fig. 1). In the multivariate Cox regression model that included initial biomarker levels, age, and KPS, VEGF change remained significant for PFS (P = .033) and OS (P = .021) (Table 3).
Finally, inverse correlations were found between MMP2 and MMP9 baseline plasma levels (P = .004) and between MMP2 and MMP9 initial changes (P = .037).
Cohort 2
Objective RR in this less selected population was 36.7%. Median PFS and OS observed in responding patients were similar to those observed in Cohort 1 (Table 1).
Based on results from Cohort 1, baseline MMP2 and MMP9 were the only prebiomarkers assessed in that cohort for which only a baseline sample was available. The cutoffs for MMP2 and MMP9 defined in Cohort 1 (227.5 ng/mL and 235 ng/mL, respectively) were applied for subsequent analyses. In that cohort, median MMP2 and MMP9 levels were 185.2 ng/mL and 202.2 ng/mL, respectively. Sixteen patients (32%) exhibited a high MMP2 level and 27 patients (54%) a low MMP9 level (Table 3). The lower number of patients with a high MMP2 level was in accordance with the lower RR of this cohort. MMP2 similarly impacted RR, with 12 objective responses (RR: 80%) observed in the 15 patients with high MMP2 level and 6 objective responses (RR:17.6%) in patients with low MMP2 level (Table 2). High plasma MMP2 level was associated with median PFS and OS of 7.1 (95% CI: 5.3–8.9) and 11.8 months (95% CI: 7.6–16.1), respectively, versus 4.2 (95% CI: 2.9–5.5) and 5.9 (95% CI: 5.4–6.4) in cases with low MMP2 level (P = .009 for PFS and P = .009 for OS; Fig. 1). MMP2 continued to be significant when analyzed as a continuous variable. In the population restricted to patients with GBM (n = 31), the MMP2 level remained significant for response (P = .004), PFS (6.6 mo vs 3.8 mo for high vs low MMP2, respectively, P = .0104), and OS (11.8 mo vs 5.8 mo for high vs low MMP2, respectively, P = .003). No correlation was observed between MMP9 level and PFS or OS (Supplementary Fig. S1).
Cohort 3
At the time of analysis, 4 patients were alive. Median follow-up was 9.4 months (range, 2.1–61.6). Median PFS and OS of Cohort 3 were 1.9 months (95% CI: 1.7–2.3) and 6.1 months (95% CI: 2.6–9.7), respectively (Table 1). Based on our results from Cohort 1 and Cohort 2, baseline MMP2 and MMP9 were the only biomarkers assessed. The cutoffs for MMP2 and MMP9 defined in Cohort 1 were applied for analysis. Median MMP2 level was 178.5 ng/mL, and 11 patients (32.4%) exhibited a high MMP2 level (≥227.5 ng/mL; Table 3). No association was found between the MMP 2 or MMP9 baseline level and PFS (MMP2, P = .757; MMP9, P = .206). As opposed to our finding in Cohort 1 and Cohort 2, low MMP2 level tended to be associated with better OS (P = .066), while MMP9 level did not affect OS (Fig. 1).
Discussion
In 2 cohorts of patients with recurrent HGG treated with bevacizumab—one prospective, the other retrospective—high MMP2 baseline plasma levels were associated with increased RR, PFS, and OS, consistent in each of the cohorts tested and between the 2 cohorts, with no impact observed in a third cohort treated without bevacizumab. To our knowledge, this study is the first to evaluate MMP2 plasma level as a potential biomarker for bevacizumab.
In regard to the increasing use, heterogeneity of benefit, and cost of antiangiogenic agents, particularly bevacizumab, biomarkers of efficacy that could drive therapeutic decision remain an unmet need in oncology.5 Hypertension and polymorphism that affect the VEGF pathway have been associated with some predictive value of bevacizumab benefit, although their limitations include lack of standardization and inconsistent effect among tumors.6 Baseline plasma biomarkers such as VEGF, soluble VEGFR1, PlGF, and SDF1α, as well as circulating endothelial cells, have been reported to be correlated with outcome. However, their predictive value has been inconsistent among studies, and none of them has been associated with response, PFS, and OS, as baseline MMP2 has in our study. With other antiangiogenic agents, VEGF-C, soluble VEGFR3, or change in plasma concentration of VEGF and interleukins has been associated with tumor control but not with survival.7,11
Despite the lack of a proper control trial, bevacizumab has been associated with a high RR in patients with recurrent GBM compared with historical controls.3 Moreover, although response assessment is challenging with the use of antiangiogenic agents, objective responses that were reported with bevacizumab appear to be correlated with a survival benefit.12 In our study, patient characteristics of the prospective cohort appear more favorable in terms of numbers of previous treatment lines and KPS distribution compared with Cohort 2 and Cohort 3. However, it should be noticed that survival associated with objective response to bevacizumab was similar between Cohorts 1 and 2, which reinforces our results. All patients with grades II and III tumors, as documented at first diagnosis, were treated with bevacizumab after multiple previous chemotherapy lines (from 2 to 6). Only 2 of the 5 grades II–III tumors analyzed presented IDH1 R132H immune positivity. Moreover, these patients with grades II–III tumors were treated at the time they presented an aggressive heterogeneous contrast-enhancing progression on MRI and experienced a survival rate similar to that observed for grade IV patients (data not shown), so that the impact of this histological heterogeneity should be moderate. In addition, MMP2 remains correlated with response, PFS, and survival when analysis was restricted to the subgroup of 31 patients with homogeneous GBM initial histology in Cohort 2. Finally, it should be noticed that neither MMP2 nor MMP9 correlated with patient or tumor characteristics such as age, steroid intake, size of enhancing or nonenhancing tumor, infiltrative pattern, or IDH1 status, thus limiting the potential bias of these results. We acknowledge that most of the patients received irinotecan in addition to bevacizumab, so that the predictive value of MMP2 was formally related to the bevacizumab-irinotecan combination. However, the role of irinotecan is now recognized to be modest, if any, so that our finding should be related mainly to bevacizumab activity.
Potential biomarkers that could predict outcome of patients treated with bevacizumab for a recurrent HGG, analyzed in tumor tissue on the sample of initial diagnosis, included VEGF correlated with response, and hypoxia-inducible carbonic anhydrase 9 and VEGF-A/VEGFR2 ratio associated with survival.13,14 Limitation of immunohistochemistry in a heterogeneous tumor tissue, as well as potential discrepancy of biology between initial and recurrent tumor, may explain the inconsistency of these results. In regard to restricted access to tumor tissue, a circulating marker is highly desirable to monitor therapy in patients with brain tumor. Among a large panel of plasma biomarkers, including MMP2, evaluated in patients with recurrent GBM treated with cediranib, a pan-VEGFR inhibitor, none of them evaluated at baseline correlated with outcome.8 Whether this lack of impact is related to the differences in the activity or the mechanism of action between these 2 agents is unknown.
Considering markers’ early kinetics (from baseline to day 15), we observed that a decrease in VEGF plasma level after the first bevacizumab dose appeared to be correlated with both PFS and OS. Assessment of circulating VEGF is impaired by VEGF bound to bevacizumab and VEGF released from activated platelets in patients with cancer.15 An increase in circulating VEGF after the start of bevacizumab has been generally observed, in contrast to a fall in concentration of circulating free VEGF, both with uncertain predictive value.6 Detailed analysis of total and free circulating VEGF should be further explored in GBM patients treated with bevacizumab. Consistent with previous studies, an increase in PlGF was observed in all patients analyzed, while changes in other potential biomarkers tested were heterogeneous. However, none of these changes appears to influence outcome as observed in most studies with bevacizumab.
MMP2 belongs to the matrix metalloproteinase family, whose activity has been implicated in proteolysis of extracellular matrix, regulation of cell adhesion and migration, processing of growth factors and cytokines, and liberation of angiogenic factors.16 Expression of MMP, particularly MMP2 and MMP9, in plasma, urine, or tumor tissue has been considered a potential biomarker that could reflect diagnosis, dissemination, prognosis, and effect of therapy in various cancers. Expression and/or activity of MMP2 and MMP9 in urine, CSF, or plasma appears to be correlated with tissue expression in bladder cancer and brain tumors.17,18 High expressions of MMP2 and MMP9 have been associated with tumor aggressiveness and poor prognosis in various cancers, although in glioma this impact is unclear.19–21
Unexpectedly, a high baseline level of MMP2 appears to be potentially predictive of bevacizumab benefit. In patients with HGG, treatment with bevacizumab has been associated with an initial decrease of urine MMP2 activity, followed by an upregulation at the time of progression in urine or tumor tissue,22,23 lending credence to consideration of MMP2 as a potential actor of infiltrative escape from bevacizumab. However, baseline MMP2 was not considered in these cases, and since numerous other genes are upregulated after bevacizumab treatment, other candidates could be involved in the invasive phenotype associated with progression under bevacizumab.24 Furthermore, a possible discrepancy between MMP activity and expression could account for this finding. MMP2 has been involved in angiogenesis and associated with pericyte recruitment and vascular maturation and functionality via multiple effectors.25,26 Interestingly, in an orthotopic mouse model of GBM completely devoid of MMP2 activity, tumor vascularity appeared to be increased but less functional with reduction of VEGFR2 expression in tumor vessels and a decrease in pericyte coverage and activation. Another study also recently reported that tumors depleted of MMP2 exhibit fewer macrophages and more destabilized vasculature, which supports the view that MMP2 could slow vessel destabilization and glioma growth.26 Among other hypotheses, it can be suggested that a high level of MMP2 is associated with an increase of bevacizumab uptake in tumor tissue and/or is required for bevacizumab activity on tumor vasculature, possibly via VEGFR2 activation.
In this study, we identify MMP2 as an attractive candidate to predict bevacizumab benefit in patients with HGG. Further studies are needed to validate this finding and to test it in other cancer patient' populations treated with bevacizumab or other antiangiogenic agents.27 The unexpected positive impact of a high circulating MMP2 level on bevacizumab-treated patient outcomes requires reassessing the biological role of MMP2 and its interaction with the VEGF pathway.
Supplementary Material
Funding
This study was supported by the patient association ARTC (Association pour la Recherche sur les Tumeurs Cérébrales) Sud and Aix-Marseille University. This work was completed in the Site de Recherche Intégrée en Cancérologie of Marseille, grant INCa-DGOS-Inserm 6038.
Supplementary Material
Acknowledgments
We thank P. M. Martin and F. Grisoli for their past valuable contribution. O.C.: hadfull access to all of the data in the study, obtained funding, and takes responsibility for the study concept and design, the integrity of the data, and the accuracy of the data analysis. E.T., F.B., M.B., M.M., C.B., D.F-B., P.M., O.C.: acquisition of data. E.T., A.L., O.C.: analysis and interpretation of data. E.T., O.C.: drafting the manuscript. E.T., A.C., M.S., L.O., O.C.: critical revision of the manuscript for important intellectual content. E.T., L.O., O.C.: statistical analysis. L.O., O.C.: administrative, technical, material support. E.T., O.C.: study supervision.
Conflict of interest statement. Dr Chinot, Dr Carpentier, and Dr Sanson are consultants for Roche. None of the other authors declares a conflict of interest. The funding sources had no role in the design or the conduct of the study, the interpretation of the data, or the conception of the manuscript.
References
- 1.Van Meter ME, Kim ES. Bevacizumab: current updates in treatment. Curr Opin Oncol. 2010;22(6):586–591. doi: 10.1097/CCO.0b013e32833edc0c. [DOI] [PubMed] [Google Scholar]
- 2.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor–induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 3.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 4.Chinot OL, de La Motte Rouge T, Moore N, et al. AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28(4):334–340. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 5.Duda DG, Ancukiewicz M, Jain RK. Biomarkers of antiangiogenic therapy: how do we move from candidate biomarkers to valid biomarkers? J Clin Oncol. 2010;28(2):183–185. doi: 10.1200/JCO.2009.24.8021. [DOI] [PubMed] [Google Scholar]
- 6.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11(12):1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 7.Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2010;28(2):193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan–vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 10.Ouafik L, Sauze S, Boudouresque F, et al. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am J Pathol. 2002;160(4):1279–1292. doi: 10.1016/S0002-9440(10)62555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26(22):3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 12.Prados M, Cloughesy T, Samant M, et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(1):143–151. doi: 10.1093/neuonc/noq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raizer JJ, Grimm S, Chamberlain MC, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 15.Niers TM, Richel DJ, Meijers JC, Schlingemann RO. Vascular endothelial growth factor in the circulation in cancer patients may not be a relevant biomarker. PLoS One. 2011;6(5):e19873. doi: 10.1371/journal.pone.0019873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008;14(8):2378–2386. doi: 10.1158/1078-0432.CCR-07-1253. [DOI] [PubMed] [Google Scholar]
- 18.Papathoma AS, Petraki C, Grigorakis A, et al. Prognostic significance of matrix metalloproteinases 2 and 9 in bladder cancer. Anticancer Res. 2000;20(3B):2009–2013. [PubMed] [Google Scholar]
- 19.Jaalinoja J, Herva R, Korpela M, Hoyhtya M, Turpeenniemi-Hujanen T. Matrix metalloproteinase 2 (MMP-2) immunoreactive protein is associated with poor grade and survival in brain neoplasms. J Neurooncol. 2000;46(1):81–90. doi: 10.1023/a:1006421112839. [DOI] [PubMed] [Google Scholar]
- 20.Colin C, Voutsinos-Porche B, Nanni I, et al. High expression of cathepsin B and plasminogen activator inhibitor type-1 are strong predictors of survival in glioblastomas. Acta Neuropathol. 2009;118(6):745–754. doi: 10.1007/s00401-009-0592-2. [DOI] [PubMed] [Google Scholar]
- 21.Brell M, Ibanez J, Felpete A, Burguera B, Frontera M, Couce ME. Quantitative analysis of matrix metalloproteinase-2 mRNA expression in central and peripheral regions of gliomas. Brain Tumor Pathol. 2011;28(2):137–144. doi: 10.1007/s10014-011-0021-9. [DOI] [PubMed] [Google Scholar]
- 22.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12(3):233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takano S, Mashiko R, Osuka S, Ishikawa E, Ohneda O, Matsumura A. Detection of failure of bevacizumab treatment for malignant glioma based on urinary matrix metalloproteinase activity. Brain Tumor Pathol. 2010;27(2):89–94. doi: 10.1007/s10014-010-0271-y. [DOI] [PubMed] [Google Scholar]
- 24.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15(14):4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 25.Du R, Petritsch C, Lu K, et al. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol. 2008;10(3):254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremblay P, Beaudet MJ, Tremblay E, Rueda N, Thomas T, Vallieres L. Matrix metalloproteinase 2 attenuates brain tumour growth, while promoting macrophage recruitment and vascular repair. J Pathol. 2011;224(2):222–233. doi: 10.1002/path.2854. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.