Abstract
Background
There are emerging reports that the family of a disintegrin and metalloproteinases (ADAM) are involved in the maintenance of the malignant phenotype of glioblastomas. Notably, ADAM proteases 10 and 17 might impair the immune recognition of glioma cells via the activating immunoreceptor NKG2D by cleavage of its ligands from the cell surface. Glioblastoma-initiating cells (GIC) with stem cell properties have been identified as an attractive target for immunotherapy. However, GIC immunogenicity seems to be low.
Methods and Results
Here,we show that ADAM10 and ADAM17 are expressed on the cell surface of GIC and contribute to an immunosuppressive phenotype by cleavage of ULBP2. The cell surface expression of ULBP2 is enhanced upon blocking ADAM10 and ADAM17, and treatment with ADAM10 and ADAM17specific inhibitors leads to enhanced immunerecognition of GIC by natural killer cells.
Conclusions
Therefore, ADAM10 and ADAM17 constitute suitable targets to boost an immune response against GIC.
Keywords: a disintegrin and metalloproteinase (ADAM), glioblastoma, immunotherapy, stem cell, NK cell, NKG2D
The treatment of glioblastoma remains a major challenge in the field of neuro-oncology. The current standard of care includes surgery, radiotherapy, and alkylating chemotherapy. The median survival times with this treatment are <12 months according to a population-based study.1 One promising approach to enhance the survival of glioblastoma patients is immunotherapy.2,3 Glioma cells are per se prone to an attack by natural killer (NK) cells due to expression of ligands for activating immunoreceptors like NKG2D.4 NKG2D is a C-type lectin-like homodimeric receptor expressed by NK, γδ T, and CD8+ αβ T cells. The ligands for NKG2D include the MHC class I chain-related proteins A and B (MICA/B) and UL16-binding proteins (ULBP1–6), which are not expressed by most normal tissues but are upregulated upon malignant transformation, infection, or cellular stress.5,6 MICA, MICB, and ULBP1-3 are expressed on the cell surface of human glioma cells.7,8 In a mouse model of glioma, the growth of syngeneic intracerebral tumors was inhibited by peripheral vaccination with MICA-overexpressing irradiated tumor cells, and vaccination resulted in NK and T-cell activation in vivo, indicating a possible therapeutic use of the NKG2D receptor-ligand system in glioblastoma.7 However, the immunosuppressive micromilieu within glioblastomas impairs the NKG2D system via downregulation of cell surface expression of MICA and ULBP2 mediated by transforming growth factor (TGF)-β and cleavage by metalloproteinases.8
Among these metalloproteinases, members of the a disintegrin and metalloproteinase (ADAM) family confer malignancy in several types of cancer (eg, breast cancer or malignant gliomas.)9 ADAMs are involved in the activation of preforms of cytokines and growth factors and have the ability to shed the extracellular domains of cell surface proteins.9 In the human glioma cell line U87, ADAM17, also known as tumor necrosis factor alpha converting enzyme (TACE), contributes to the malignant phenotype of these cells including promotion of cell growth, viability, invasiveness, and neo-angiogenesis in vitro and tumor growth in vivo, which is in part mediated by epidermal growth factor receptor-phosphoinositide 3-kinase/AKT signaling.10 ADAM10 promotes glioma cell migration by cleavage of the adhesion molecule N-cadherin from the cell surface in a protein kinase C-dependent manner.11 Moreover, ADAM10 and ADAM17 might even be involved in the maintenance of the stem cell phenotype of glioblastoma stem cells (see next paragraph).12 Notably, ADAM10 and ADAM17 cleave MICA and ULBP2 from the cell surface of B cell line C1R, the embryonic fibroblast cell line 293T, and cervical, mammary, prostate, and pancreatic carcinoma cell lines.13,14 However, to date little is known about a possible role of ADAM10 and ADAM17 in the regulation of cell surface expression of NKG2D ligands (NKG2DL) and thus a possible modulation of immunogenicity in glioma cells.
A crucial issue for an effective immunotherapy is the choice of target. In recent years, there has been growing evidence for the presence of glioma-initiating cells within glioblastomas possessing stem cell properties.15 Here we refer to these cells as glioma-initiating cells (GIC) in the following text. In a hierarchical tumor model, GIC are crucial for the initiation and maintenance of glioblastomas and therefore constitute an attractive therapeutic target. GIC are defined by the stem cell properties of self-renewal, multipotency, and tumorigenicity, forming tumors resembling the initial human tumors.16,17 Current treatments might spare enough GIC to allow regrowth of the tumors. Despite the expression of ligands on GIC for activating immunoreceptors like NKG2D or NKp46,18,19, several immunosuppressive mechanisms of GIC have been described that might lead to immune evasion. These include the induction of regulatory T cells or the inhibition of proliferation and the apoptosis of T cells in vitro that is in part mediated by signal transducer and activator of transcription 3 (STAT3).20,21 A defective antigen processing mechanism in GIC enhances their ability to evade a T cell-mediated immune response.19 We have previously defined a contribution of the atypical human leukocyte antigen (HLA)-E to this immunosuppressive phenotype of GIC towards innate immunity.22
In the present work, we describe the modulation of immunogenicity of GIC by membrane-bound ADAM10 and ADAM17. Blocking of ADAM10 and ADAM17 with specific inhibitors or the use of small interfering RNA (siRNA) decreases cleavage from the cell surface and therefore, as a direct consequence, the cell surface expression of ULBP2 is enhanced. Treatment with ADAM10 and ADAM17 specific inhibitors leads to enhanced immune recognition of GIC in cytotoxicity assays and to enhanced release of interferon (IFN)-γ by NK cells in co-culture with these GIC. Therefore, ADAM10 and ADAM17 constitute suitable targets to boost an immune response against GIC.
Materials and Methods
Materials and Cell Lines
The human malignant glioma cell line LN-229 was originally provided by Dr N. de Tribolet (Lausanne, Switzerland) and renamed LNT-229 for clarification (T for Tübingen). The cells were maintained in Dulbecco's Modified Eagle Medium supplemented with 2 mM L glutamine (Invitrogen) and 10% fetal calf serum (FCS) (Invitrogen). The GIC lines GS-2, GS-5, GS-7, and GS-9 had been characterized for stemness properties earlier.23 In summary, the cell lines expressed the stemness markers Sox2 and nestin as undifferentiated cultures and either neuronal (MAP2, neurofilament), oligodendroglial (galactocerebroside C), or glial markers (glial fibrillary acidic protein) upon differentiation. Moreover, the GIC lines were characterized by self-renewal, and their tumorigenicity was confirmed in vivo. All GIC lines were cultured in 75 cm2 culture flasks and maintained in neurobasal medium with B-27 supplement (20 µL/mL; Invitrogen) and glutamax (10 µL/mL; Invitrogen) fibroblast growth factor-2 (20 ng/mL; Peprotech), epidermal growth factor (20 ng/mL; Peprotech), and heparin (32 IE/mL; Ratiopharm). Stem cell factors were supplemented twice a week, and complete medium was changed once a week. Cells were passaged when spheres reached an estimated diameter of 500 μm or an estimated density of 5 × 104 cells/cm2. Spheres were dissociated mechanically and enzymatically. Briefly, we spun down the cells and resuspended the pellet in 1 mL Accutase (PAA). After mechanical dissection by pipetting up and down, we incubated the cells at 37°C for 5 minutes. From previous work, we knew that Accutase does not alter the expression level of NKG2DL on the cell surface of glioma cells.4,8 The NK cell line NKL24 was cultured in RPMI 1640 medium (PAA) containing 15% FCS, 2 mM L-glutamine (Gibco Life Technologies), penicillin (100 IU/mL)/streptomycin (100 mg/mL) (Gibco), 1 mM sodium pyruvate (6-PAA Laboratories GmbH, PAA-Strasse 1, 4061 Pasching, Germany), and 100 U/mL interleukin 2 (Peprotech). Specific inhibitors for ADAM10 (GI254023X) and ADAM10 and ADAM17 (GW280264X) were purchased from Glaxo Smith Kline. GI254023X is considered to be 100× more specific in blocking ADAM10 than ADAM17.25 SD-208,26 a transforming growth factor (TGF)-β receptor I kinase inhibitor specifically targeting activin receptor-like kinase 5, was purchased from Tocris Bioscience.
Monoclonal Antibodies and Flow Cytometry
Cell surface expression was assessed with the following monoclonal antibodies (mAbs): ADAM10 (clone 163003, APC-conjugated; mouse IgG2b; R&D Systems) and ADAM17 (clone 111633, fluorescein-conjugated; mouse IgG1; R&D Systems), and hybridoma supernatants MICA (clone AMO1, unconjugated, mouse IgG1), MICB (clone BMO1, unconjugated, mouse IgG1), ULBP1-3 (clones AUMO1; BUMO1, and CUMO3, all unconjugated, mouse IgG127). Isotype-matched antibodies unconjugated IgG1 (clone MOPC 21; Sigma-Aldrich), FITC-conjugated IgG1 (clone MOPC 21; Sigma-Aldrich), and APC-conjugated IgG2b (clone MPC-11; Biolegend) were used as controls. The PE-conjugated goat anti-mouse IgG (Dako; dilution 1:20) was used as secondary antibody where appropriate. The cells were dissociated as described, preincubated in phosphate-buffered saline (PBS) with 2% FCS and stained with specific mAb or matched mouse Ig isotype for 30 minutes on ice, followed by incubation with the PE-conjugated secondary antibody for 30 minutes where appropriate. Then the cells were washed with PBS containing 2% FCS. Flow cytometry was performed with a CyAn ADP Analyzer (Beckman Coulter). Specific fluorescence index (SFI) was calculated by dividing median fluorescence obtained with the specific antibody by median fluorescence obtained with the control antibody.
Viability and Proliferation
2.5 × 105 GIC were seeded in a 24-well plate in the presence of increasing concentrations of GI254023X, GW280264X (both 3 µM), or dimethyl sulfoxide (DMSO) for 48 hours. Viability and cell concentration were assessed by Trypan blue staining using Vi-CELL™ XR (Beckman Coulter). The cell count was calculated based on the cell concentration and the predetermined cell culture volume.
Limiting Dilution Sphere Formation Assay
For the sphere formation assays, GS-5 cells were mechanically and enzymatically dissociated to a single cell suspension with Accutase and diluted and seeded in triplicate at 10, 100, 1 000, and 10 000 cells/well in a 96-well plate under stem cell culture conditions. After 24 hours, 50 μL stem cell medium containing GI254023X, GW280264X (both to a final concentration of 3 μM), or DMSO were added. After 7 days, another 100 μl of stem cell medium containing inhibitors (final concentration 3 μM) or DMSO was supplemented. The number of spheres was counted in each well after 14 days.
Immunocytochemistry
For undifferentiated GIC, cytospin samples were prepared. For differentiation, GIC were dissociated and dissolved in neurobasal A medium containing B-27 supplement (20 µl/mL), FCS (10%), and glutamax (10 µl/mL) (all Invitrogen) as well as cyclic adenosine monophosphate (cAMP) (0.75 mM) and retinoic acid (1 mM) (both Sigma-Aldrich).23 GIC were seeded on glass coverslips in a 24-well plate (both TPP Techno Plastic Products) for 8 days. Cytospin samples and coverslips were either fixed in methanol for glial fibrillary acidic protein (GFAP) or 4% paraformaldehyde for nestin, ß-3-tubulin ,and galactocerebroside C (GalC), treated with H2O2, and blocked with PBS containing 10% swine serum and 0.3% Triton X. The following antibodies were used for primary stem cell staining: nestin (clone 10C2, IgG1; Zytomed; diluted 1:100), GFAP (ab Z0334, polyclonal rabbit IgG; Dako; diluted 1:1000), ß-3-tubulin (ab18207, polyclonal rabbit IgG; Abcam; diluted 1:1000), and GalC (MAB342, mouse IgG3; Millipore; diluted 1:200). As isotype controls, we used mouse IgG1 (Dianova), mouse IgG3 (eBioscience), or polyclonal rabbit IgG (Vector Laboratories). The cells were incubated at 4°C overnight. Goat anti-mouse IgG Alexa Fluor 594-coupled secondary antibody (Invitrogen) or goat anti-rabbit IgG Alexa Fluor 594-coupled secondary antibody (Invitrogen) were used at 1:100. 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) was used at 1:2 000 for nuclear counterstain. Slides were mounted using fluorescence mounting medium (Dako). Images were acquired by using an Axioplan II fluorescence microscope (Carl Zeiss AG).
Real-Time Polymerase Chain Reaction
RNA was prepared from the cell lines using NucleoSpin RNA II Kit (Macherey-Nagel). Subsequently, cDNA was generated using SuperScript II Reverse Transcriptase (Invitrogen). RNA and DNA concentrations were determined using NanoDrop (Thermo Scientific).
cDNA amplification was monitored using SYBRGreen chemistry on the ABI PRISM7000 Sequence Detection System (Applied Bio-Systems) using the following specific primers (forward/reverse): GAPDH: described in;22 ADAM10: 5′-GGATGGAAAAGGAACCCAAT-3, 5′-ACGAAGAACAGGGAACATGG-3′; ADAM17 5′-TTGAGCGATTTTGGGATTTC-3′, 5′-AAACCAGAACAGACCCAACG-3′. Primers for MICA, MICB and ULBP1-3 have been published.28 Data analysis was done using the DDCT method for relative quantification. Briefly, threshold cycles (CT) for GAPDH RNA (reference) and specific primers (sample) were determined in duplicate. The expression levels were determined following the formula: rI = 2-[CTTarget - CT GAPDH] and then referred to whole normal brain cDNA obtained from Lonza.
RNA Interference
To silence ADAM10 and ADAM17 expression, the glioma cells were transfected with ADAM10 and ADAM17 ON-TARGETplus SMARTpool siRNA (Dharmacon) or Non-targeting SMARTpool siRNA (Dharmacon) as a control respectively. Glioma cells were seeded into 6-well plates overnight and subsequently transfected with siRNA using lipofectamine RNAi (Invitrogen). Gene silencing was verified by real-time PCR and by flow cytometry 72 hours after transfection.
Enzyme-linked Immunosorbent Assay
For the detection of soluble ULBP2 (sULBP2) in cell culture supernatants, 5 × 105 cells were cultured in 500 μL B-27 supplement free-medium and treated as indicated. The supernatants were collected after 48 hours and then analyzed by sandwich enzyme-linked immunosorbent assay (ELISA) for sULBP2 using the Duoset ELISA Kit (R&D Systems).
To assess IFN-γ secretion by NKL, 2 × 104 GIC were pretreated with either GW 280264X or DMSO as a control for 24 hours and subsequently co-cultured with 4 × 105 NK cells in 500 μl serum free RPMI medium for 12 hours. The conditioned media from this co-culture were collected and analyzed using the IFN-γ ELISA MAX standard kit obtained from Biolegend.
Cytotoxicity Assay
We used a flow cytometry-based cytotoxicity assay29 that employs the membrane dye PKH-26 and the viability dye ToPro3, as described.22 Briefly, the target cells were incubated with PKH-26 dissolved in Diluent C (1:250; both Sigma–Aldrich) for 3 minutes. Staining was stopped by washing with RPMI 1640 medium containing 10% FCS. Then the effector and target cells were coincubated at varying effector-to-target ratios for 3 hours. ToPro3 (Invitrogen) was added to the vials prior to measurement and incubated for 30 seconds. The percentage of target cell lysis was determined by flow cytometry using the CyAn ADP Analyzer (Beckman Coulter). Background elimination was done by subtraction of spontaneous lysis of target cells only. All experiments were done in duplicate.
Statistical Analysis
The experiments were performed 2 to 3 times as indicated. Statistical significance was calculated by Student 2-tailed t test (* P < .05; ** P < .01) or by ANOVA, using GraphPad Prism software, version 5.0 (La Jolla) where indicated.
Results
GIC Lines Express NKG2DL
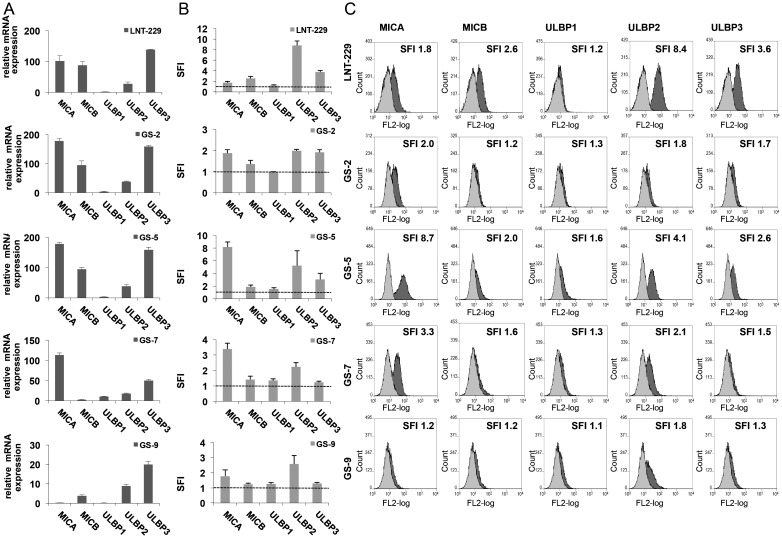
The expression of NKG2D ligands in the glioma cell line LNT-229 and in GIC lines GS-2, GS-5, GS-7, and GS-9 was assessed by real-time PCR and flow cytometry (Fig. 1). All investigated cell lines variably expressed NKG2DL on the cell surface, predominantly MICA and ULBP2 (Fig. 1B and C). This was only roughly paralleled by the expression on mRNA level (Fig. 1A), indicating additional mechanisms of posttranscriptional and/or posttranslational regulation, as they are well described for regulation of NKG2DL cell surface expression.30
Fig. 1.
GIC lines express NKG2D ligands. The expression of NKG2DL in GIC was assessed on the transcriptional and cell membrane levels. (A) MICA, MICB and ULBP1-3 mRNA expression was analyzed by real-time PCR. Data are expressed relative to normal brain cDNA and represent mean relative expression ± SD from 3 independent experiments. (B, C) GIC were stained with mAbs for MICA (clone AMO1), MICB (clone BMO1), ULBP1 (clone AUMO1), ULBP2 (clone BUMO1), ULBP3 (clone CUMO3) or isotype-matched Ig, followed by staining with a PE-labeled anti-mouse secondary antibody. Flow cytometry was performed and data in (B) represent mean SFI values ± SD from 3 independent experiments (SFI = 1 is marked with dotted line); **P < .01, *P < .05; Student 2-tailed t test). In (C), representative histograms from flow cytometry, including the respective SFI values, are shown (curve in light grey: isotype control, dark grey: specific MICA,B or ULBP1-3 mAb).
GIC Lines Express ADAM10 and ADAM17 on the Cell Surface
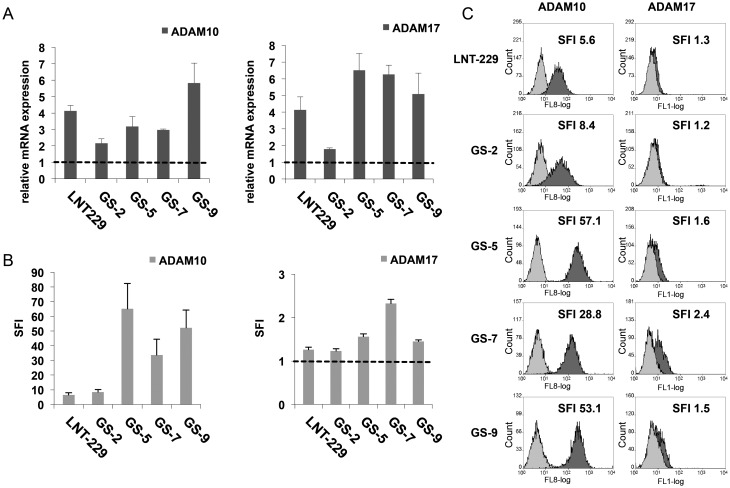
The expression of ADAM10 and ADAM17 in GIC was assessed by real-time PCR and flow cytometry (Fig. 2). ADAM10 and ADAM17 genes were transcribed in all of the investigated GIC lines with expression levels being higher in GS-5, GS-7 and GS-9 compared with GS-2 (Fig. 2A). In parallel, ADAM10 and, to a lesser extent, ADAM17 protein could also be detected on the cell surface of GIC (Fig. 2B and C). In line with the relative low mRNA expression levels of ADAM10 and ADAM17 in GS-2, cell surface protein expression for ADAM10 was lower than in the other GS lines and only marginal for ADAM17 (Fig. 2B and C). While exhibiting strong expression of ADAM10, the cell surface level of ADAM17 in GS-9 was low (Fig. 2B and C). GS-7 showed the strongest cell surface expression level of ADAM17 of the GIC lines tested here and also showed high levels of ADAM10.
Fig. 2.
GIC lines express ADAM10 and ADAM17 on the cell surface. (A) ADAM10 and ADAM17 mRNA expression was determined by real-time PCR. Data are expressed relative to the expression of normal brain cDNA and represent mean relative expression ± SD from 3 independent experiments. (B) GIC were stained with directly labeled mAbs for either ADAM10 (clone 163003), ADAM17 (clone 111633), or isotype-matched Ig. Data represent mean SFI values ± SD from 3 independent experiments (SFI = 1 is marked with dotted line). In (C), representative histograms from flow cytometry, including the respective SFI values, are shown (curve in light grey: isotype control, dark grey: specific ADAM10 or ADAM17 mAb).
The Inhibition of ADAM10 and ADAM17 Leads to an Upregulation of ULBP2 Cell Surface Expression on Glioma-initiating Cells
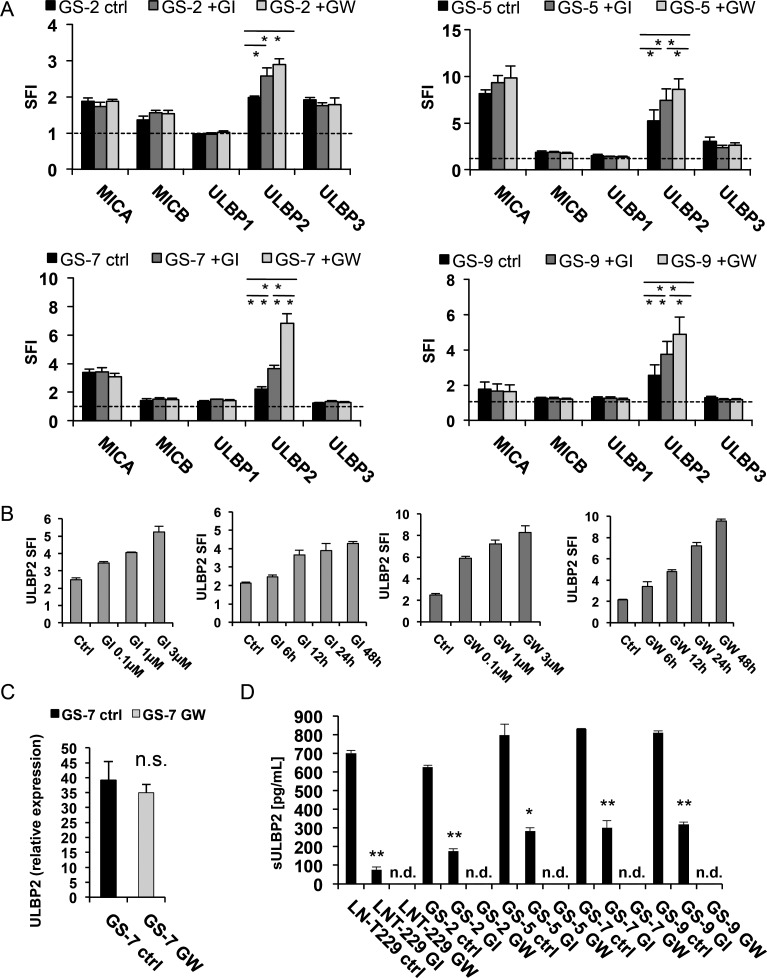
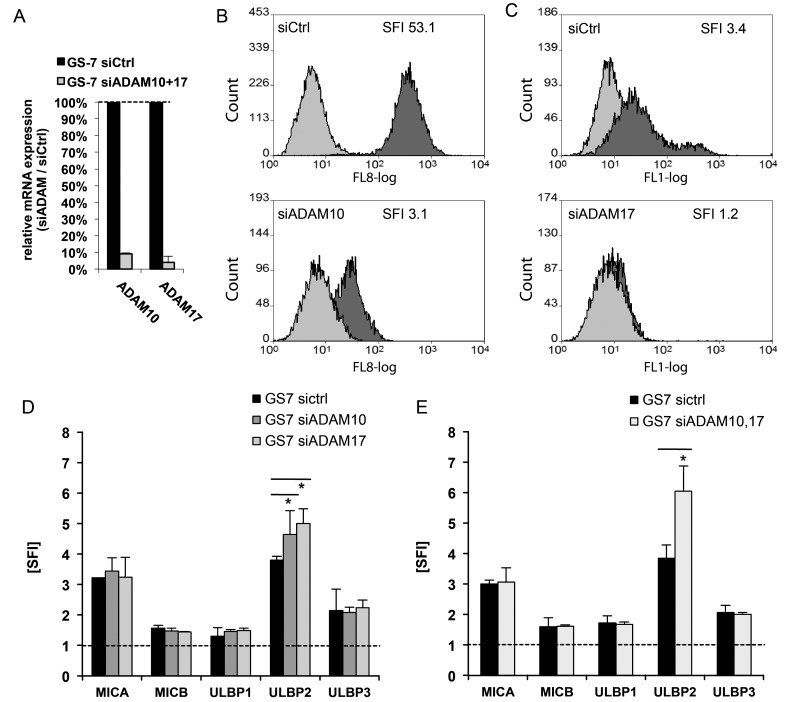
We next assessed the role of ADAM10 and ADAM17 in the regulation of NKG2DL expression. GS-2, GS-5, GS-7, and GS-9 cells were treated with specific inhibitors GI254023X (for ADAM10) and GW280264X (for ADAM10 and ADAM17). The level of NKG2DL was assessed by flow cytometry. We found a significant upregulation of ULBP2 in all investigated cell lines (Fig. 3A) in a time- and concentration-dependent manner (shown exemplarily for GS-7, Fig. 3B). While the expression levels of MICB, ULBP1, and ULBP3 were unaltered, we observed a trend towards upregulation of MICA in GS-5 (Fig. 3A). Next, we assessed the presence of sULBP2 in conditioned cell culture supernatants of LNT-229 and GIC cultures by ELISA. sULBP2 concentrations in the supernatants decreased significantly following treatment with GI254023X and were below the detection limit upon treatment with GW280264X, while the level of ULBP2 on the mRNA level remained largely unaltered as assessed by real-time PCR (Fig. 3C and D). In order to confirm the specificity of the effects mediated by the inhibitors, we used siRNA to transiently silence ADAM10 and ADAM17 gene expression in GS-7. We achieved an efficacy of gene silencing of more than 90% as assessed by real-time PCR (Fig. 4A). The knockdown of ADAM10 and ADAM17 cell surface expression was verified by flow cytometry (Fig. 4B). The knockdown of either ADAM10 or ADAM17 gene expression, as well as the combined knockdown, led to a significant upregulation of ULBP2 on the cell surface, while the expression of the other NKG2DL was largely unaffected (Fig. 4D and E), similar to the effect mediated by the inhibitors.
Fig. 3.
Inhibition of ADAM10 and ADAM17 using specific inhibitors leads to an upregulation of ULBP2 cell surface levels and to decrease of sULBP2 in cell culture supernatants. (A) The GIC lines GS-2, GS-5, GS-7, and GS-9 were incubated with GI254023X (for ADAM10) or GW280264X (for ADAM10 and ADAM17), respectively (3 µM; 48 h). The cells were subsequently stained with mAbs for MICA, MICB, ULBP1-3 or isotype-matched Ig, followed by staining with a PE-labeled anti-mouse secondary antibody. Flow cytometry data represent mean SFI ± SD from 3 independent experiments (SFI = 1 is marked with a dotted line; ** P < .01, * P < .05; Student 2-tailed t test). (B) GS-7 cells were treated with increasing concentrations of GI254023X or GW280264X for various time points. The cells were subsequently stained with mAb for ULBP2 or isotype-matched Ig, followed by staining with a PE-labeled anti-mouse secondary antibody. Flow cytometry data represent mean SFI ± SD from 3 independent experiments. (C) GS-7 cells were treated with GW280264X (3 µM; 48 h). ULBP2 mRNA expression was determined by real-time PCR. Data are expressed relative to the expression of normal brain cDNA and represent mean relative expression ± SD from 3 independent experiments. (D) GIC or LNT-229 cells were treated with DMSO, GI254023X (3 µM) or GW280264X (3 µM) for 48 hours, then cell culture supernatants were harvested. The levels of sULBP2 were assessed by ELISA. Data represent mean concentrations ± SD from 2 independent experiments.
Fig. 4.
ADAM10 and ADAM17 gene silencing leads to an upregulation of ULBP2 cell surface expression. The GIC line GS-7 was transfected with control (siCtrl) or siRNA targeting ADAM10 (siADAM10) or ADAM17 (siADAM17). (A,B) The cells were harvested 72 hours later, and knockdown efficiency was confirmed by real-time PCR (A). Data are expressed as percentage of ADAM10 and 17 expression in cells following ADAM10/ADAM17 gene silencing relative to control siRNA and represent mean relative expression ± SD from 3 independent experiments. (B) The knockdown efficiency was further confirmed by flow cytometry with ADAM10 or ADAM17 specific mAB (curve in light grey: isotype control, dark grey: specific ADAM10 or ADAM17 mAb). (C, D) GS-7 cells transfected with either siADAM10, siADAM17, or both were stained with mAbs for MICA, MICB, ULBP1-3, or isotype-matched Ig, followed by staining with a PE-labeled anti-mouse secondary antibody. Flow cytometry data represent mean SFI values ± SD from 3 independent experiments (** P < .01, * P < .05; Student 2-tailed t test).
We assessed the proliferation and survival of GIC upon treatment with the ADAM inhibitors or knockdown of ADAM expression. The viability of GIC was not altered following treatment with the inhibitors or upon knockdown. This is shown exemplarily for the inhibitors in GS-7 (Supplementary Fig. S1A). The proliferation of GS-7 cells was significantly reduced upon treatment with GW280264X or ADAM10/17 co-knockdown (Supplementary Fig. S1B and data not shown) respectively, consistent with previous data.12
Blocking of TGF-β Signaling Does Not Alter Upregulation of ULBP2 Cell Surface Levels Mediated by Inhibition of ADAM10 and ADAM17
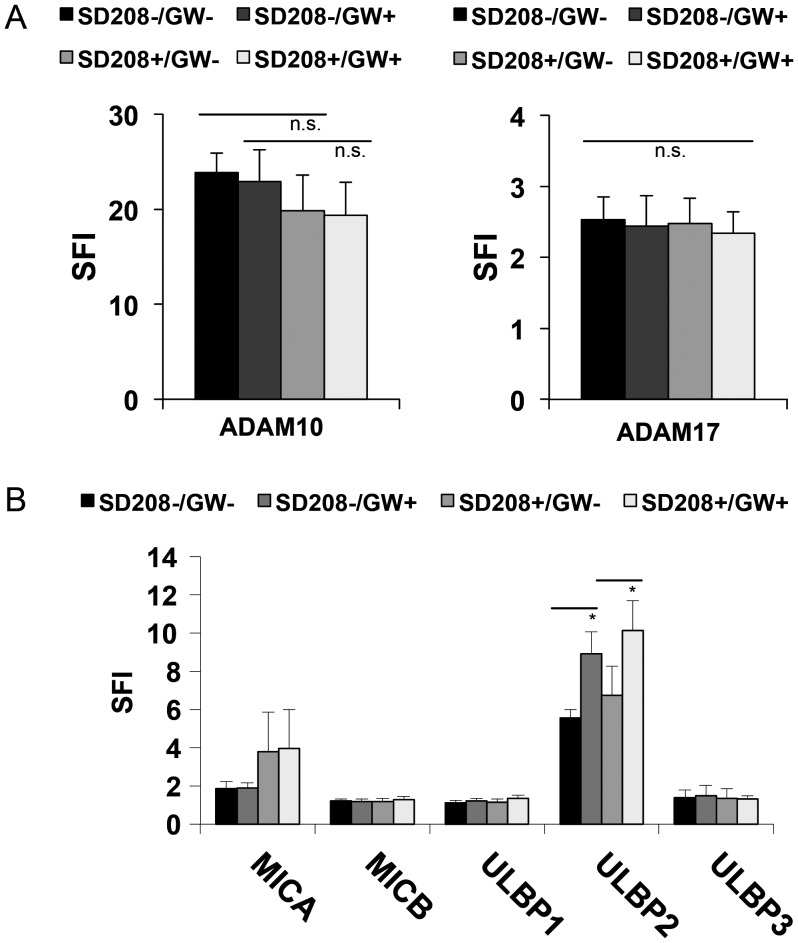
In order to investigate a possible involvement of endogenous TGF-β in the ADAM10 and ADAM17-mediated effects on ULBP2 cell surface expression, we used SD-208, a TGF-beta receptor I kinase inhibitor that efficiently blocks TGF-β signaling in glioma cells.26 GS-7 cells were treated with SD-208 for 48 hours, and blockade of SMAD2 phosphorylation was verified by immunoblot (data not shown). The expression of ADAM10 and ADAM17 remained unaltered as assessed by flow cytometry (Fig. 5A). We observed a tendency to enhanced expression of MICA and ULBP2, though not statistically significant (Fig. 5B). Next, the cells were treated with GW280264X for 24 hours, and no further increase in MICA expression was observed. However, expression of ULBP2 in SD-208 treated cells was markedly increased (Fig. 5B), suggesting that cleavage of ULBP2 by ADAM10 and ADAM17 is largely independent of TGF-β.
Fig. 5.
Blocking of TGF-β signaling does not alter upregulation of ULBP2 cell surface levels mediated by inhibition of ADAM10 and ADAM17. (A) GS-7 cells were treated with SD-208 (2 μM) or DMSO for 48 hours. The levels of ADAM10 and ADAM17 at the cell surface were assessed by flow cytometry. In (B) the cells were treated as in (A), followed by treatment with either GW280264X (3 µM, 24 h) or DMSO. The levels of MICA, MICB and ULBP1-3 were assessed by flow cytometry. Flow cytometry data represent mean SFI values ± SD from 3 independent experiments.
The Inhibition of ADAM10 and ADAM17 Enhances Immunogenicity of Glioma-initiating Cells
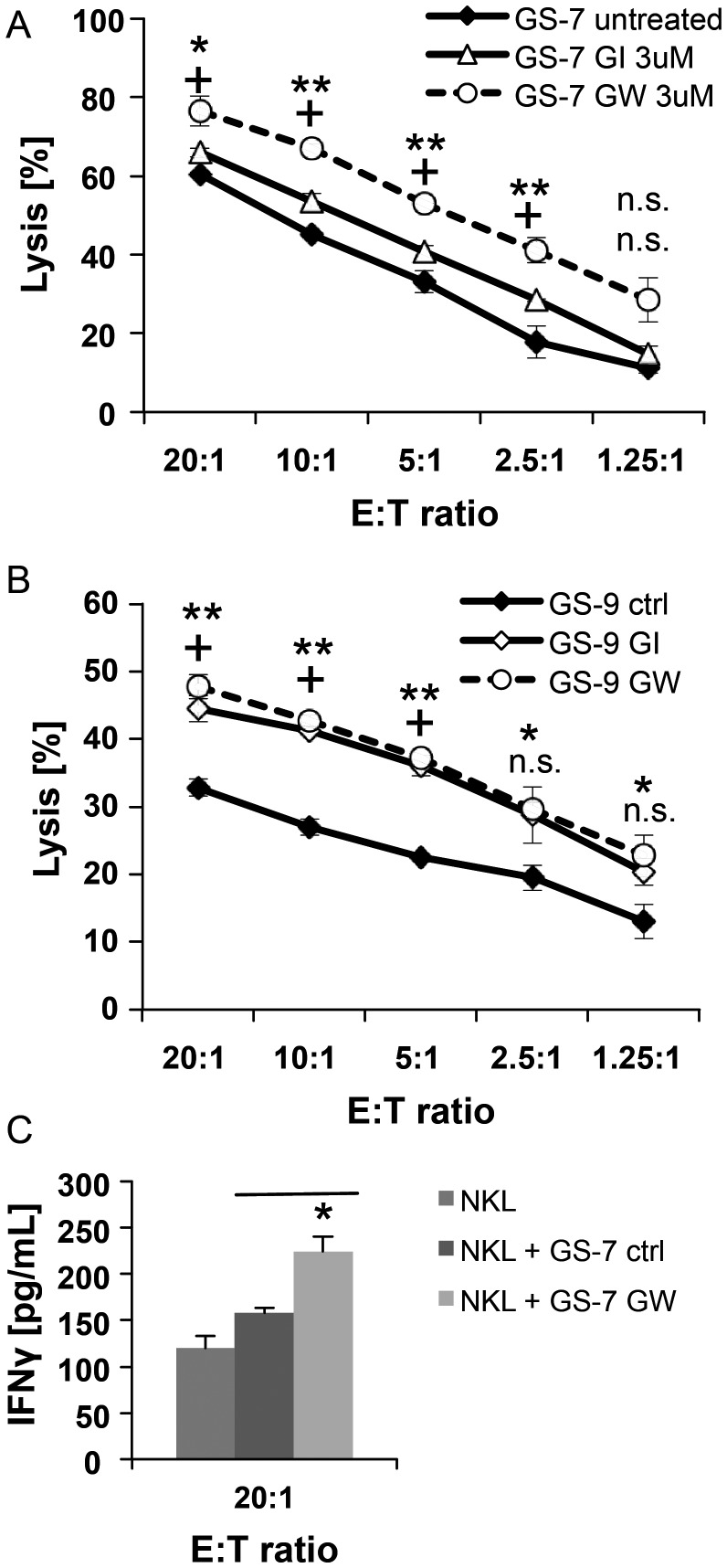
To define a role for ADAM10 and ADAM17 in modulating the immunogenicity of GIC, we used either GI254023X- or GW280264X-pretreated GS-7 or GS-9 cells as targets in a cytotoxicity assay with NKL cells as effectors. We observed a significantly increased susceptibility towards NK cell mediated lysis following treatment with the ADAM10 and ADAM17-specific inhibitor GW280264X and to a lesser extent by the ADAM10-specific inhibitor GI254023X in GS-7 (Fig. 6A). In GS-9, treatment with ADAM inhibitors led to enhanced susceptibility towards NK cells; however, the effect of GW280264X was not superior to GI254023X (Fig. 6B). The relative increase of ULBP2 expression following treatment with GW280264X compared with GI254023X was highly significant in GS-7 cells (P = .008) and present to a lesser degree in GS-9 (P = .02) (Fig. 3A). Thus, the slight additional upregulation of ULBP2 levels in GS-9 by GW280264X compared with GI254023X might not be sufficient to be translated into an enhanced immune recognition by NKL cells. Since the secretion of IFN-γ by activated NK cells is considered an important link between the innate and adaptive immune response, we analyzed the conditioned media of NKL and GS-7 co-cultures for the level of IFN-γ by ELISA. NKL cells were co-cultured with GS-7 cells, pretreated or not with GW280264X (3 μM for 48 h). The levels of IFN-γ in the supernatant of co-cultures with GS-7 cells pretreated with GW280264X were significantly increased (Fig. 6C).
Fig. 6.
The inhibition of ADAM10 and ADAM17 enhances immunogenicity of GIC. (A) GS-7 and (B) GS-9 cells were incubated with GI254023X or GW280264X (3 µM; 48 h) and then used as targets in a cytotoxicity assay with NKL cells as effectors. Target cells were stained with the membrane dye PKH-26 and then co-cultured with NKL cells at various effector-to-target (E:T) ratios for 3 hours in duplicate. The cells were stained with the viability marker ToPro3 to identify the lysed cells by flow cytometry. Specific lysis was calculated by subtraction of background lysis from the fraction of dead cells (n = 2). Statistics were done by ANOVA (** P = .01*, P = .05 for GI254023X pretreated, + P = .05 for GW280264X pretreated cells.) (C) GS-7 cells were pretreated with either GW280264X (3 µM, 48 h) or DMSO and then co-cultured with NKL cells (12 h, effector-to-target ratio 20:1). Conditioned media were collected from co-cultures or NKL cells alone, and concentration of IFN-γ was assessed by ELISA. Results represent mean concentrations ± SD of 2 independent experiments.
Discussion
In this study, we define a role of ADAM10 and ADAM17 for the modulation of immunogenicity of GIC by influencing the NKG2D receptor-ligand system. The NKG2DL MICA, MICB and ULBP1-3 are expressed on the cell surface of GIC lines. The expression of NKG2DL in gliomas, especially of MICA and ULBP2, is known to be influenced by various factors, including downregulation by TGF-β and cleavage by metalloproteinases.8 ADAM10 and ADAM17 belong to a family of proteases involved in the shedding of extracellular domains of cell surface proteins.9 Both proteins cleave NKG2DL, namely MICA and ULBP2, as demonstrated in malignant and nonmalignant cell lines.14,31 A single report provides evidence that ADAM17 is crucially involved in the shedding of soluble MICB from the cell surface of U373 transfectants overexpressing MICB within detergent-resistant membrane microdomains.32 In this work, we investigated the role of ADAM10 and ADAM17 in modulating the cell surface expression of NKG2DL in GIC. These GIC lines have been described before for their stemness properties.23 We adhered to the stem cell-permissive culture conditions to assure maintenance of the stem cell phenotype and verified self-renewal capacity and multipotency of GIC. ADAM10 was expressed on all GIC lines investigated here. The strongest ADAM17 expression was present in GS-7. Interference with ADAM10 and ADAM17 function by specific pharmacological inhibitors or gene silencing with siRNA led to a strong upregulation of ULBP2 expression on the cell surface. sULBP2 concentrations in the supernatants decreased significantly following treatment with the inhibitors, while the level of ULBP2 on the mRNA level remained unaltered, underscoring the view that ADAM10 and ADAM17 decrease ULBP2 cell surface expression via proteolytic cleavage.
Few reports indicate a link between effects mediated by ADAM proteases and TGF-β. TGF-β-mediated effects on glioma cell motility and the invasiveness that could be abrogated in part by blocking ADAM1733 and ADAM12 expression was upregulated by TGF-β in mammary carcinoma cells.34 The cleavage of ULBP2 from the cell surface by ADAM10 and ADAM17, however, seems to be largely independent from TGF-β. Previously, the abrogation of TGF-β signaling led to an upregulation of MICA and ULBP2 on non-GIC.8 We observed a tendency to enhanced expression of MICA and ULBP2 on the cell surface of GS-7 cells treated with SD-208, though not statistically significant. These cells were subsequently treated with GW280264X for 24 hours, and no further increase in MICA expression was observed. However, the levels of ULBP2 in these cells with blocked TGF-β signaling were markedly increased, showing that increased ULBP2 levels upon inhibition of ADAM10 and ADAM17 are largely independent from TGF-β but rather are due to a direct blockade of ADAM-mediated ULBP2 cleavage.
In contrast to previous observations in HeLa cervix carcinoma cells,14 we found a minor upregulation of MICA only in GS-5 following impairment of ADAM10 and ADAM17. The sheddase activity of ADAM10 and ADAM17 is not dependent on a specific sequence motif but rather on the length of the stalk region of the NKG2DL.14 Notably, the most common MICA variant, MICA08, is not cleaved by ADAM proteases but rather is released in endosomes.35 This might explain variations in NKG2DL cleavage observed in different cell lines of different tissue origin14,32 and our findings. Moreover, shedding of MICA seems to depend critically on its association to endoplasmic reticulum protein 5.36 An impairment of this association in our GIC lines might be involved in the lack of shedding of MICA, a possible mechanism that warrants further investigation. The inhibitors used here are highly specific for ADAM10 (GI254023X) or ADAM10 and ADAM17 (GW280264X), respectively. The specificity of GI254023X and GW280264X has been repeatedly confirmed, and these inhibitors have been used in various subsequent investigations.12–14,25,37 Moreover, we used RNA interference to silence ADAM10 and ADAM17 expression in a sequence-specific manner using appropriate controls and obtained the same results as with the pharmacological inhibitors.
Collectively, our data provide strong evidence that ULBP2 expression on GIC is substantially reduced through cleavage by ADAM10 and ADAM17, which is in accord with the present literature. We do not assume that the cleavage of NKG2DL is a specific mechanism in GIC since the sheddase activity of ADAM10 and ADAM17 depends on the length of the stalk region of the NKG2DL, as described above. However, it is of greater importance that the GIC are not spared when targeting ADAM proteases to enhance immune response against glioma cells.
To date, little is known about the role of ADAM proteases in regulating immune function in glioma. We show here that inhibition of ADAM10 and ADAM17 leads to enhanced lysis of GIC by NK cells. Moreover, we found significantly increased levels of IFN-γ in the supernatant of co-cultures of NK cells with GIC pretreated with an ADAM inhibitor. This is in line with the results from a recent report investigating tumor entities other than glioma.13 Since IFN-γ is a main regulator of T-cell activity, inhibition of ADAM10 and ADAM17 and subsequent upregulation of ULBP2 might therefore also enhance adaptive immune responses through induction of IFN-γ secretion by NK cells.
As outlined earlier, ADAM10 and ADAM17 are further involved in the maintainance of the malignant phenotype of glioma cells regarding migration, invasion, neo-angiogenesis, or proliferation.10,11 Recently, a role for ADAM10 and ADAM17 for the maintenance of the stem cell phenotype of human glioma cells kept under stem cell culture conditions was proposed.12 Here we focused on the modulation of the immunophenotype of GIC by ADAM proteases rather than the stem cell phenotype. Nevertheless, we reproduced the finding of reduced proliferation and decreased number of spheres in a limiting dilution assay upon inhibition of ADAM10 and ADAM17 (Supplementary Fig. S3), as previously reported.12
In summary, we demonstrated that ADAM10 and ADAM17 are involved in the modulation of immunogenicity of glioblastoma stem-like cells. ADAM10 and ADAM17 cleave ULBP2 from the cell surface and therefore warrant further investigation as possible targets to boost an immune response towards these cells.
Supplementary Material
Funding
Swiss National Science Foundation (National Center of Competence In Research (NCCR) Neuro - Neural Plasticity and Repair to M.W.)
Supplementary Material
Acknowledgments
We thank Prof. K. Lamszus, Department of Neurosurgery, University Hospital, Eppendorf, Hamburg, for sharing the GS cell lines and Silvia Dolski for technical assistance.
Conflict of interest statement. None declared.
References
- 1.Johnson DR, Ma DJ, Buckner JC, Hammack JE. Conditional probability of long-term survival in glioblastoma: A population-based analysis. Cancer. 2012;118(22):5608–5613. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell DA, Sampson JH. Toward effective immunotherapy for the treatment of malignant brain tumors. Neurotherapeutics. 2009;6(3):527–538. doi: 10.1016/j.nurt.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogbomo H, Cinatl J, Jr., Mody CH, Forsyth PA. Immunotherapy in gliomas: limitations and potential of natural killer (NK) cell therapy. Trends Mol Med. 2011;17(8):433–441. doi: 10.1016/j.molmed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Friese MA, Steinle A, Weller M. The innate immune response in the central nervous system and its role in glioma immune surveillance. Onkologie. 2004;27(5):487–491. doi: 10.1159/000080371. [DOI] [PubMed] [Google Scholar]
- 5.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89(2):216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Messina L, Reyburn HT, Vales-Gomez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol. 2012;3:299. doi: 10.3389/fimmu.2012.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friese MA, Platten M, Lutz SZ, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003;63(24):8996–9006. [PubMed] [Google Scholar]
- 8.Eisele G, Wischhusen J, Mittelbronn M, et al. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129(Pt 9):2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MJ, Mullooly M, O'Donovan N, et al. The ADAMs family of proteases: new biomarkers and therapeutic targets for cancer? Clin Proteomics. 2011;8(1):9. doi: 10.1186/1559-0275-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Jiang F, Katakowski M, Lu Y, Chopp M. ADAM17 promotes glioma cell malignant phenotype. Mol Carcinog. 2012;51(2):150–164. doi: 10.1002/mc.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohutek ZA, diPierro CG, Redpath GT, Hussaini IM. ADAM-10-mediated N-cadherin cleavage is protein kinase C-alpha dependent and promotes glioblastoma cell migration. J Neurosci. 2009;29(14):4605–4615. doi: 10.1523/JNEUROSCI.5126-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulstrode H, Jones LM, Siney EJ, et al. A-Disintegrin and Metalloprotease (ADAM) 10 and 17 promote self-renewal of brain tumor sphere forming cells. Cancer Lett. 2012;326(1):79–87. doi: 10.1016/j.canlet.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Chitadze G, Lettau M, Bhat J, et al. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: Heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer. 2013;133(7):1557–1566. doi: 10.1002/ijc.28174. [DOI] [PubMed] [Google Scholar]
- 14.Waldhauer I, Goehlsdorf D, Gieseke F, et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68(15):6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23(43):7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 16.Tabatabai G, Weller M. Glioblastoma stem cells. Cell Tissue Res. 2011;343(3):459–465. doi: 10.1007/s00441-010-1123-0. [DOI] [PubMed] [Google Scholar]
- 17.Park DM, Rich JN. Biology of glioma cancer stem cells. Mol Cells. 2009;28(1):7–12. doi: 10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 18.Castriconi R, Daga A, Dondero A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182(6):3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 19.Di Tomaso T, Mazzoleni S, Wang E, et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16(3):800–813. doi: 10.1158/1078-0432.CCR-09-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J, Barr J, Kong LY, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9(1):67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Barr J, Kong LY, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16(2):461–473. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Wolpert F, Roth P, Lamszus K, Tabatabai G, Weller M, Eisele G. HLA-E contributes to an immune-inhibitory phenotype of glioblastoma stem-like cells. J Neuroimmunol. 2012;250(1–2):27–34. doi: 10.1016/j.jneuroim.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Gunther HS, Schmidt NO, Phillips HS, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27(20):2897–2909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 24.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24(3):406–415. [PubMed] [Google Scholar]
- 25.Ludwig A, Hundhausen C, Lambert MH, et al. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8(2):161–171. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- 26.Uhl M, Aulwurm S, Wischhusen J, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64(21):7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 27.Welte SA, Sinzger C, Lutz SZ, et al. Selective intracellular retention of virally induced NKG2D ligands by the human cytomegalovirus UL16 glycoprotein. Eur J Immunol. 2003;33(1):194–203. doi: 10.1002/immu.200390022. [DOI] [PubMed] [Google Scholar]
- 28.Schreiner B, Voss J, Wischhusen J, et al. Expression of toll-like receptors by human muscle cells in vitro and in vivo: TLR3 is highly expressed in inflammatory and HIV myopathies, mediates IL-8 release and up-regulation of NKG2D-ligands. FASEB J. 2005;20(1):118–120. doi: 10.1096/fj.05-4342fje. [DOI] [PubMed] [Google Scholar]
- 29.Lee-MacAry AE, Ross EL, Davies D, et al. Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J Immunol Methods. 2001;252(1–2):83–92. doi: 10.1016/s0022-1759(01)00336-2. [DOI] [PubMed] [Google Scholar]
- 30.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res. 2006;66(5):2520–2526. doi: 10.1158/0008-5472.CAN-05-2520. [DOI] [PubMed] [Google Scholar]
- 32.Boutet P, Aguera-Gonzalez S, Atkinson S, et al. Cutting edge: the metalloproteinase ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding of the MHC class I-related chain B protein. J Immunol. 2009;182(1):49–53. doi: 10.4049/jimmunol.182.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Jiang F, Zheng X, et al. TGF-beta1 promotes motility and invasiveness of glioma cells through activation of ADAM17. Oncol Rep. 2011;25(5):1329–1335. doi: 10.3892/or.2011.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray A, Dhar S, Ray BK. Transforming growth factor-beta1-mediated activation of NF-kappaB contributes to enhanced ADAM-12 expression in mammary carcinoma cells. Mol Cancer Res. 2010;8(9):1261–1270. doi: 10.1158/1541-7786.MCR-10-0212. [DOI] [PubMed] [Google Scholar]
- 35.Ashiru O, Boutet P, Fernandez-Messina L, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70(2):481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser BK, Yim D, Chow IT, et al. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447(7143):482–486. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 37.Hundhausen C, Misztela D, Berkhout TA, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102(4):1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.