Abstract
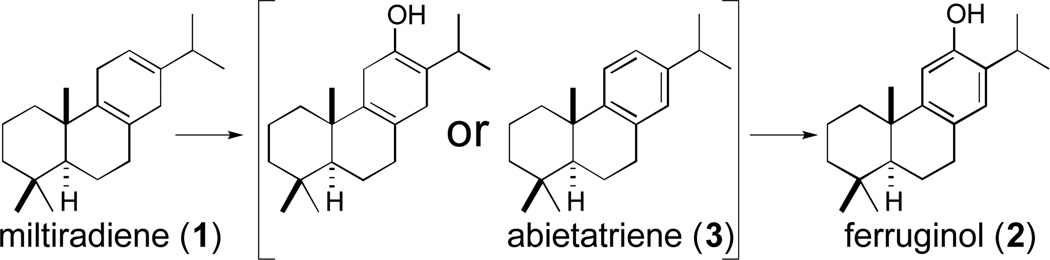
Miltiradiene (1), is the precursor of phenolic diterpenoids such as ferruginol (2), requiring aromatization and hydroxylation. While this has been attributed to a single cytochrome P450 (CYP76AH1), characterization of the rosemary ortholog CYP76AH4 led to the discovery that these CYPs simply hydroxylate the facilely oxidized aromatic intermediate abietatriene (3).
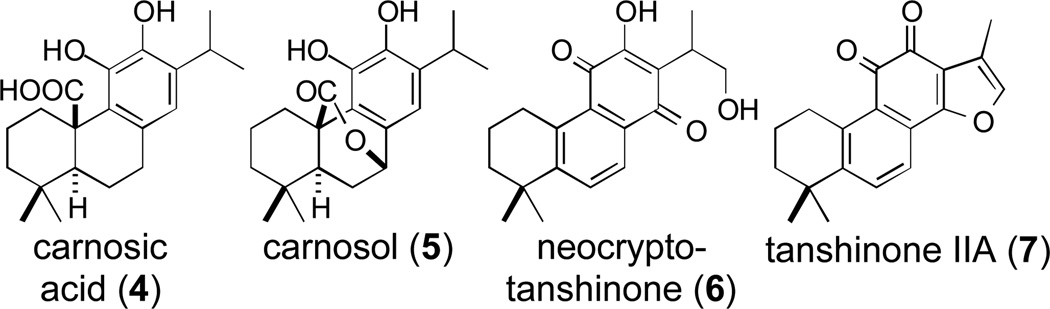
Rosemary (Rosmarinus officinalis) has long been used as a flavoring agent, and more recently as a nutritional supplement, as well as in cosmetics, due to its antioxidant properties.1 Carnosic acid (4) and carnosol (5) are the major phenolic diterpenoid constituents of rosemary, are responsible for much of its antioxidant activity, and exhibit a variety of other effects, including acting as anti-HIV and anticancer agents.2, 3 Moreover, 4 and 5 are likely intermediates in the biosynthesis of more elaborated phenolic diterpenoids, such as the tanshinones (e.g., 6 and 7; Figure 1) that make up the bioactive lipophilic constituents of the widely used Chinese medicinal herb Danshen (Salvia miltiorrhiza).4 Accordingly, there is significant interest in elucidation of phenolic diterpenoid biosynthesis.
Figure 1.
Representative phenolic diterpenoids.
Phenolic diterpenoids such as 2 – 7 fall within the labdane-related superfamily of natural products, whose biosynthesis is initiated by a sequential pair of cyclization and/or rearrangement reactions.5 This most often results in the production of an olefin, such as 1, which requires further elaboration to produce bioactive natural products, particularly the incorporation of oxygen catalysed by cytochromes P450 (CYPs). While these heme thiolate mono-oxygenases typically catalyse hydroxylation reactions,6 members of this enzymatic superfamily are known to catalyse more complex reactions, including aromatization and multiple reaction cycles with certain substrates.7, 8
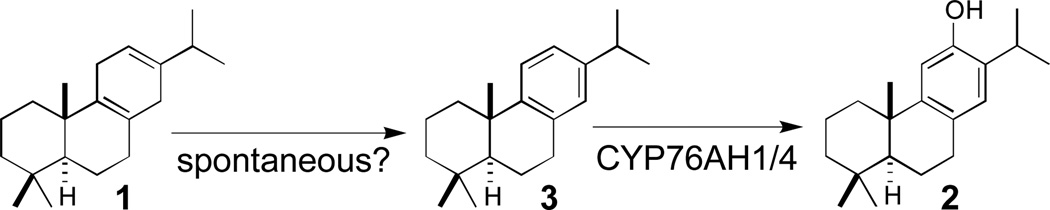
Previous work has identified two diterpene synthases from S. miltiorrhiza that together cyclize the general diterpenoid precursor (E,E,E)-geranylgeranyl diphosphate into the tricyclic olefin 1, with initial bicyclization catalysed by DsCPS, and subsequent cyclization and rearrangement catalysed by DsKSL.9 This abietane contains a planar cyclohexa-1,4-diene ring that is poised for aromatization, and recently was shown to be the precursor of the phenolic diterpenoids 2 and 6 via labelling studies.10 In addition, via an RNA-Seq approach, the S. miltiorrhiza CYP76AH1 was identified and reported to catalyse both aromatization and hydroxylation of 1 to form 2.10 However, it was unclear in what order aromatization and hydroxylation occur (Scheme 1).
Scheme 1.
Reactions putatively catalysed by CYP76AH1.10
Although phenolic diterpenoid biosynthesis is widely distributed in the Lamiaceae plant family (e.g., the production of 4 and 5 in rosemary), it was unclear how applicable the S. miltiorrhiza results were to other species from this family. Fortuitously, RNA-Seq data for a number of medicinal plants, including rosemary, has recently become available (http://medicinalplantgenomics.msu.edu). Thus, it is possible to simply carry out BLAST searches of the rosemary transcriptome, which is of quite good quality. Of particular interest here, using CYP76AH1 as the query sequence, four full-length homologs, CYP76AH4-7, were found. These share 60 – 85% amino acid (aa) sequence identity with CYP76AH1 and 59 – 92% aa sequence identity with each other (Figure S1), with CYP76AH4 exhibiting the highest identity with CYP76AH1.
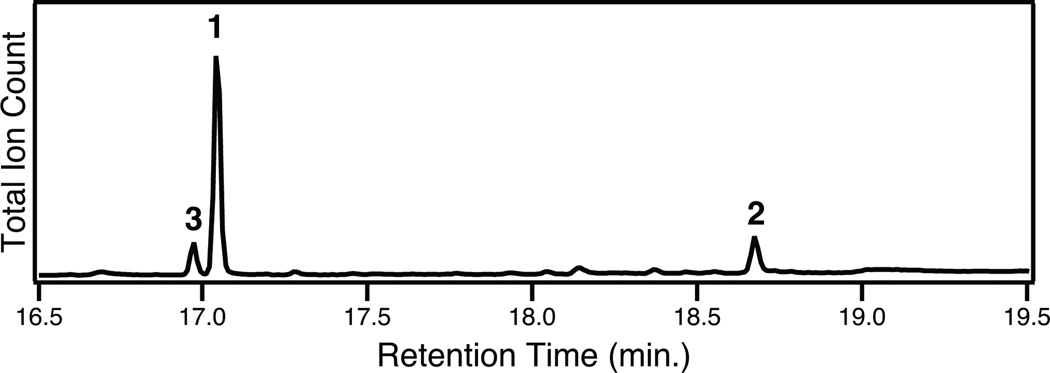
The role of CYP76AH4, as well as the other CYP76AH sub-family members from rosemary (i.e., CYP76AH5-7), in production of 2 was investigated via a synthetic biology approach. In particular, we have previously demonstrated that whole gene codon optimization, as well as replacement of the N-terminal membrane anchor region by a 10 amino acid long lysine- and serine-rich leader peptide, can be required for functional heterologous expression of plant P450s in E. coli.11–14 Accordingly, this approach was applied to all four candidate CYP76AH sub-family members from rosemary via gene synthesis. These were then co-expressed in E. coli with the requisite CYP reductase, along with overexpression of key genes from the endogenous upstream isoprenoid precursor biosynthetic pathway,15 a GGPP synthase, and DsCPS and DsKSL for production of the putative substrate 1, using a previously described modular metabolic engineering system.16 As expected, 2 is easily detectable in cultures expressing CYP76AH4, indicating that this is the rosemary ortholog to the S. miltiorrhiza CYP76AH1 (Figure 2).
Figure 2.
GC-MS chromatogram of extract from E. coli co-expressing CYP76AH4 and enzymes for the production of 1.
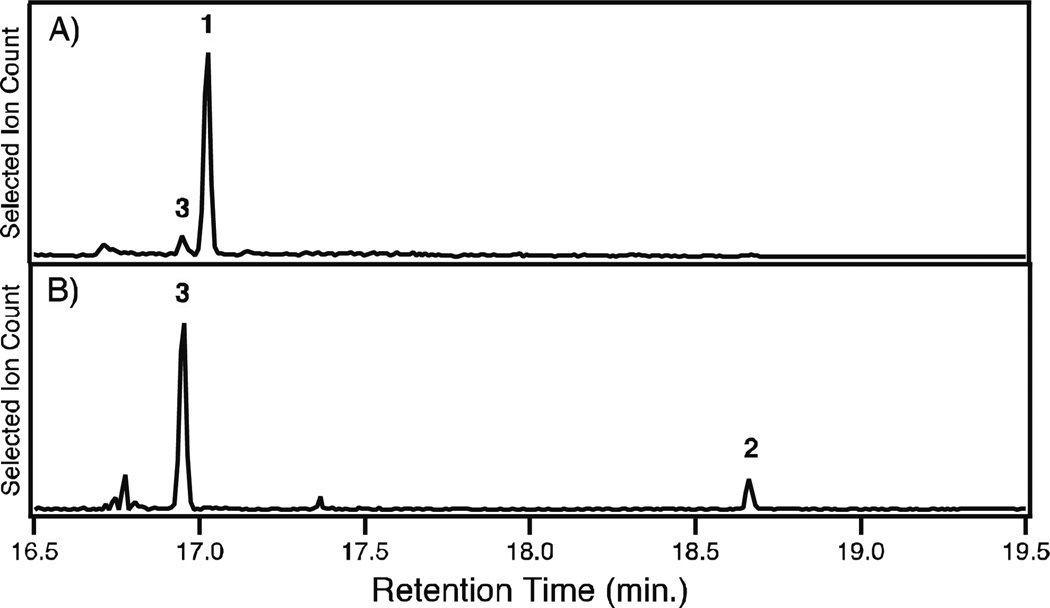
Having demonstrated that the CYP76AH sub-family plays a broader role in phenolic diterpenoid biosynthesis in the Lamiaceae, we began investigating the order of the presumed dual aromatization and hydroxylation reactions. In particular, it was possible to easily convert 1 (3 mg obtained from metabolically engineered E. coli) to 3, as verified by NMR analysis (Figures S2–7 and Table S1), simply via exposure to UV-irradiation. In vitro assays indicated that CYP76AH4 readily converted 3 to 2 (Figure 3A), with KM = 25 ± 6 µM. Surprisingly, a significant amount of 3 was found in the preparations of 1, due the presence of 3 in the metabolic engineering system (Figure 2), even in the absence of any CYP (Figure S8). Moreover, even following careful purification of 1, increasing amounts of 3 was always observed upon storage, indicating that 3 can arise from facile spontaneous oxidation. In addition, with fresh preparations of pure 1, CYP76AH4 was unable to produce significant amounts of 2, with the trace amounts that were found likely attributable to the remaining 3 and/or spontaneous oxidation of 1 to 3 occurring during the course of the enzymatic reaction (Figure 3B). Thus, it appears that CYP76AH4 catalyses only hydroxylation of 3. To determine if this also was true for the originally reported activity of CYP76AH1,10 an analogous series of studies were carried out, including use of an N-terminally modified and codon optimized gene construct, yielding the same results – i.e., CYP76AH1 similarly catalyses only hydroxylation of 3, and does not appear to catalyse aromatization of 1 (Figure S9).
Figure 3.
GC-MS chromatograms of in vitro assays of CYP76AH4 with either A) purified 1 (selected ions m/z = 272 + 286) or B) 3 (selected ions m/z = 255 + 286).
To determine if it would be possible to separate the facile spontaneous oxidation and enzymatic hydroxylation, we investigated the mechanism by which aromatization occurs. In particular, the role of molecular oxygen (O2), which was investigated by incubation of 1 in phosphate buffer in the presence or absence of O2 (removed via bubbling N2 through the solution and capping in a gas-tight vial). Significant conversion to 3 was only observed in the presence of O2 (Figure S10). Given the requirement for O2 in CYP catalysed reactions as well, it is then not possible to completely separate these transformations.
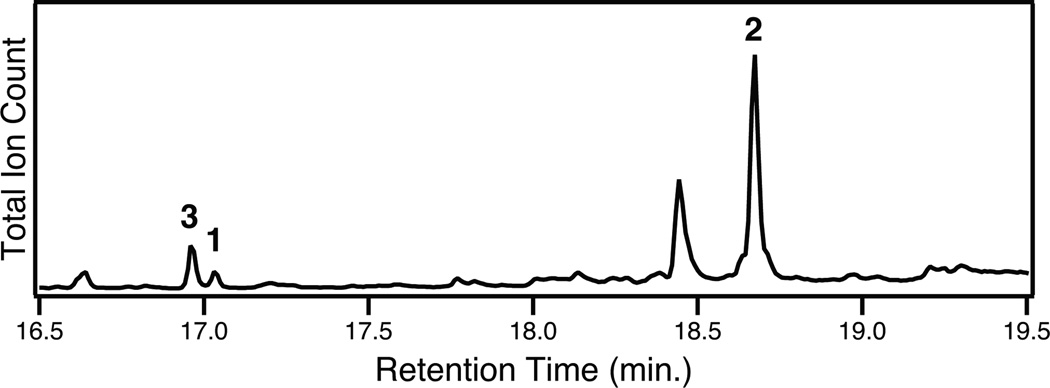
To further investigate the potential role of 3 in plant phenolic diterpenoid biosynthesis, we carried out phytochemical analysis of both rosemary and S. miltiorrhiza to determine if 3 could be found, along with 1 (which also has not yet been reported from rosemary) and 2. All three compounds were found in hexane extracts of both aerial tissues of rosemary and hairy root cultures of S. miltiorrhiza (Figures 4 & S11). This is consistent with the hypothesis that 3 is the relevant intermediate for conversion from 1 to 2. In planta, 3 is present at higher concentrations than 1, which is the inverse of the ratio observed in our bacterial metabolic engineering system (c.f., Figures 2 & 4), which suggests that aromatization of 1 to 3 may be enzymatically catalysed in planta.†
Figure 4.
GC-MS chromatogram of rosemary extract.
Conclusions
In summary, our results clarify the biosynthesis of phenolic diterpenoids, confining the role of the characterized CYP76AH sub-family members to C12-hydroxylation of the aromatic intermediate 3 (Scheme 2). The presence of 3, along with 1 and 2, in both rosemary and S. miltiorrhiza, is consistent with such a role for 3 in biosynthesis of phenolic diterpenoids. Thus, the initially formed olefin intermediate 1 first undergoes aromatization to 3 prior to formation of 2. While the conversion of 1 to 3 does occurs spontaneously, it seems likely that this aromatization reaction is enzymatically catalysed in planta, although the relevant enzyme is yet to be determined, providing a target for future investigation.
Scheme 2.
Actual role of CYP76AH sub-family members in plant phenolic diterpenoid biosynthesis.
Supplementary Material
Acknowledgements
This work was supported by a grant from the NIH (GM076324) and funds from Iowa State University to R.J.P., who also thanks the Alexander von Humboldt Foundation for sabbatical fellowship support during the preparation of this manuscript.
Footnotes
Intriguingly, if non-enzymatic oxidation plays a role in the conversion of 1 to 3 in planta, our findings may provide a rationale for the observed seasonal variation in content of 5 in rosemary, which seems to heavily depend on photoperiod.17 We speculate that sunlight promotes the conversion of 1 to 3, increasing the rate at which the natural antioxidant 5 is formed, providing another means by which these plants protect themselves from the UV-irradiation.
Electronic Supplementary Information (ESI) available: Description of materials and methods. Supplemental figures and table. Sequences of synthetic genes for CYP76AH1 & 4–7. See DOI: 10.1039/c000000x/
Notes and references
- 1.Zheng W, Wang SY. J Agr Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 2.Paris A, Strukelj B, Renko M, Turk V, Pukl M, Umek A, Korant BD. Journal of natural products. 1993;56:1426–1430. doi: 10.1021/np50098a031. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JJ. Cancer Lett. 2011;305:1–7. doi: 10.1016/j.canlet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Morris-Natschke SL, Lee K-H. Med. Res. Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 5.Peters RJ. Nat. Prod. Rep. 2010;27:1521–1530. doi: 10.1039/c0np00019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz de Montellano PR. Chem. Rev. 2010;110:932–948. doi: 10.1021/cr9002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guengerich FP. Chem. Res. Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 8.Mizutani M, Sato F. Arch Biochem Biophys. 2011;507:194–203. doi: 10.1016/j.abb.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Gao W, Hillwig ML, Huang L, Cui G, Wang X, Kong J, Yang B, Peters RJ. Org. Lett. 2009;11:5170–5173. doi: 10.1021/ol902051v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J, Zhou YJ, Hillwig ML, Shen Y, Yang L, Wang Y, Zhang X, Liu W, Peters RJ, Chen X, Zhao ZK, Huang L. Proc Natl Acad Sci U S A. 2013;110:12108–12113. doi: 10.1073/pnas.1218061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Hillwig ML, Okada K, Yamazaki K, Wu Y, Swaminathan S, Yamane H, Peters RJ. J. Biol. Chem. 2012;287:6159–6168. doi: 10.1074/jbc.M111.305599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Hillwig ML, Peters RJ. Plant J. 2011;65:87–95. doi: 10.1111/j.1365-313X.2010.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Hillwig ML, Wu Y, Peters RJ. Plant Physiol. 2012;158:1418–1425. doi: 10.1104/pp.111.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Hillwig ML, Wang Q, Peters RJ. FEBS Lett. 2011;585:3446–3451. doi: 10.1016/j.febslet.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrone D, Lowry L, Determan MK, Hershey DM, Xu M, Peters RJ. Appl. Microbiol. Biotechnol. 2010;85:1893–1906. doi: 10.1007/s00253-009-2219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cyr A, Wilderman PR, Determan M, Peters RJ. J. Am. Chem. Soc. 2007;129:6684–6685. doi: 10.1021/ja071158n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidalgo PJ, Ubera JL, Tena MT, Valcarcel M. J Agr Food Chem. 1998;46:2624–2627. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.