Phyllodes tumour is an unusual fibroepithelial tumour of the breast constituting only 0.3% to 0.5% of all breast lesions.1,2 It usually occurs in middle-aged women. The median size of phyllodes tumour is 4 cm; however, 20% of these tumours grow larger than 10 cm and are known as giant phyllodes tumours. We report here a rare case of a middle-aged woman who presented to her family physician with an unusually large breast tumour which measured 24 × 22 × 20 cm in size, weighed 5.5 kg, showed multiple ulcerations, and resulted in gross cosmetic disfigurement. The tumour was subsequently diagnosed as a borderline-grade giant phyllodes tumour. To our knowledge, such a large phyllodes tumour (weighing more than 5 kg) has rarely been reported in the medical literature.
Case
A 42-year-old woman noticed a lump in her right breast while bathing 3 years ago. She visited her family physician, who examined her and found a lump in the inferolateral quadrant of her breast that was approximately 3 × 2 cm in size. It was firm, mobile, and nontender. He recommended an excision biopsy but the patient refused as she feared it would be diagnosed as a malignancy. Moreover, both she and her husband disliked the thought of losing a breast. Instead, they resorted to traditional forms of medication in the hope that the mass would resolve.
However, the mass continued to steadily increase in size and 19 months later she revisited her physician, as the mass was now too big to bear and had begun to ulcerate at 2 places (Figure 1). Her physician immediately referred her to the surgical outpatient department at the local hospital. On examination, the surgeon noticed that the right breast was grossly enlarged and lobulated and the skin over the breast appeared stretched, shiny, and hyperpigmented, with dilated superficial veins. The right nipple appeared stretched and flattened out. However, it was neither ulcerated nor retracted and there was no discharge from the nipple. No peau d’orange was noted. The mass in the right breast was measured and found to be 24 × 22 × 20 cm in size. It was firm, nontender, mobile, and not attached to the underlying structures. The mass also appeared to be well demarcated, with a lobulated surface. Two ulcerations were noted over the lateral aspect of the mass measuring 2 × 1 cm and 3.5 × 2.5 cm (Figure 2, black arrows). The left breast was normal on examination. Cervical and axillary lymph nodes were not palpable. Results of chest x-ray scans and high-resolution computed tomography of the thorax were normal. The lung fields were clear and there was no evidence of enlarged hilar or mediastinal glands. Ultrasonography of the abdomen revealed no abnormality. The liver, spleen, pancreas, and kidneys appeared normal. There was no evidence of enlarged abdominal lymph nodes. A core tissue biopsy was performed. On microscopy, a well-circumscribed tumour was seen, showing slitlike ducts and pushing borders (Figure 3A), with focal fibroadipose tissue invasion. The tumour consisted of stromal proliferation with elongated leaflike papillae protruding into dilated spaces. These structures were lined by ductal epithelium and myoepithelial cells. The spaces were filled with eosinophilic fluid and blood. Hyalinization and myxoid changes were seen throughout the tumour (Figure 3B). Scattered rounded acini were seen lined by hyperplastic epithelial cells without atypia. The stromal cells showed moderate cellularity and spindle-shaped nuclei with some areas of cells exhibiting ovoid, slightly plump nuclei. Occasional mitosis was seen (Figure 4A). Areas of chondromyxoid (cartilaginous) and osseous metaplasia were also seen (Figure 4B). There was no evidence of Paget disease of the nipple. The ulcerated skin was covered by exudate and necrotic debris, with underlying granulation tissue. The tumour showed infiltration at the inferior and lateral margins. Consequently, a diagnosis of borderline-grade giant phyllodes tumour was made, and the patient was advised to undergo simple mastectomy. Surgery was performed and the tumour mass was found to weigh 5.5 kg (Figure 5).
Postoperatively, the resected tumour mass was subjected to multiple sections (1 section per centimetre of tissue). Most sections showed histopathologic evidence of borderline-grade giant phyllodes tumour, with no section showing areas of focal malignancy.
Figure 1.
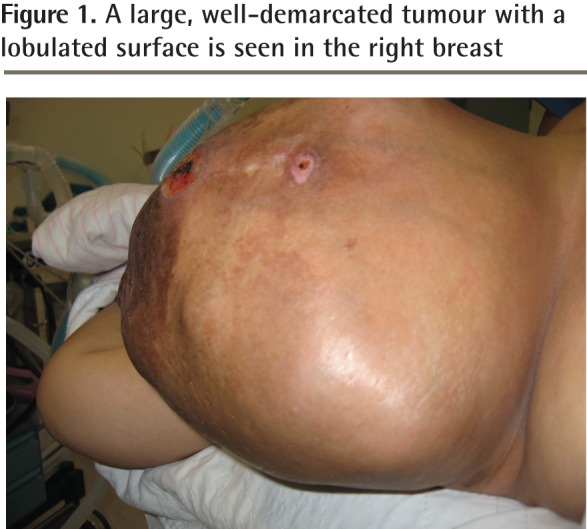
A large, well-demarcated tumour with a lobulated surface is seen in the right breast
Figure 2.
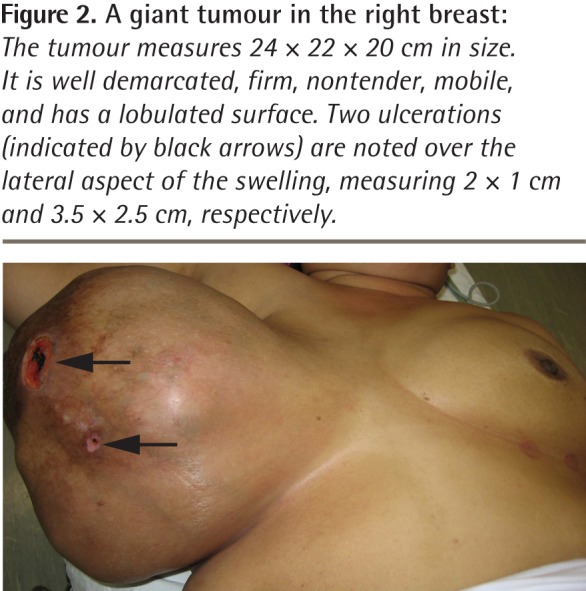
A giant tumour in the right breast: The tumour measures 24 × 22 × 20 cm in size. It is well demarcated, firm, nontender, mobile, and has a lobulated surface. Two ulcerations (indicated by black arrows) are noted over the lateral aspect of the swelling, measuring 2 × 1 cm and 3.5 × 2.5 cm, respectively.
Figure 3.
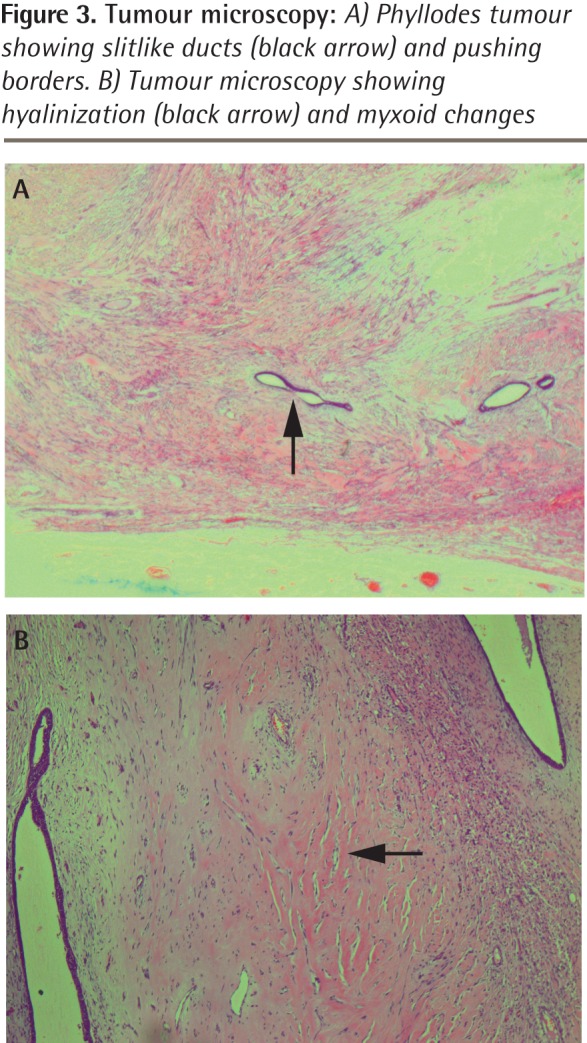
Tumour microscopy: A) Phyllodes tumour showing slitlike ducts (black arrow) and pushing borders. B) Tumour microscopy showing hyalinization (black arrow) and myxoid changes
Figure 4.
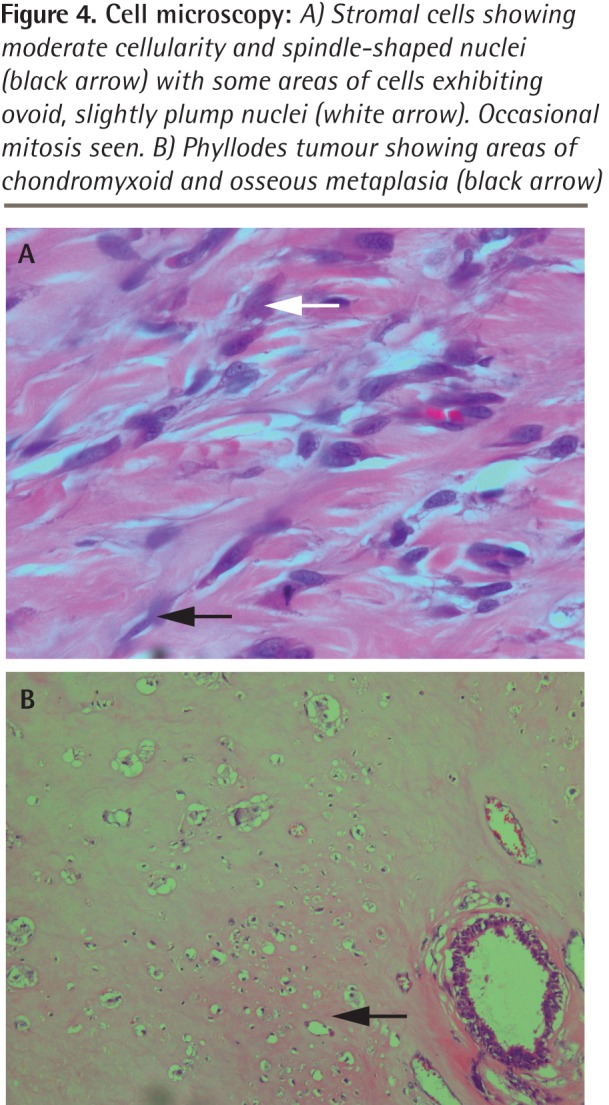
Cell microscopy: A) Stromal cells showing moderate cellularity and spindle-shaped nuclei (black arrow) with some areas of cells exhibiting ovoid, slightly plump nuclei (white arrow). Occasional mitosis seen. B) Phyllodes tumour showing areas of chondromyxoid and osseous metaplasia (black arrow)
Figure 5.
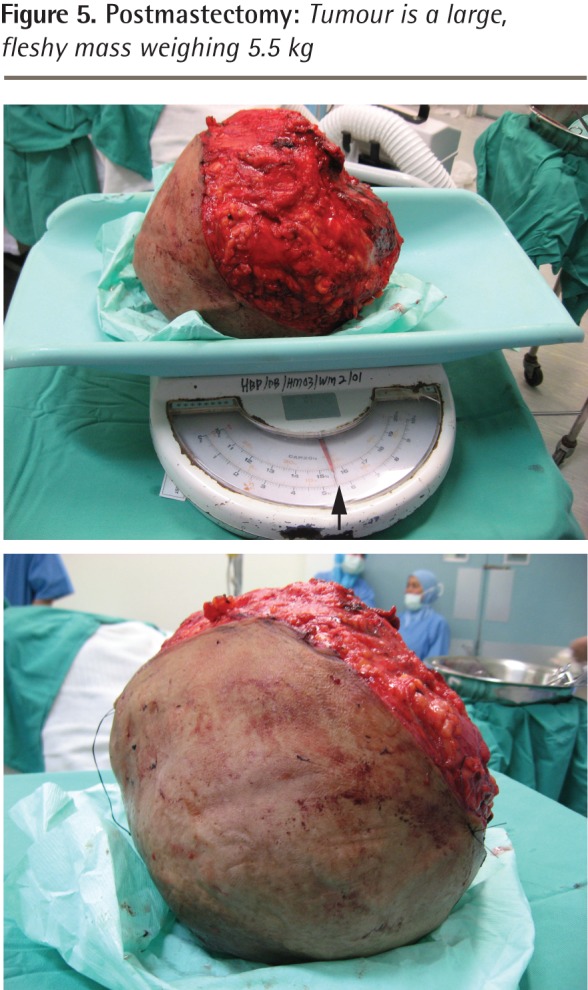
Postmastectomy: Tumour is a large, fleshy mass weighing 5.5 kg
Discussion
Phyllodes tumour was originally described by Johannes Müller in 1838.3 It is an unusual fibroepithelial tumour of the breast constituting only 0.3% to 0.5% of all breast lesions.1,2 It is defined as mixed epithelial and stromal-mesenchymal proliferation of breast tissue characterized by increased stromal cellularity and broad leaflike papillae inserted into cleftlike spaces. It usually occurs in middle-aged women but occasionally occurs at a younger age and in men. The mean age of patients presenting with phyllodes tumour of the breast is about 41 years.4–8 This is approximately 10 to 15 years older than the mean age of patients presenting with fibroadenomas of the breast,9 but both conditions occur over a wide age range (15 to 67 years for phyllodes tumours).5 The tumour is more common in the upper and outer quadrant of the breast but has no predilection for side. Bilateral tumours are also rare (1%). The patient usually presents with a breast lump that is firm, mobile, and nontender. The lump is usually well defined, with distinct borders, and is difficult to distinguish from a fibroadenoma. Malignant phyllodes tumour is usually suspected in older patients, those with a strong family history of breast cancer, those with clinical signs such as skin dimpling or vein reticula, and irregular or indistinct margins on tumour palpation. Complications such as bleeding and ulceration over the lesion, lymphedema, brachial plexus injuries, hematoma formation, cosmetic disfiguration due to the abnormally large size of the tumour mass, and metastasis to the lungs, bone, liver, and distant lymph nodes are also hallmarks of a malignant phyllodes tumour. The differential diagnosis is predominantly fibroadenoma; however, other conditions such as metastatic carcinoma, angiosarcoma, pleomorphic adenoma, pure sarcoma, and myofibroblastoma also need to be considered. The median size of phyllodes tumour is 4 cm.2 However, 20% of these tumours grow larger than 10 cm and are known as giant phyllodes tumours. These tumours can get even larger, growing up to 40 cm.10 Phyllodes tumours are divided into benign, borderline, and malignant histotypes based on the microscopic appearance of the stromal component. Approximately 15% to 30% of all phyllodes tumours are classified as malignant.4,11,12 Various diagnostic procedures are available for the diagnosis of phyllodes tumour. However, the optimal diagnostic test is still debated. While mammography typically shows lobulated, benign-appearing opacities, microcalcification is rare.13,14 In one study, mammography reported benign features in 6 of 8 patients with borderline or malignant histologic features.5 Ultrasonography as a diagnostic tool has also been used. It shows smooth contours, low-level internal echoes, absence of posterior shadowing, and intramural cysts.14,15 One study reported the role of thermography in distinguishing phyllodes tumour from fibroadenomas by demonstrating increased heat generation from the enhanced stromal cell activity seen in phyllodes tumours.16 However, to date there is insufficient evidence to support the role of thermography in the diagnosis of phyllodes tumour. Most studies are also in agreement that clinical examination, mammography, and ultrasonography are not reliable tools for the diagnosis of phyllodes tumour or the preoperative prediction of the histologic type.13,14,17,18 The diagnosis of phyllodes tumour is confirmed by histologic examination after surgical removal. Core needle biopsy is the mainstay in the preoperative diagnosis of phyllodes tumour. In comparison, fine-needle aspiration cytology has certain shortcomings, as its accuracy depends on an adequate and representative sample. Phyllodes tumours are by nature heterogeneous tumours with focal areas of hypercellular fragments. Consequently, sampling problems can occur, and fine-needle aspiration cytology might be inaccurate if sampling has been done from a relatively hypocellular, myxoid, or hyalinized area of stromal tissue. Hence, the value of fine-needle aspiration cytology in diagnosis of phyllodes tumour remains debatable, with an overall accuracy of 63%.19
Phyllodes tumours have a similar basic structure to intracanalicular fibroadenomas but have a greater degree of stromal cellularity and contain leaflike or club-like epithelial-lined papillary projections pushing into cystic spaces.20 This difference in the connective tissue element is the essential feature distinguishing them from fibroadenomas. Hawkins et al21 examined the histologic features that predicted malignant behaviour and found that stromal overgrowth, high stromal cell mitotic activity (≥ 10 mitoses per 10 high-power fields), severe nuclear pleomorphism, and infiltrating margins were substantially associated with later metastasis. However, histologic appearance does not always correlate with clinical behaviour,4,11,22,23 as both malignant and borderline tumours have been shown to be capable of metastasizing. Also, while benign phyllodes tumours might recur, borderline phyllodes tumours show higher propensity to recur locally. In our patient, the tumour was graded as borderline, as there was stromal overgrowth, evidence of occasional mitotic activity, and infiltration at the inferior and lateral margins. It was not graded as malignant because there were neither sarcomatous stroma nor a high mitotic index. Moreover, there was no evidence of local metastasis to the axillary lymph nodes or distant metastasis to the liver or lungs. Chest x-ray scans, high-resolution computed tomography of the thorax, and abdominal ultrasonography had normal results. The lung fields were clear and there was no evidence of enlarged hilar or mediastinal glands, and the liver, spleen, pancreas, and kidneys appeared to be normal. Follow-up of the patient 17 months after surgery showed no evidence of local recurrence or metastasis to the axillary lymph nodes (Figure 6), liver, bone, or lungs. Repeat chest x-ray scans and abdominal ultrasonography done at this time also had normal results.
Figure 6.
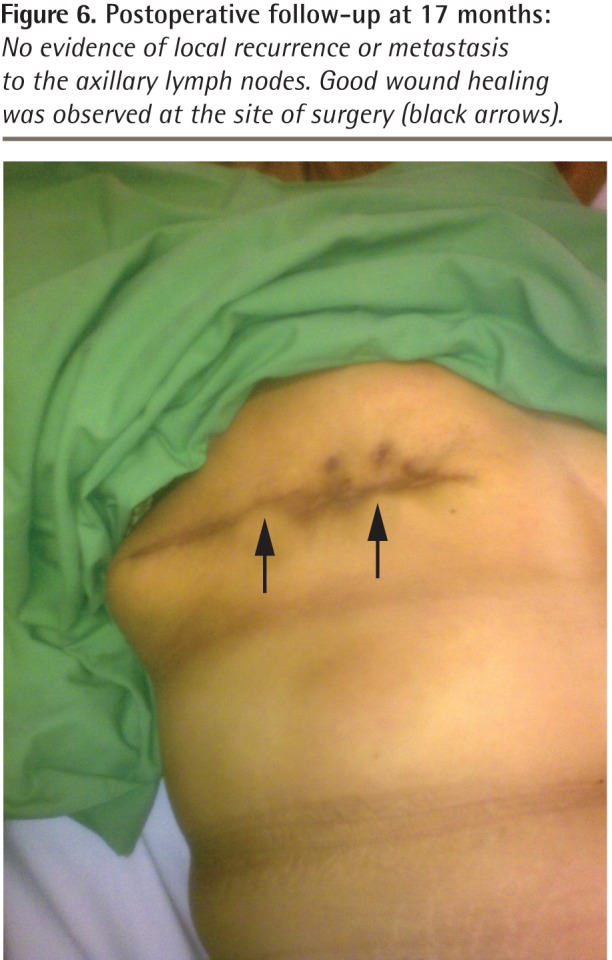
Postoperative follow-up at 17 months: No evidence of local recurrence or metastasis to the axillary lymph nodes. Good wound healing was observed at the site of surgery (black arrows).
Management of giant phyllodes tumour presents unique challenges to the treating surgeon.24 While core tissue biopsy represents an attractive means for preoperative diagnosis and helps in the differentiation of phyllodes tumours from fibroadenomas, simple mastectomy is usually the treatment of choice for most of these tumours. In most cases of phyllodes tumour, a wide local excision is performed with a rim of normal tissue. There are no fixed rules for margin size; however, a 2-cm margin for small tumours (< 5 cm in size) and a 5-cm margin for large tumours (≥ 5 cm in size) have been suggested. If the tumour-to-breast ratio is sufficiently high to preclude a satisfactory result by segmental excision, total mastectomy is recommended. More radical surgical resection is usually not necessary, and axillary lymph node resection is generally not required in the absence of suspicious nodes. Adjuvant chemotherapy and radiotherapy have not proven to be useful in the treatment of phyllodes tumour and response to chemotherapy and radiotherapy is known to be poor in case of metastasis. Hormonal therapy has also been unsuccessful in the treatment of phyllodes tumour. Axillary lymph node metastasis is usually rare, and dissection should be limited to patients with evidence of metastasis to these sites.
While metastases are seen in 25% to 31% of breast tumours,2,11 they are seen in only 4% of all phyllodes tumours.12 However, 25% of malignant phyllodes tumours show evidence of metastasis during the course of the disease process. Skin involvement by the tumour does not appear to be a predictor of metastasis.25 Metastases are hemopoietic and usually appear within 5 years of the diagnosis. Family physicians who follow up with their patients diagnosed with borderline-grade giant phyllodes tumours need to remember that isolated lung metastases can develop years later, which might be resectable if detected early. Hence, detailed follow-up accompanied by chest x-ray scans at regular intervals is useful in such patients.
Expected outcome essentially depends on the histologic type (benign, borderline, malignant) of phyllodes tumour. In one large study10 involving 170 women with phyllodes tumour, the overall 5-year survival rate was 82.9% (141 patients). Patients with benign phyllodes tumours had a 5-year survival rate of 95.7%, while the 5-year survival rates in patients with borderline and malignant phyllodes tumours were 73.7% and 66.1%, respectively. Another study that combined benign and borderline tumours into a single category found 5-year survival rates of 91% for benign tumours and 82% for malignant tumours. However, 10-year survival rates dropped to 79% and 42%, respectively.11 A recent review and clinical follow-up of 33 cases concluded that histopathologic classification is the strongest prognostic factor for this disease.26 However, others have failed to duplicate the correlation between histotype and survival.22
Conclusion
Breast swellings are a common problem encountered by family physicians in the course of their busy medical practices. The causes usually include fibroadenoma, metastatic carcinoma, pure sarcoma, hamartoma, myofibroblastoma, phyllodes tumour, and angiosarcoma.
A diagnosis of phyllodes tumour is typically confirmed on histologic examination after excision. Core needle biopsy is the usual method of tissue collection for making a preoperative diagnosis of phyllodes tumour. Expected outcome depends on the histologic type (benign, borderline, malignant). Our patient was diagnosed with a borderline-grade tumour, and follow-up 17 months after surgery revealed no evidence of local recurrence or metastasis to the axillary lymph nodes.
EDITOR’S KEY POINTS
Phyllodes tumour is defined as an unusual mixed epithelial and stromal-mesenchymal proliferation of breast tissue characterized by increased stromal cellularity and broad leaflike papillae inserted into cleftlike spaces. Tumours larger than 10 cm are known as giant phyllodes tumours.
The diagnosis of phyllodes tumour is confirmed by excision and histologic examination. Core needle biopsy is the mainstay in the preoperative diagnosis of phyllodes tumour.
POINTS DE REPÈRE DU RÉDACTEUR
La tumeur phyllode est définie comme une prolifération épithéliale et stromale-mésenchymale mixte inhabituelle de tissus du sein, qui se caractérise par une cellularité stromale accrue et de larges papilles foliées insérées dans des espaces semblables à des fissures. Les tumeurs de plus de 10 cm sont considérées comme des tumeurs phyllodes géantes.
Le diagnostic d’une tumeur phyllode est confirmé par l’excision et un examen histologique. La biopsie au trocart est à la base d’un diagnostic préopératoire d’une tumeur phyllode.
Footnotes
This article has been peer reviewed. Cet article a fait l’objet d’une révision par des pairs.
Competing interests
None declared
References
- 1.Kario K, Maeda S, Mizuno Y, Makino Y, Tankawa H, Kitazawa S. Phyllodes tumor of the breast: a clinicopathologic study of 34 cases. J Surg Oncol. 1990;45(1):46–51. doi: 10.1002/jso.2930450111. [DOI] [PubMed] [Google Scholar]
- 2.Rowell MD, Perry RR, Hsiu JG, Barranco SC. Phyllodes tumors. Am J Surg. 1993;165(3):376–9. doi: 10.1016/s0002-9610(05)80849-9. [DOI] [PubMed] [Google Scholar]
- 3.Fiks A. Cystosarcoma phyllodes of the mammary gland—Müller’s tumor. For the 180th birthday of Johannes Müller. Virchows Arch A Pathol Anat Histol. 1981;392(1):1–6. doi: 10.1007/BF00430543. [DOI] [PubMed] [Google Scholar]
- 4.Norris HJ, Taylor HB. Relationship of histologic features to behavior of cystosarcoma phyllodes. Analysis of ninety-four cases. Cancer. 1967;20(12):2090–9. doi: 10.1002/1097-0142(196712)20:12<2090::aid-cncr2820201206>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Stebbing JF, Nash AG. Diagnosis and management of phyllodes tumour of the breast: experience of 33 cases at a specialist centre. Ann R Coll Surg Engl. 1995;77(3):181–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Pietruszka M, Barnes L. Cystosarcoma phyllodes: a clinicopathologic analysis of 42 cases. Cancer. 1978;41(5):1974–83. doi: 10.1002/1097-0142(197805)41:5<1974::aid-cncr2820410543>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Haagensen CD, editor. Diseases of the breast. 3rd ed. Philadelphia, PA: WB Saunders; 1986. Cystosarcoma phyllodes; pp. 284–312. [Google Scholar]
- 8.Bernstein L, Deapen D, Ross RK. The descriptive epidemiology of malignant cystosarcoma phyllodes tumors of the breast. Cancer. 1993;71(10):3020–4. doi: 10.1002/1097-0142(19930515)71:10<3020::aid-cncr2820711022>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Umpleby HC, Moore I, Royle GT, Guyer GB, Taylor I. An evaluation of the preoperative diagnosis and management of cystosarcoma phyllodes. Ann R Coll Surg Engl. 1989;71(5):285–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Reinfuss M, Mituś J, Duda K, Stelmach A, Ryś J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77(5):910–6. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Chaney AW, Pollack A, McNeese MD, Zagars GK, Pisters PW, Pollock RE, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89(7):1502–11. doi: 10.1002/1097-0142(20001001)89:7<1502::aid-cncr13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Salvadori B, Cusumano F, Del Bo R, Delledonne V, Grassi M, Rovini D, et al. Surgical treatment of phyllodes tumors of the breast. Cancer. 1989;63(12):2532–6. doi: 10.1002/1097-0142(19890615)63:12<2532::aid-cncr2820631229>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Page JE, Williams JE. The radiological features of phyllodes tumour of the breast with clinico-pathological correlation. Clin Radiol. 1991;44(1):8–12. doi: 10.1016/s0009-9260(05)80217-x. [DOI] [PubMed] [Google Scholar]
- 14.Buchberger W, Strasser K, Heim K, Müller E, Schröcksnadel H. Phyllodes tumor: findings on mammography, sonography and aspiration cytology in 10 cases. Am J Roentgenol. 1991;157(4):715–9. doi: 10.2214/ajr.157.4.1654022. [DOI] [PubMed] [Google Scholar]
- 15.Cole-Beuglet C, Soriano R, Kurtz AB, Meyer JE, Kopans DB, Goldberg BB. Ultrasound, x-ray mammography, and histopathology of cystosarcoma phylloides. Radiology. 1983;146(2):481–6. doi: 10.1148/radiology.146.2.6294737. [DOI] [PubMed] [Google Scholar]
- 16.Pierart J, Burmeister R, Steinberg J, Schalper J, Cid L. Use of thermography in the differential diagnosis of phyllodes tumour. Br J Surg. 1990;77(7):783–4. doi: 10.1002/bjs.1800770721. [DOI] [PubMed] [Google Scholar]
- 17.Cosmacini P, Zurrida S, Veronesi P, Bartoli C, Coopmans de Yoldi GF. Phyllode tumor of the breast: mammographic experience in 99 cases. Eur J Radiol. 1992;15(1):11–4. doi: 10.1016/0720-048x(92)90194-e. [DOI] [PubMed] [Google Scholar]
- 18.Ciatto S, Bonardi R, Cataliotti L, Cardona G. Phyllodes tumor of the breast: a multi-center series of 59 cases. Eur J Surg Oncol. 1992;18(6):545–9. [PubMed] [Google Scholar]
- 19.Scolyer RA, McKenzie PR, Achmed D, Lee CS. Can phyllodes tumours of the breast be distinguished from fibroadenomas using fine needle aspiration cytology? Pathology. 2001;33(4):437–43. doi: 10.1080/00313020120083151. [DOI] [PubMed] [Google Scholar]
- 20.Sloane JP, Trott PA. Biopsy pathology of the breast. London, UK: Chapman and Hall; 1985. pp. 49pp. 216–8. [Google Scholar]
- 21.Hawkins RE, Schofield JB, Fisher C, Wiltshaw E, McKinna JA. The clinical and histologic criteria that predict metastasis from cystosarcoma phyllodes. Cancer. 1992;69(1):141–7. doi: 10.1002/1097-0142(19920101)69:1<141::aid-cncr2820690125>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Sheen-Chen SM, Chou FF, Chen WJ. Cystosarcoma phylloides of the breast: a review of clinical, pathological and therapeutic option in 18 cases. Int Surg. 1991;76(2):101–4. [PubMed] [Google Scholar]
- 23.Tan PH, Jayabaskar T, Chuah KL, Lee HY, Tan Y, Hilmy M, et al. Phyllodes tumors of the breast: the role of pathologic parameters. Am J Clin Pathol. 2005;123(4):529–40. doi: 10.1309/U6DV-BFM8-1MLJ-C1FN. [DOI] [PubMed] [Google Scholar]
- 24.Liang MI, Ramaswamy B, Patterson CC, McKelvey MT, Gordillo G, Nuovo GJ, et al. Giant breast tumors: surgical management of phyllodes tumors, potential for reconstructive surgery and a review of literature. World J Surg Oncol. 2008;6:117. doi: 10.1186/1477-7819-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browder W, McQuitty JT, Jr, McDonald JC. Malignant cystosarcoma phylloides. Treatment and prognosis. Am J Surg. 1978;136(2):239–41. doi: 10.1016/0002-9610(78)90236-2. [DOI] [PubMed] [Google Scholar]
- 26.Lenhard MS, Kahlert S, Himsl I, Ditsch N, Untch M, Bauerfeind I. Phyllodes tumor of the breast: clinical follow-up of 33 cases of this rare disease. Eur J Obstet Gynecol Reprod Biol. 2008;138(2):217–21. doi: 10.1016/j.ejogrb.2007.08.002. Epub 2007 Sep 14. [DOI] [PubMed] [Google Scholar]


