Abstract
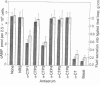
The immune response against six synthetic peptides corresponding to various segments of the B subunit of cholera toxin was evaluated. Conjugates in which the peptides were covalently linked to tetanus toxoid served for immunization of rabbits. As previously reported, four of these conjugates elicited antibodies cross-reactive with intact cholera toxin. We report here that antisera against two of these synthetic peptides inhibit the entire spectrum of activities of the intact cholera toxin. This is manifested both on the biochemical level (adenylate cyclase induction) and on the biological effect (intestinal fluid secretion). These results indicate that these peptides may serve as suitable candidates for preparation of a synthetic anticholera vaccine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R., Maron E., Sela M., Anfinsen C. B. Antibodies reactive with native lysozyme elicited by a completely synthetic antigen. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1450–1455. doi: 10.1073/pnas.68.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R., Sela M., Parant M., Chedid L. Antiviral response elicited by a completely synthetic antigen with built-in adjuvanticity. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6769–6772. doi: 10.1073/pnas.77.11.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R., Shapira M., Jacob C. O. Synthetic vaccines. J Immunol Methods. 1983 Jul 29;61(3):261–273. doi: 10.1016/0022-1759(83)90220-x. [DOI] [PubMed] [Google Scholar]
- Audibert F., Jolivet M., Chedid L., Arnon R., Sela M. Successful immunization with a totally synthetic diphtheria vaccine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5042–5046. doi: 10.1073/pnas.79.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azurin J. C., Cruz A., Pesigan T. P., Alvero M., Camena T., Suplido R., Ledesma L., Gomez C. Z. A controlled field trial of the effectiveness of cholera and cholera El Tor vaccines in the Philippines. Bull World Health Organ. 1967;37(5):703–727. [PMC free article] [PubMed] [Google Scholar]
- Benenson A. S., Mosley W. H., Fahimuddin M., Oseasohn R. O. Cholera vaccine field trials in east Pakistan. 2. Effectiveness in the field. Bull World Health Organ. 1968;38(3):359–372. [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Possibilities of immunization against cholera and related enterotoxic enteropathies. Dev Biol Stand. 1976;33:102–107. [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Glass R. I., Holmgren J., Khan M. R., Hossain K. M., Huq M. I., Greenough W. B. A randomized, controlled trial of the toxin-blocking effects of B subunit in family members of patients with cholera. J Infect Dis. 1984 Apr;149(4):495–500. doi: 10.1093/infdis/149.4.495. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Selection and characteristics of a Vibrio cholerae mutant lacking the A (ADP-ribosylating) portion of the cholera enterotoxin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2052–2056. doi: 10.1073/pnas.76.4.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Sela M., Arnon R. Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7611–7615. doi: 10.1073/pnas.80.24.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. Y. The chemistry and biology of cholera toxin. CRC Crit Rev Biochem. 1980;9(3):171–206. doi: 10.3109/10409238009105434. [DOI] [PubMed] [Google Scholar]
- Langbeheim H., Arnon R., Sela M. Antiviral effect on MS-2 coliphage obtained with a synthetic antigen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4636–4640. doi: 10.1073/pnas.73.12.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B. Automated synthesis of peptides. Science. 1965 Oct 8;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- Müller G. M., Shapira M., Arnon R. Anti-influenza response achieved by immunization with a synthetic conjugate. Proc Natl Acad Sci U S A. 1982 Jan;79(2):569–573. doi: 10.1073/pnas.79.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M., Polin D., Hurwitz S. Urinary cyclic AMP excretion in birds: dependence on parathyroid hormone activity. Gen Comp Endocrinol. 1983 Jan;49(1):90–96. doi: 10.1016/0016-6480(83)90011-4. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Pierzchala W. A., Bonde G., McCann T., Rubin B. A. Development of a purified cholera toxoid. III. Refinements in purification of toxin and methods for the determination of residual somatic antigen. Infect Immun. 1976 Sep;14(3):687–693. doi: 10.1128/iai.14.3.687-693.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Sack D. A., Holmgren J., Bardhan P. K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982 Feb 6;1(8267):305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- Van Heyningen S., King C. A. Short communications. Subunit A from cholera toxin is an activator of adenylate cyclase in pigeon erythrocytes. Biochem J. 1975 Jan;146(1):269–271. doi: 10.1042/bj1460269. [DOI] [PMC free article] [PubMed] [Google Scholar]