Abstract
Background and Objectives
Despite the dramatic increase in the use of buprenorphine for the treatment of opioid dependence, clinical outcomes of this treatment approach continue to need evaluation. This study examines factors associated with relapse and retention during buprenorphine treatment in a sample of opioid dependent outpatients.
Methods
In a retrospective chart review of 62 patients with opioid dependence, relapse was determined by self-report, urine toxicology screens, and by checking the state controlled substance monitoring database. Data was analyzed using two-way tests of association and logistic regression.
Results
Patients with comorbid anxiety disorders, active benzodiazepine use (contrary to clinic policy), or active alcohol abuse, were significantly more likely to relapse. Patients who relapsed were also more likely to be on a higher buprenorphine maintenance dose.
Conclusion
This study identifies relapse risk factors during buprenorphine treatment for opioid dependence. Future research is needed to determine whether modifying these factors may lead to improved treatment outcomes.
Opioid misuse continues to be a significant problem in the United States. In 2010, an estimated 200,000 persons reported heroin use in the past month. Besides heroin, the abuse of opioid pain medication appears to be dramatically increasing. In the same year, there were 5.1 million persons aged 12 or older who reported nonmedical use of prescription pain relievers in the past month. The number of persons with opioid analgesic dependence or abuse increased between 2002 and 2010 (from 1.5 million to 1.9 million). The number receiving specialty treatment for a problem with nonmedical analgesic use more than doubled during this period, from 199,000 to 406,000 per year (1). Deaths from overdose on opioid pain medication have more than tripled in the past decade in the United States confirming the devastating effects of the current increasing trend (2).
Traditionally, treatment for opioid dependence has been provided in federally regulated programs such as methadone clinics. However, over the past decade, buprenorphine has been increasingly used in the office-based treatment of opioid dependence, both for detoxification and maintenance. Several factors favor the use of buprenorphine, including convenient access to the medication through office-based provision of care, minimization of stigma, and the ability to customize care to the needs of the patient (3). The availability of buprenorphine has significantly improved quality of life for many opioid dependent patients (4). Buprenorphine has been shown to have similar efficacy to moderate dose methadone and levomethadyl acetate in reducing the illicit use of opioids (5). However, outcomes for patients treated in “real world” office-based buprenorphine maintenance have not been extensively studied. A recently published randomized controlled trial examined relapse rates during and after a brief course of office-based buprenorphine treatment specifically for prescription opioid dependence, and suggested that the likelihood of relapse is high during and after treatment (6). A recent study suggested that youth presenting to buprenorphine treatment with previous 30-day injection drug use and more active medical/psychiatric problems were less likely remain abstinent at week 12 of treatment (7). A recent meta-analysis has suggested that higher buprenorphine dose (16–32 mg per day) predicted better retention in treatment compared with a dose less than 16 mg per day (8).
Our study, conducted in a university addiction psychiatry clinic, retrospectively examines factors associated with relapse and treatment retention in opioid dependent outpatients who had agreed to participate in maintenance treatment using buprenorphine. For the purposes of this study, relapse was defined specifically as any other opioid use during the treatment period. It was hypothesized that comorbid substance use, comorbid psychiatric disorders, lower buprenorphine dose, and previous drug use history would increase the likelihood of relapse in the sample.
Methods
Chart Review
A retrospective chart review of 62 patients with opioid dependence treated at the Vanderbilt University Addiction Psychiatry Clinic was conducted. The Vanderbilt Institutional Review Board approved the chart review. The study examined a cohort of patients who sought and had received any treatment with buprenorphine-naloxone (Suboxone, Reckitt-Benckiser) for opioid dependence in the clinic from July 2008 to June 2009. Records were reviewed from the beginning of a patient’s opioid treatment in the clinic, through Dec 31, 2009, to ensure an opportunity for at least 6 months of data collection, even if the patient dropped out of treatment. Patient visits ranged in frequency from weekly to monthly depending on clinical stability. The clinic treatment team included a clinic nurse, two addiction board certified psychiatrists, psychiatry residents, and a part-time social worker who provided group therapy. Clinic policy required weekly psychoeducational group therapy attendance during stabilization, which was subsequently diminished to monthly attendance. 12-step meeting attendance was strongly encouraged. Patients were all seen by at least one of the senior authors and a resident and a consensus diagnosis was derived over time for each patient after discussion with the treatment team.
Patients’ medical records were accessed via the Vanderbilt electronic medical record database (StarPanel, Nashville, Tennessee) to collect data regarding demographics, drug use history, opioid use prior to buprenorphine treatment, current buprenorphine use, and relapse indicators. In order to achieve some standardization of opioid use, an opioid equianalgesic table was devised based on published data and was used to convert self-reported average opioid use to daily morphine equivalent dosage (9). Due to the unreliability of self-report regarding heroin dose that was used, the few patients who had been actively abusing intravenous heroin prior to treatment were arbitrarily assigned a daily morphine equivalence of 1000mg.
The primary outcome (“relapse”) was defined as any opioid use despite the clinic contract that mandates total abstinence. This was determined by a combination of patient self-report, family report, evidence of opioid use in urine toxicology screens, and as recorded in the Tennessee Prescription Monitoring Program database. Urine drug screens were obtained at the hospital laboratory but chain-of-custody procedures were not the rule. The Tennessee Prescription Monitoring Program central database mandates all pharmacies in the state to report controlled substance prescriptions for all patients, and is available for providers to monitor for opioid and other controlled substance prescriptions obtained by patients from all physician sources. The database does not show controlled substances acquired without prescription. Secondary outcomes included time to relapse, treatment retention, and abuse of other illicit substances as discovered on urine toxicology screens, the controlled substance database, or from family or patient self-report.
Statistical Analysis
Descriptive statistics were presented as median with interquartile range (IQR) or percentage (N) as appropriate. Wilcoxon rank sum tests were used to compare the patients who relapsed to those who did not relapse for continuous variables. Categorical variables were compared using Pearson Chi-squared tests. The associations between relapse and insurance type, comorbid substance use, comorbid psychiatric disorders, and previous drug history were then assessed separately using multivariable logistic regression with adjustment for age and gender. All tests were two-tailed, with a significance level of 5%. All statistical analyses were performed using open source R statistical software (version 2.13.0, Vienna, Austria).
Results
As shown in Table 1, the sample size was 62 (32 male). The median age was 32 years. Half were married and 37 (60%) were employed. The majority had health insurance (34 private, 25 public). The treatment period ranged from one to 49 months in the clinic (median 12 months, mean 14 months). Twenty (32%) patients had comorbid anxiety disorders (16 with Post-traumatic Stress Disorder, two with Panic Disorder, and two with Anxiety Not Otherwise Specified) and 16 (26%) had comorbid depression (including both Dysthymia and Major Depressive Disorder, and excluding Bipolar Disorder and Substance Induced Mood Disorder). All patients reported daily or nearly daily opioid use prior to treatment. The median daily opioid use was 240mg (expressed in morphine equivalents). The median daily starting buprenorphine dose was 10mg and median daily maintenance dose was 20mg. The median time to relapse was 5 months. 31 patients (50%) were found to have relapsed at least once during the period of observation. Fourteen of these (23% of the sample population) dropped out of treatment completely and did not return.
Table 1.
Sample Demographic Information
| N | ||
|---|---|---|
| Age | 62 | 26 32 42 (35±11) |
| Gender | 62 | |
| Female | 48% | |
| Male | 52% | |
| Marital Status | 62 | |
| Single | 50% | |
| Married | 50% | |
| Insurance Type | 62 | |
| Private | 55% | |
| Public | 40% | |
| Uninsured | 5% | |
| Employment | 62 | |
| Unemployed | 40% | |
| Employed | 60% | |
| History of IV Drug Use (any substance) | 60 | |
| No | 45% | |
| Yes | 55% | |
| History of Residential Treatment | 62 | |
| No | 45% | |
| Yes | 55% | |
| Comorbid Anxiety Disorder | 62 | |
| No | 68% | |
| Yes | 32% | |
| Severity of Opioid Use (mg) | 62 | 101 240 908 (410±375) |
| Starting Buprenorphine Dose(mg) | 62 | 8.0 10.0 16.0 (11.3± 5.5) |
| Buprenorphine Maintenance Dose (mg) | 62 | 16 20 24 (19± 7) |
| Time to Relapse (months) | 30 | 3 5 16 (10±10) |
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables. x ± s represents X ± 1 SD.
N is the number of non-missing values.
Comparisons of relapsers and non-relapsers are shown in Table 2. Wilcoxon rank sum tests and Pearson Chi-squared tests revealed no association between relapse and severity of prior opioid use (in daily morphine equivalents) or a history of intravenous drug use. No association was found between relapse and comorbid depression. Among those patient with comorbid anxiety disorders, 75% relapsed compared to 38% among those without (p=0.007). Patients actively using benzodiazepines (despite clinic policy to discourage any use) relapsed more often (70% vs. 42%, p=0.043). Of the 19 patients with anxiety disorders, 10 were found to have used benzodiazepines and 9 were not, while 10 out of 39 patients without anxiety diagnoses were found to have used benzodiazepines. 82% of patients who reported alcohol use during buprenorphine treatment (despite clinic policy to discourage any use) relapsed compared to 43% of those who did not (p=0.022). The median buprenorphine maintenance dose for patients who relapsed was 24mg compared to 16mg for patients who did not relapse (p<0.001).
Table 2.
Comparison of patients who relapsed with patients who did not relapse
| Non-Relapse N = 31 | Relapse N = 31 | p-value | |
|---|---|---|---|
| Age | 25 29 40 (34±12) | 30 35 44 (37±10) | 0.1501 |
| Gender | 0.1302 | ||
| Female | 39% | 58% | |
| Male | 31% | 42% | |
| Insurance Type | 0.0832 | ||
| Public | 31% | 53% | |
| Private | 69% | 47% | |
| History of Residential Treatment | 0.3102 | ||
| No | 52% | 39% | |
| Yes | 48% | 61% | |
| History of IV Drug Use (any substance) | 0.1902 | ||
| No | 53% | 37% | |
| Yes | 47% | 63% | |
| Benzodiazepine Use during Treatment | 0.0432 | ||
| No | 79% | 53% | |
| Yes | 21% | 47% | |
| Alcohol Abuse during Treatment | 0.0222 | ||
| No | 93% | 69% | |
| Yes | 7% | 31% | |
| Smoking during Treatment | 0.0862 | ||
| No | 50% | 26% | |
| Yes | 50% | 74% | |
| Comorbid Anxiety Disorder | 0.0072 | ||
| No | 84% | 52% | |
| Yes | 16% | 48% | |
| Severity of Opioid Use (mg) | 100 170 1000 (403± 403) | 120 300 615 (416± 352) | 0.3901 |
| Buprenorphine Maintenance Dose (mg) | 16.0 16.0 20.0 (15.5± 7.3) | 20.0 24.0 24.0 (21.5± 5.3) | < 0.0011 |
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables. x ± s represents X ± 1 SD.
Tests used:
Wilcoxon test;
Pearson chi-squared test
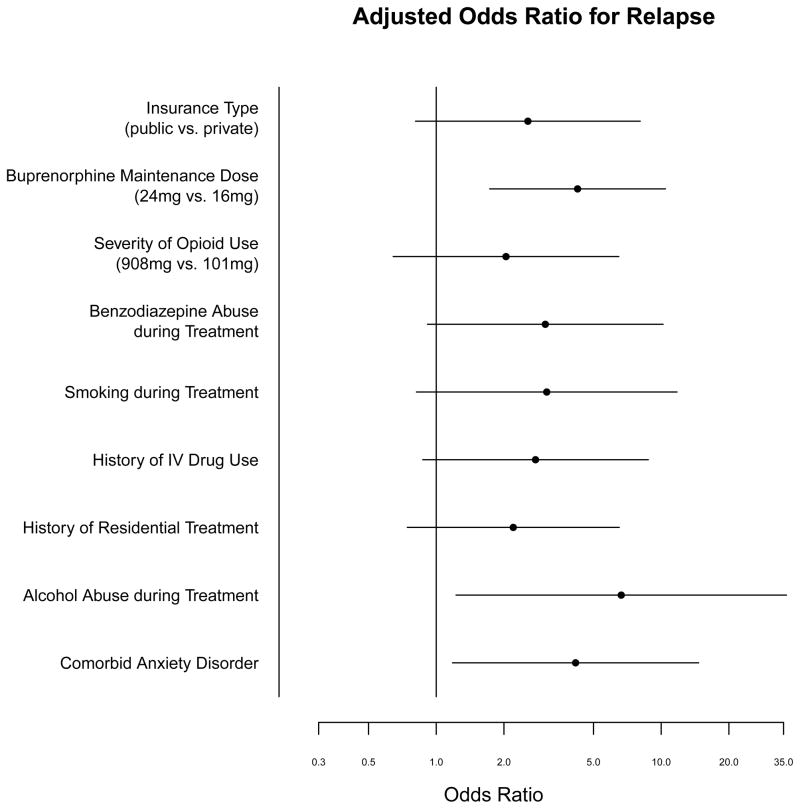
The adjusted odds ratios from the multivariable logistic regression are shown in Figure 1. Severity of previous opioid use was not significantly associated with relapse (OR=1.27, 95% CI=0.41–3.91). The following variables were significantly associated with relapse when adjusted for age and gender: higher buprenorphine maintenance doses (OR=4.08, 95% CI=1.64–10.13; p=0.002); comorbid anxiety disorders (OR=4.16, 95% CI=1.18–14.67; p=0.026); alcohol abuse during buprenorphine treatment (OR=6.64, 95% CI=1.22–36.05; p=0.030). The associations identified between relapse and insurance type (OR 2.56), a history of intravenous drug use (OR 2.76), comorbid benzodiazepine use (OR 3.05), and a previous history of residential substance abuse treatment (OR 2.20) were suggestive of a trend but were not statistically significant in the logistic regression analysis (Figure 1). Logistic regression was performed for buprenorphine maintenance dose after adjusting for previous opioid use severity which confirmed a significant association of buprenorphine dose with relapse (OR 3.97, 95% CI=1.58–9.97; p=0.003). A logistic regression of relapse for both benzodiazepine use and comorbid anxiety disorders revealed an odds ratio for benzodiazepine use of 2.60 (95% CI 0.75–9.01, p=0.131) and for comorbid anxiety of 2.93 (95% CI 0.79–10.86, p=0.107).
Figure 1.
Adjusted Odds Ratio for Relapse
Discussion
The major findings of this study were the associations between comorbid anxiety disorders, comorbid alcohol abuse, and higher buprenorphine maintenance dose with poor outcome as reflected by illicit opioid use during treatment. The study also suggested an association between comorbid benzodiazepine use and relapse to opioid use. Logistic regression of relapse including both benzodiazepine use and diagnosis of anxiety disorders indicated that when both variables were included in the model, an effect is still present (as seen from the odds ratios), but the limited sample size lead to non-significant p-values. The association of comorbid anxiety as a correlate of relapse during buprenorphine maintenance has been suggested in a recent randomized controlled trial in pregnant women (10). Although one study has suggested that comorbid depression is associated with higher retention in a clinical sample of heroin-dependent patients, our data did not replicate this finding (11).
The fact that no association was found between the severity of daily opioid use in morphine equivalents prior to buprenorphine treatment may be related to the equianalgesic conversion process itself. It may also be related to the limitations of the available data, namely we did not quantify lifetime opioid use nor formally determine other dependence severity criteria other than recent average use.
Perhaps the most interesting finding is the association between a higher buprenorphine maintenance dose and the likelihood of relapse (OR 4.25). This association contradicts a number of studies which found that higher dose was associated with greater treatment retention, as summarized in a recent metanalysis (8). However, the relationship reported in our study was also noted in a recent large study that found a higher rate of continued opioid use throughout treatment in patients treated with 24mg vs. lower doses of buprenorphine (12). The study authors discussed a number of possible explanations. First, physicians may choose buprenorphine dose based on perceived substance use severity. Second, that the higher dose group may not have received enough drug (i.e. needed more than 24mg). And third, that buprenorphine may not be the best agent for treatment of patients with severe opioid dependence who may require further mu-receptor stimulation beyond the ceiling effect of buprenorphine to alleviate cravings. This is contrary to what has been observed with methadone maintenance, namely that higher doses tend to be more effective in achieving retention and abstinence. (For example, in a randomized study comparing high-dose and low-dose methadone (5), the number of patients with 12 consecutive opioid-negative urine specimens was 28% in the high-dose group and 8% in the low-dose group.) Gerra et al. (2004) reported that higher buprenorphine dose was associated with lower rates of opioid-positive urine screens, but not associated with retention (11). We propose that our findings suggest significant differences in the pharmacology of partial and full agonists in treatment of opioid dependence (13).
Limitations
Although this study is a well-designed retrospective analysis in a “real world” university clinic practice setting, there are significant limitations which warrant discussion. While the study results necessarily reflect the local clinical practice and clinic environment, they nevertheless offer some generalizable findings that may be helpful. The sample size is small which limits the power to identify all differences that might be present. The study design is retrospective and requires interpreting data not collected as part of a formal clinical trial. The use of a chart review, however detailed, limits the ability to control quality of assessment and collection of more detailed cohort characteristics. For instance, better and more consistent history of recent and lifetime drug use severity may have allowed the analysis to identify a more robust relationship between previous drug use and future relapse. Also, the quality of the diagnostic assessment was not well controlled (e.g. it was based on real practice-associated psychiatric interviews by attending psychiatrists, psychiatry residents, and nurses, and not necessarily aimed at rigorous standardized diagnosis). The time of observation was not standardized for all patients, although it was of at least six months duration for all patients. The study was designed to capture “relapse” events as defined by any opioid use during the treatment. This definition required complete abstinence and differs from more recent attempts to define a “good clinical outcome” which allows for a few such relapses (e.g. in the COMBINE study for alcohol dependence) (14).
Conclusion
The results of this study suggest that patients with a higher buprenorphine maintenance dose, comorbid anxiety disorders, alcohol abuse, or benzodiazepine abuse, are more likely to relapse during treatment. A larger sample size and prospective longitudinal study design would be helpful to better characterize these and other relevant factors. Future research should address whether addressing these variables in the treatment process may improve outcomes.
Acknowledgments
Statistical support provided in part by the Vanderbilt CTSA grant UL1 RR024975 from NCRR/NIH. This paper was presented in part as a poster at the annual meeting of the American Academy of Addiction Psychiatry in December 2010.
References
- 1.Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- 2.CDC. CDC Grand Rounds: Prescription Drug Overdoses – a U.S. Epidemic. Morbidity and Mortality Weekly (MMWR) 2012;61(01):10–13. [PubMed] [Google Scholar]
- 3.Feillin D, O’Connor P. Office-Based Treatment of Opioid-Dependent Patients. N Engl J Med. 2002;347:817–823. doi: 10.1056/NEJMcp013579. [DOI] [PubMed] [Google Scholar]
- 4.Fudala PJ, Bridge TP, Herbert S, et al. Office-Based Treatment of Opiate Addiction with a Sublingual-Tablet Formulation of Buprenorphine and Naloxone. N Engl J Med. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 6.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive Counseling During Brief and Extended Buprenorphine-Naloxone Treatment for Prescription Opioid Dependence: A 2-Phase Randomized Controlled Trial. Arch Gen Psychiat. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramaniam GA, Warden D, Minhajuddin A, et al. Predictors of abstinence: National Institute of Drug Abuse multisite buprenorphine/naloxone treatment trial in opioid-dependent youth. J Am Acad Child Adolesc Psychiatry. 2011 Nov;50(11):1120–1128. doi: 10.1016/j.jaac.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fareed A, Vayalapalli S, Casarella J, Drexler K. Effect of buprenorphine dose on treatment outcome. J Addict Dis. 2012;31(1):8–18. doi: 10.1080/10550887.2011.642758. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed June 2010];Equianalgesic Chart. www2.massgeneral.org/painrelief/Equianalgesia.pdf.
- 10.Benningfield MM, Heil SH, Jones HE, et al. Co-occurring psychiatric symptoms, treatment efficacy, and retention in opioid agonist treatment of pregnant women. Addiction. 2012 (in press) [Google Scholar]
- 11.Gerra G, Borella F, Zaimovic A, et al. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug Alcohol Depend. 2004 Jul 15;75(1):37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Hillhouse M, Canamar CP, Doraimani G, Thomas C, Hasson A, Ling W. Participant characteristics and buprenorphine dose. Am J Drug Alcohol Abuse. 2011;37:453–459. doi: 10.3109/00952990.2011.596974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin PR, Patel S, Swift RM. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 3. Lippincott Williams & Wilkins; Philadelphia: 2012. Pharmacology of drugs of abuse; pp. 284–309. [Google Scholar]
- 14.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]