Abstract
Glutamate signaling plays an essential role in drug-seeking behavior. Using reinstatement of conditioned place preference (CPP), we determined whether ceftriaxone, a β-lactam antibiotic known to increase the expression and activity of the glutamate transporter (EAAT2) on glial cells, blocks methamphetamine-triggered reinstatement of CPP. Rats acquired methamphetamine CPP following 7 consecutive days of conditioning, during which each animal received pairings of alternating morning methamphetamine (2.5 mg/kg, IP) and afternoon saline (IP). Animals showing CPP were successfully extinguished with repeated twice daily saline administration over a 7-day period. Ceftriaxone (200 mg/kg, IP) was administered (vs. saline) once a day for 7 days during the extinction period. Upon successful extinction, animals received a single dose of methamphetamine (2.5 mg/kg, IP) for reinstatement and were tested for CPP one day later. Using real time PCR, EAAT2 mRNA levels in the nucleus accumbens (NAc) and medial prefrontal cortex (mPFC) were quantified in response to ceftriaxone. Ceftriaxone blocked methamphetamine-triggered reinstatement of CPP and significantly increased EAAT2 mRNA levels in the mPFC, with a trend towards significance in the NAc. In conclusion, Ceftriaxone modulated the expression of the glutamate transporter in a critical region of the cortico-striatal addiction circuitry and attenuated drug-seeking behavior in rats. Further research is needed to test the efficacy of compounds targeting the EAAT2 in human methamphetamine-dependent users.
Keywords: Glutamate, Ceftriaxone, Methamphetamine, Relapse, Conditioned place preference, Glutamate transporter, Excitatory amino acid transporter, Addiction
1. Introduction
Several lines of evidence demonstrate that the prefrontal cortex (PFC) and the nucleus accumbens (NAc) are essential for relapse-like behavior in reinstatement animal models (Goldstein and Volkow, 2002; Kalivas, 2004; Kalivas and Stewart, 1991; Robinson and Becker, 1986). The PFC is involved in decision making (Feltenstein and See, 2008) and in the prediction of rewarding behaviors by determining the salience of environmental stimuli and directing the intensity of the behavioral response (Feltenstein and See, 2008), while the NAc mediates reward-related activities and drug-seeking behavior (Kalivas and Volkow, 2005). Although dopamine-mediated signaling has been traditionally viewed as the primary mechanism initiating drug reinstatement (Self, 1998; Willuhn et al., 2010), recent research suggests a significant role for glutamate (Moussawi and Kalivas, 2010). Glutamate in the PFC stimulates dopamine release in the ventral tegmental area and the NAc (Krystal et al., 2003). A direct infusion of glutamate into the NAc induces reinstatement of extinguished cocaine-seeking behavior (Cornish et al., 1999). Methamphetamine causes an immediate release of glutamate in the PFC (Shoblock et al., 2003; Stephans and Yamamoto, 1995) and NAc (Dalia et al., 1998; Ito et al., 2006; Labarca et al., 1995; Shoblock et al., 2003; Xue et al., 1996) that is sustained in abstinence (Sailasuta et al., 2010; Stephans and Yamamoto, 1995). Extracellular levels of glutamate are regulated by a family of high affinity transporters known as the excitatory amino-acid transporters (EAATs) (Robinson, 1998). Five major subtypes of EAAT have been identified. EAAT1 is present in glial cells throughout the CNS and at high levels in Bergmann glia of the cerebellum. EAAT2 is almost exclusively glial, and is widespread and highly abundant throughout the CNS. The transporters EAAT3 and EAAT4 are present predominantly in neurons; EAAT3 is abundant throughout the CNS, whereas EAAT4 is predominantly localized to cerebellar Purkinje cells, with low levels of expression also present in the forebrain. EAAT5 is present in rod photoreceptor and bipolar cells of the retina (Reviewed by Beart and O’Shea, 2007)
Acute methamphetamine administration causes an elevation of glutamate due to initial reversal and subsequent inhibition (through the production of reactive oxygen species) of the glutamate transporter (Wolf et al., 2000). Furthermore, glutamate transporter activation (Nakagawa et al., 2005), or expression of the transporter through gene transfer into the NAc (Fujio et al., 2005) attenuates methamphetamine conditioned place preference.
Compounds that upregulate EAAT2 such as ceftriaxone, a ß-lactam antibiotic known to increase glutamate reuptake through the induction of glial EAAT2 (Rothstein et al., 2005), might be used to offset this glutamate elevation and, therefore, are candidate compounds for relapse prevention. A recent report (Sari et al., 2009) tested the effect of ceftriaxone on blocking the reinstatement of cocaine self-administration (0.125 mg per IV dose) in rats using a lever-pressing task in a daily 2-hour session for 10–14 days, followed by 5 days of extinction training. Immediately after each extinction session, rats received ceftriaxone (200 mg/kg IP). On the following day, presentation of the cue (light and tone) previously associated with cocaine self-administration reinstated lever pressing in rats treated with vehicle, whereas ceftriaxone blocked this response. Another study from the Kalivas group (Knackstedt et al., 2010), used a similar self-administration paradigm; ceftriaxone (200 mg IP for 5–10 days during extinction training) successfully blocked cue- and cocaine-primed reinstatement of drug-seeking behavior. Recent reports also showed ceftriaxone’s efficacy in reducing ethanol consumption in alcohol-preferring rats (Sari et al., 2011), attenuating amphetamine-induced hyperactivity and behavioral sensitization in rats (Rasmussen et al., 2011), and decreasing the acquisition of cocaine, but not glucose, reinforced behavior (Ward et al., 2011).
Conditioned place preference (CPP) is a valid and reliable method used to assess the rewarding properties of various drugs of abuse, including methamphetamine (for a review see Feltenstein and See, 2008). This study aimed to test the efficacy of ceftriaxone in blocking methamphetamine-triggered reinstatement of CPP in a rat model of the acute rewarding effects of methamphetamine addiction and to investigate the effect of ceftriaxone on the expression of the glutamate transporter (EAAT2) in the medial prefrontal cortex (mPFC) and NAc.
2. Results
2.1. Ceftriaxone reduces methamphetamine-induced reinstatement of CPP
After receiving methamphetamine 2.5 mg/kg IP for 7 consecutive days, a CPP to methamphetamine was established. Animals (n=16) spent significantly more time in the methamphetamine-paired chamber (mean±SEM: 13.68±0.46 min) compared to saline-paired chambers (4.17±0.5 min) during 20 min of testing for CPP (t=13.99, df=30, p<0.0001). CPP was significantly decreased following 7 consecutive days of twice daily saline (or saline+ceftriaxone) injection. The mean total time spent in the methamphetamine-paired chamber following partial extinction (8.71±0.42 min.) was significantly less than the time spent during CPP (13.68±0.46 min.: t=6.95, df=30, p<0.0001). No significant difference in partial extinction time (min) was noted between ceftriaxone and saline treatment [mean±SEM in methamphetamine-paired chamber following partial extinction for ceftriaxone vs. saline: 8.05±1.4 min. vs. 9.37±1.1 min., p=ns].
MANOVA indicated a significant overall effect of drug group (ceftriaxone vs. saline) on total time spent in the various chambers (Wilk’s Lambda F (6,8)=6.09; p<0.01) during the reinstatement component of the experiment. Subsequent univariate ANOVA indicated that the ceftriaxone group effect was present only in reinstatement, specifically in the methamphetamine chamber (F (1,13)=30.9; p<.001) and the saline chamber (F (1,13)= 23.6; p<.001). Repeated measures (testing days 1–3) [testing for (1) CPP, (2) partial extinction, (3) reinstatement]. ANOVA indicated no significant main effects of drug group (ceftriaxone vs. saline), days, or chamber (methamphetamine vs. saline). However, there was a significant interaction of drug group×chamber preference (F (1,14)=33; p<.001) and of days×drug group×chamber preference (F (3,42)=3.7; p<.02). Compared to controls (n=8), ceftriaxone-treated animals (n=8) spent significantly less time in the methamphetamine-paired chamber during testing for methamphetamine-triggered reinstatement of CPP [controls vs. ceftriaxone-treated animals (mean±SEM): 12.83±0.74 min. vs. 4.14±0.5 min, two-tailed independent t-test=27.5, df=14, p<0.001] (Figs. 1C and 2).
Fig. 1.
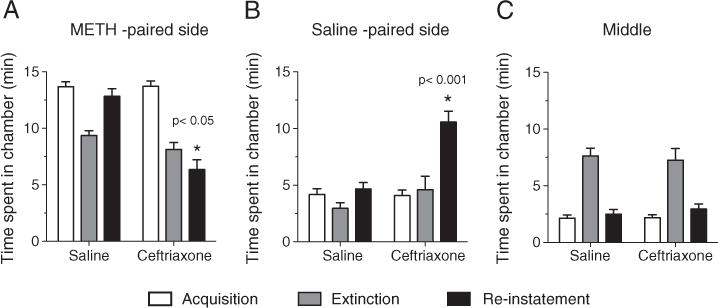
Acquisition (A), extinction (B) and reinstatement (C) of CPP in methamphetamine dependent rats assigned to ceftriaxone vs. saline groups (Ceftriaxone group recived ceftriaxone 200 mg/kg IP only during the extinction phase of the study). Time spent in methamphatmine-paired chamber during reinstatement (n=8) (mean±SEM): 12.83±0.74 vs. 4.14±0.5 two-tailed independent t-test=27.5, df=14, p<0.001.
Fig. 2.
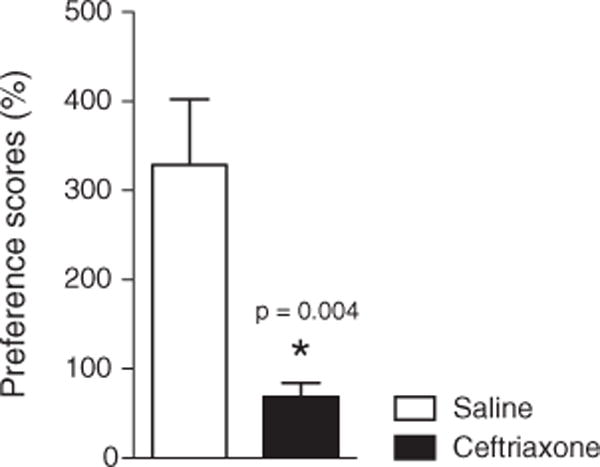
Ceftriaxone reduces the preference score (i.e. time spent in methamphetamine-paired/saline-paired chambers) during reinstatement: t(14)=3.450, p=0.0039.
Preference score was calculated as the time spent in methamphetamine-paired side/ time spent in saline paired side in the last day of testing (for reinstatement). Ceftriaxone group had significantly less preference to the methamphetamine-paired side compared to the control group: t(14)=3.450, p=0.0039.
2.2. Ceftriaxone when administered alone does not significantly affect conditioned place preference
Ceftriaxone given daily for 7 days did not significantly alter place preference for the compartment in which the ceftriaxone had been administered: time (min) spent in ceftriaxone-paired vs. saline-paired chamber: mean± SEM 10.06±1.75 min vs. 6.49±1.78 min, two-tailed independent t-test, p>0.17. No significant difference was observed in overall motor activity as measured by the number of crossings between the two compartments in the CPP cage (total crossings/20 min: mean±SEM; ceftriaxone 95.5±8.3; saline 97.9±8.4).
2.3. Ceftriaxone has no effect on basal locomotor activity
To address the possible confound that the decreased time spent in the methamphetamine-paired chamber during reinstatement was related to a possible negative impact of ceftriaxone on locomotor activity, a separate study was done in which 8 animals were administered daily methamphetamine 2.5 mg/kg+ceftriaxone 200 mg/kg IP, while a second group (n=8) received methamphetamine 2.5 mg/kg+saline 0.1 ml IP in the home-cage for 7 consecutive days. Measurement of both horizontal and vertical locomotor activity of individual animals was performed over a two hour period using an infrared beam break system. Repeated measures ANOVA indicated a decline in locomotor activity over consecutive 30 min intervalsinthe XY plane (F (3,18) = 24.8; p<.001), and in the Z dimension (F (3,18)=26.4; p<.001). However, no significant main effect of drug group or drug group interaction with the replicate was observed (F=(0,98)=26.36, p<0.39) (Fig. 3). Thus, it appears that the observed blocking effect of ceftriaxone on methamphetamine-triggered reinstatement of CPP was not related to an overall change in locomotor activity. As noted above, ceftriaxone administered by itself also resulted in no change in locomotor activity compared with a saline control.
Fig. 3.
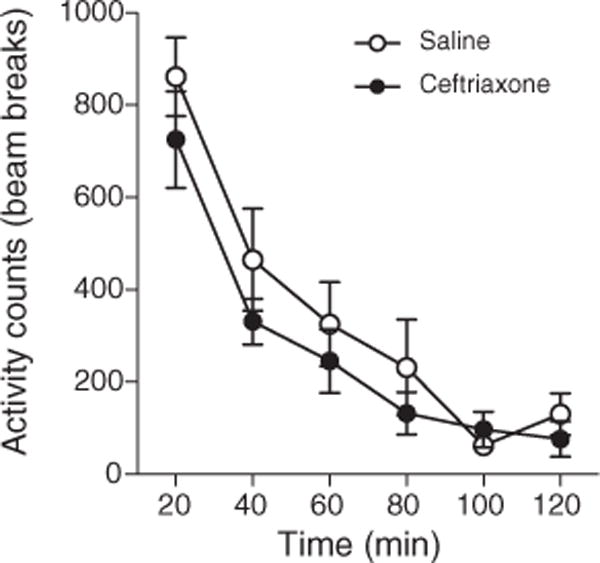
No effect of ceftriaxone on locomotor activity (n=8) (F=(0,98)=26.36, p<0.39).
2.4. Ceftriaxone increases EAAT2 mRNA expression differentially in the NAc and mPFC
We examined the expression levels of the high affinity glutamate transporter EAAT2 in two specific brain regions of the cortico-striatal circuitry, the mPFC and the NAc, during addiction training [4h following the last ceftriaxone injection (Fig. 4)] and during extinction treatment [72h following the last ceftriaxone injection (Fig. 5)]. Given the paucity in research investigating the temporal effect of ceftriaxone on EAAT2, we chose to test the effect of ceftriaxone immediately following behavioral testing for reinstatement which was 72 hour following the last injection and chose the 4 hour time point to test for subacute effect of ceftriaxone on EAAT2 mRNA. In the first experiment (4-hour assay following last ceftriaxone administration during addiction training), a significant Wilk’s lambda in the MANOVA [F (2,13)=7.306, p<0.007] showed the two dependent variables together discriminated the groups. Subsequent ANOVA showed that the difference was driven mainly by the mPFC [F (1,14)=7.101, p<0.018], with no significant difference in the NAc [F (1,14)=3.340, p<0.089], while in the second experiment (72-hour assay following last ceftriaxone administration during extinction) Wilk’s lambda for MANOVA [F (2,11)=5.955, p<0.018] showed the two dependent variables together discriminated the groups (Figs. 4 and 5).
Fig. 4.
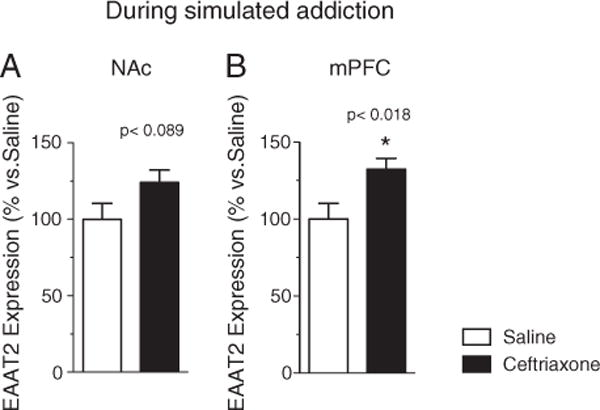
EAAT2 mRNA concentration in (A) NAc (n=16) [F (1,14)=3.340, p<0.089] and (B) mPFC (n=16) [F (1,14)=7.101, p<0.018], in ceftriaxone+methamphetamine-treated (black columns) and saline+methamphetamine (clear columns) control groups at 4 h following the last ceftriaxone administered during simulated addiction (p<0.018).
Fig. 5.
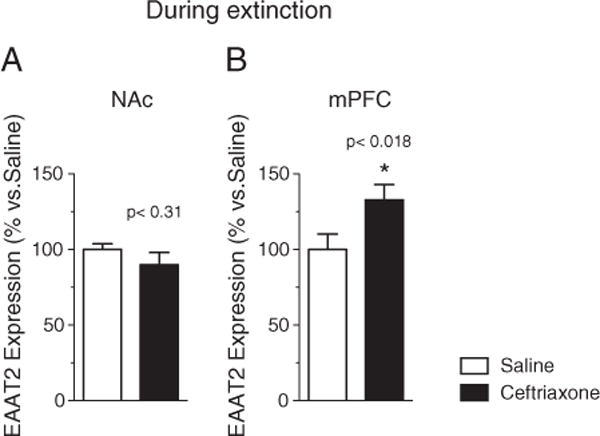
EAAT2 mRNA concentration in (A) NAc [F (1,14)=2.14, p<0.31] (n=16) and (B) mPFC(n=16) [F (2,11)=5.955, p<0.018] in ceftriaxone+methamphetamine-treated (black columns) and saline+methamphetamine (clear columns) control groups at 72 h following the last ceftriaxone administered during extinction.
3. Discussion
In the present study, we found that the β-lactam antibiotic ceftriaxone prevented reinstatement of methamphetamine-triggered CPP and increased the expression of EAAT2 in the mPFC and to a lesser extent in the NAc when given during extinction. The mPFC acts as an inhibitory control region for drug-seeking behavior (Kalivas, 2009), while the NAc receives projections from emotion regulating cortical regions, including the mPFC (Rigoard et al., 2011), ventral pallidum (Jones and Mogenson, 1980), thalamus (Kelley and Stinus, 1984), basolateral amygdala (Groenewegen et al., 1996), and hypothalamus (Yu and Han, 1989), and acts as a gateway to enhancing or inhibiting input from these emotion centers to the motor control regions (Ito et al., 2006). Consistent with prior work (Daza-Losada et al., 2007; DeMarco et al., 2009; Hiroi and White, 1991), methamphetamine given daily for 7 consecutive days in our study produced CPP in which animals spent significantly more time in the methamphetamine-paired chamber. Subsequently, repeated daily saline administration for 7 days successfully partially extinguished this methamphetamine-induced CPP. Consistent with prior work (Anggadiredja et al., 2004; Davidson et al., 2007; Yan et al., 2006), a single dose of methamphetamine reinstated a preference for the drug-paired chamber. However, the reinstatement of CPP was significantly less in the animals that received ceftriaxone compared to those that received saline.
In our working model, we hypothesized extracellular glutamate to be elevated during the reinstatement, and we used ceftriaxone to offset this increase in extracellular glutamate by enhancing glutamate uptake to the astrocytes through the induction of the expression of EAAT2, as measured at the mRNA level. Several lines of evidence support this working model. Methamphetamine increases glutamate concentration in the prefronto-accumbal pathway by a mechanisms involving downregulation of the glutamate transporter (Wolf et al., 2000). Elevated glutamate neurotransmission is considered a key factor in methamphetamine reinstatement (Crombag et al., 2008; Voorn et al., 2004; Wilson and Kawaguchi, 1996). Exposure to drug or drug contexts activates glutamate neurons that project to the NAc, and consequently lead to reinstatement of drug seeking behavior (Meredith, 1999). Interventions that offset that excess glutamate release, such as direct inactivation of the PFC (McFarland et al., 2003) or the selective mGluR5 antagonist, have been shown to reduce intravenous methamphetamine self-administration (Gass et al., 2009). More importantly, increasing expression of the glutamate transporter by gene transfer to the NAc (Fujio et al., 2005) or by co-administration of MS-153 (a glutamate transporter activator) with methamphetamine, attenuates the methamphetamine-induced CPP (Nakagawa et al., 2005). Glutamate-regulating ceftriaxone may play an important role in neuroprotection by protecting glutamate receptors from tonic stimulation by excessive glutamate (Anderson et al., 2003). One question from these results is whether ceftriaxone facilitates extinction of the CPP (an effect which could conceivably carry over to the reinstatement trial) or if ceftriaxone directly impairs reinstatement. Since there was no difference in extinction between ceftriaxone and saline treatment [mean±SEM time (min)] in methamphetamine-paired chamber during partial extinction for ceftriaxone vs. saline: [8.05±1.4 vs. 9.37±1.1, p =ns], it seems unlikely that ceftriaxone facilitated extinction. Rather, it is likely that ceftriaxone impaired subsequent association of contextual methamphetamine-related cues in the reinstatement trial, thus attenuating the CPP. Since ceftriaxone had similar effects on mPFC EEAT2 in animals in behavioral partial extinction, as well as in simulated addiction, one might speculate that ceftriaxone could be an effective agent for either preventing the early acquisition of drug dependence or its reacquisition following the failure of abstinence (“falling off the wagon”, often as a result of long-lasting drug craving).
Another approach is to consider these data from the viewpoint of reinforcement theory. Methamphetamine is a primary reinforcer, probably because it stimulates release and blocks reuptake of dopamine. Contextual cues, such as the visual, olfactory and tactile features of the chambers in which methamphetamine is administered in this study, become secondary reinforcers due to their association with methamphetamine, the primary reinforcer. Thus, a CPP develops as the animal associates the contextual cues of the chamber with methamphetamine during initial acquisition. Extinction weakens the strength of the secondary reinforcers and the CPP diminishes. The subsequent administration of methamphetamine is a reacquisition trial and the CPP again appears. The blocking effect of ceftriaxone could not be explained by a conditioned place aversion; on the contrary, a nonsignificant trend towards eliciting CPP was observed. In addition, we and others have found that the blocking effect of ceftriaxone could not be explained by a non-specific effect on locomotor activity (Knackstedt et al., 2010; Rawls et al., 2010; Sari et al., 2009).
We chose to test the effect of ceftriaxone on EAAT2 at two time points: four hours after dosing, and at 72 h following the last injection (i.e. 7 days extinction training (plus/minus ceftriaxone), 1 day of testing for extinction, 1 day of methamphetamine-triggered reinstatement and 1 day of testing for re-instatement). This allowed us to explore both subacute and persistent (subchronic) effects of ceftriaxone.
The EAAT2 mRNA assay done at 72 h after the last dose of ceftriaxone was performed following simulated addiction. These two experiments were different, not only in the time points (4 h. vs. 72 h), but also in the experimental manipulations leading up to the last dose of ceftriaxone. In the 72 h. experiment, ceftriaxone upregulated the EAAT2, potentially offsetting the glutamatergic surge elicited during methamphetamine triggered reinstatement. This was associated with an attenuation of CPP and reduced relapse following extinction. On the other hand, ceftriaxone in the 4 h. assay attenuated the inhibition of the EAAT2, suggesting that ceftriaxone given during active methamphetamine may prevent addiction; however we did not assess this possibility through behavioral testing. Although we did not perform a systematic analysis of synaptic glutamate concentration or EAAT2 activity levels, there is a considerable amount of data indicating that methamphetamine administration is associated with elevated synaptic glutamate concentration and down regulation of EAAT2 concentration (e.g. Wolf et al., 2000). Further research is needed to establish the link between measuring the mRNA for the EEAT2 and synaptic levels of glutamate.
In conclusion, our study highlights the efficacy of ceftriaxone in blocking relapse in a CPP animal model of methamphetamine addiction and in inducing the expression of the glutamate transporter in key brain regions critical for the functionality of the cortico-striatal circuitry. The EAAT2 data suggest the potential efficacy of ceftriaxone in preventing methamphetamine addiction and relapse prevention. Further research focused on the development of glutamatergic neurotransmission modulators may provide novel, evidence-based therapeutics for humans suffering from methamphetamine addiction.
4. Experimental procedures
4.1. Animals
Adult, male Sprague Dawley rats (Harlan, CA, 10 weeks old, 300 g, n=52) were housed 3 per cage with free access to food and water and maintained under a 12:12 light:dark cycle (lights on at 6.00 AM), with temperature and humidity kept constant. Animals were allowed 7 days of habituation to the new environment, during which each animal was handled every day for 2–3 min to reduce anxiety during the subsequent experiment. All study procedures and protocol were approved by the Institutional Animal Care and Use Committee of the University of Southern California.
4.2. Conditioned place preference (CPP)
The CPP cage consisted of two pairing chambers (24″×24″×24″) and a smaller middle chamber (3″×24″×24″). The two pairing chambers differed in visual and tactile cues, one with horizontal yellow and black stripes and a rough floor, and the second one with vertical stripes anda smooth hole-punched floor. The middle chamber was totally black with a featureless floor. Two guillotine doors separated the middle chamber from the 2 pairing chambers. Methamphetamine hydrochloride (Sigma Aldrich, St. Louis MO) was dissolved in saline (1 mg in 1 ml) and prepared fresh every day for IP injection at 2.5 mg/kg. Ceftriaxone sodium (Roche, Basel, Switzerland)1 g was dissolved in 10 ml saline and prepared fresh every day for IP injection at 200 mg/kg. Cage priming, to create equivalent odor cues, was done using a separate group of male animals (n=6) placed in the CPP cages for a half hour every morning throughout the entire experiment. Animals (n=16) were equally and randomly assigned to receive the methamphetamine in either chamber (rough or smooth floor), with this assignment remaining fixed for the duration of the experiment. Conditioning was performed over 7 consecutive days of 2 daily sessions: morning methamphetamine (2.5 mg/kg, IP at 6:00 a.m.) injection in one chamber, alternating with daily afternoon saline injection (0.1 ml, IP at 2:00 p.m.) in the other chamber. Each animal remained confined to a chamber (with guillotine doors closed) for 20 min following injection. Animals were allowed access to water only following the morning sessions and were allowed free access to food and water after the afternoon sessions. In the morning of day 8 of the conditioning, each animal was placed in the middle chamber for 3 min, with no injection given that day. Both guillotine doors were removed and the animal was allowed free access to both chambers for 20 min. The amount of time spent in each chamber was recorded with a digital video camera mounted above the CPP cage. Coding for the total time spent in each chamber and number of crossings was done manually by a blinded rater defining the location of the head as the location of the rat.
4.3. Effect of ceftriaxone on extinction of methamphetamine-induced CPP
Animals showing CPP (≥60% total time spent in the methamphetamine-paired chamber) were included in the study (Fujio et al., 2005; Hiroi and White, 1991). These were randomly assigned to receive ceftriaxone+saline or saline alone in the chamber previously paired with the methamphetamine administration. Extinction was performed over 7 consecutive days in a similar pattern of 2 daily sessions. Animals were randomized to receive ceftriaxone+saline or saline alone during the morning sessions and saline only during afternoon sessions. On the day following extinction, each rat was placed in the middle chamber (no injections given) for 3 min before the guillotine doors were lifted and the animal was allowed free access to both chambers. Total time spent in each chamber was recorded and coded as noted above. Extinction was defined as ≥25% reduction in total time spent in the methamphetamine-paired chamber compared to time recorded during training (Fujio et al., 2005; Hiroi and White, 1991).
4.4. Reinstatement of methamphetamine-induced CPP
The day following extinction testing, each animal received a single dose of methamphetamine (2.5 mg/kg, IP) and was confined to the methamphetamine-paired chamber for 20 min with guillotine doors closed. One day later, all animals were tested again for CPP in the same way as before.
4.5. Effects on conditioned place preference of ceftriaxone administered alone
To test if ceftriaxone causes aversion, another group of animals (n=16) was equally and randomly assigned to receive ceftriaxone (200 mg/kg IP) in either chamber (rough or smooth floor), with this assignment remaining fixed for the duration of the experiment. Procedures were identical to those followed in the methamphetamine trial. Conditioning was performed in rats (n=8) over 7 consecutive days of 2 daily sessions: morning ceftriaxone injection (200 mg/kg in 0.1 ml saline IP) in one chamber, alternating with daily afternoon saline injection (0.1 ml, IP) in the other chamber. Each animal remained confined to the specified chamber (with guillotine doors closed) for 20 min following injection. The chamber in which animals received ceftriaxone was randomized between animals. On the morning of day 8 of the experiment, CPP was evaluated as described above. Comparison was made to a separate group of rats (n=8) that had received saline for both the morning and afternoon injections.
4.6. Effect of ceftriaxone on locomotor activity
Another set of animals (n=8) was given daily IP methamphetamine (2.5 mg/kg)+ceftriaxone (200 mg/kg) versus methamphetamine (2.5 mg/kg)+saline in the home-cage for 7 consecutive days. Testing was performed in the morning following the last ceftriaxone injection. Testing for both horizontal and vertical locomotor activity of individual animals was performed over a two hour period using an infrared beam break system (Opto-M3, Columbus Instruments, Columbus, OH) mounted around a standard vivarium cage supplied with fresh bedding.
4.7. Measurement of EAAT2 mRNA levels
4.7.1. RNA isolation
Brain tissues from two groups of animals were used for this experiment. First, the behavioral group from the CPP experiment described before (n=16) received the ceftriaxone during extinction. Animals went through the following sequence of exposures: methamphetamine×7 days→ceftriaxone vs. saline×7 days→testing for extinction→single reinstatement dose of methamphetamine→testing for reinstatement→euthanasia 72 h following the last dose of ceftriaxone vs. saline. The second group (n=16) was administered ceftriaxone in simulated addiction protocol. Animals received methamphetamine (2.5 mg/kg IP)+ceftriaxone (200 mg/kg IP), (n=8) or methamphetamine (2.5 mg/kg IP) +saline (n=8)×7 days→euthanasia 4 h following the last dose of methamphetamine+ceftriaxone vs. methamphetamine+saline. Animals were euthanized by rapid sedation (3.5% isoflurane in 30% oxygen/70% nitrous oxide×2 min) followed by decapitation by guillotine. Brains were rapidly removed and dissected on ice to isolate the mPFC (~Bregma 4.2–5.6 mm including cingulate, infralimbic, prelimbic cortex) and the NAc (~Bregma 3.0–1.3 mm) (Paxinos and Watson, 2007). Dissection of NAc and PFC was done within 3 min from euthanasia. Samples were flash frozen on dry ice and kept at−70° C until RNA isolation was performed. Total RNA from tissues was isolated using an RNAeasy-Mini kit (Qiagen, Valencia, CA).
4.7.2. Real-time RT-PCR
To measure mRNA levels, real-time quantitative RT-PCR was performed with the iCycler IQ Real-Time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) using a QuantiTect SYBR Green RT-PCR Kit (Qiagen). Gene-specific primers for EAAT2 and GAPDH were purchased from Qiagen (Qiagen) and the following real-time RT-PCR protocol was used for all genes: reverse transcription step for 30 min at 50 °C, denaturation at 95 °C for 15 min to activate the HotStart enzyme followed by an additional 45 cycles of amplification and quantification (15 s at 94 °C; 10 s at 55 °C; 30 s at 72 °C) each with a single fluorescence measurement. EAAT2 mRNA level was normalized to GAPDH, a housekeeping gene. The percentage changes were calculated by subtracting mean GAPDH Ct values from Ct values for the gene of interest using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
4.8. Statistical analysis
Data analysis was performed using SYSTAT software (version 13). All data were expressed as means±SEM. Multivariate analysis of variance (MANOVA) was employed to determine if there was a difference between the ceftriaxone and saline-treated groups in time spent in the various chambers, utilizing the dependent variables % time in methamphetamine chamber during acquisition (MA), extinction (ME), and reinstatement (MR), and the corresponding times for the saline chamber (SA, SE, SR and the middle chamber (XA, XE, XR). Repeated measures ANOVA determined whether group differences varied over testing days 1–3) [testing for (1) CPP, (2) partial extinction, (3) reinstatement]. F values, degrees of freedom and levels of significance are reported in the results section.
Locomotor activity data were analyzed via repeated measures ANOVA on each dependent variable (movement in XY or Z dimensions) with drug group as the between variable, and four consecutive 30 min measures of locomotor activity as the replicate (T1–T4).
MANOVA was performed for the EAAT2 analysis, utilizing the between groups variable (ceftriaxone vs. saline for the 72 h assay; methamphetamine+ceftriaxone vs. methamphetamine+saline for the 4 h assay), and the dependent variables were NAc and mPFC levels of EAAT2 mRNA. Separate analyses were performed on the 4 h and the 72 h assays since the behavioral histories were different. Wilk’s lambda (the proportion of variance in the combination of dependent variables that is unaccounted for by the independent variable) was used in the MANOVA, similar to the role an F-test performs in a one-way ANOVA, to test for differences between the means of the identified groups. ANOVA was then performed on each brain region (dependent variables=NAc and mPFC), utilizing the same between groups variable. p values less than 0.05 were considered significant for all tests.
Acknowledgments
This work was supported by internal funding from the Department of Psychiatry at the University of Southern California and by grants from the NIH/NCRR CTSA KL2 to Dr. Abulseoud (RR024151), National Institutes of Health to D.S.C. (AA018779, AA017830-Project 1), and R21 DA026970 to D.P.H. We would like to thank David Xiong, Marco Ocampo, Yumei Guo, Jesse Costales, Kalisa G Myers, and Moonnoh R. Lee for their assistance with the experiments.
Footnotes
Financial disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Sakimura K, Hiranita T, Yamamoto T. Naltrexone attenuates cue- but not drug-induced methamphetamine seeking: a possible mechanism for the dissociation of primary and secondary reward. Brain Res. 2004;1021:272–276. doi: 10.1016/j.brainres.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia A, Uretsky NJ, Wallace LJ. Dopaminergic agonists administered into the nucleus accumbens: effects on extracellular glutamate and on locomotor activity. Brain Res. 1998;788:111–117. doi: 10.1016/s0006-8993(97)01518-7. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gopalan R, Ahn C, Chen Q, Mannelli P, Patkar A, Weese D, Lee H, Ellinwood H. Reduction in methamphetamine induced sensitization and reinstatement after combined pergolide plus ondansetron treatment during withdrawal. Eur J Pharmacol. 2007;565:113–118. doi: 10.1016/j.ejphar.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Daza-Losada M, Ribeiro Do Couto B, Manzanedo C, Aguilar MA, Rodriguez-Arias M, Miñarro J. Rewarding effects and reinstatement of MDMA-induced CPP in adolescent mice. Neuropsychopharmacology. 2007;32:1750–1759. doi: 10.1038/sj.npp.1301309. [DOI] [PubMed] [Google Scholar]
- DeMarco A, Dalal RM, Pai J, Aquilina SD, Mullapudi U, Hammel C, Kothari SK, Kahanda M, Liebling CN, Patel V, Schiffer WK, Brodie JD, Dewey SL. Racemic gamma vinyl-GABA (R, S-GVG) blocks methamphetamine-triggered reinstatement of conditioned place preference. Synapse. 2009;63:87–94. doi: 10.1002/syn.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, Satoh M, Kaneko S. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminal areas. Brain Res. 1991;552:141–152. doi: 10.1016/0006-8993(91)90672-i. [DOI] [PubMed] [Google Scholar]
- Ito K, Abekawa T, Koyama T. Relationship between development of cross-sensitization to MK-801 and delayed increases in glutamate levels in the nucleus accumbens induced by a high dose of methamphetamine. Psychopharmacology. 2006;187:293–302. doi: 10.1007/s00213-006-0423-2. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection: electrophysiological and iontophoretic investigations. Brain Res. 1980;188:93–105. doi: 10.1016/0006-8993(80)90559-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Recent understanding in the mechanisms of addiction. Curr Psychiatry Rep. 2004;6:347–351. doi: 10.1007/s11920-004-0021-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L. The distribution of the projection from the parataenial nucleus of the thalamus to the nucleus accumbens in the rat: an autoradiographic study. Exp Brain Res. 1984;54:499–512. doi: 10.1007/BF00235475. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Labarca R, Gajardo MI, Seguel M, Silva H, Jerez S, Ruiz A, Bustos G. Effects of D-amphetamine administration on the release of endogenous excitatory amino acids in the rat nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:467–473. doi: 10.1016/0278-5846(94)00027-f. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Elsevier; Amsterdam: 2007. [DOI] [PubMed] [Google Scholar]
- Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug and Alcohol Depend. 2011 Nov 1;118(2–3):484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Yuvasheva E, Raffa RB. First evidence that drugs of abuse produce behavioral sensitization and cross sensitization in planarians. Behav Pharmacol. 2010;21:301–313. doi: 10.1097/FBP.0b013e32833b0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoard P, Buffenoir K, Jaafari N, Giot JP, Houeto JL, Mertens P, Velut S, Bataille B. The accumbofrontal fasciculus in the human brain: a microsurgical anatomical study. Neurosurgery. 2011;68:1102–1111. doi: 10.1227/NEU.0b013e3182098e48. [DOI] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Harris KC, Ross BD. Glial dysfunction in abstinent methamphetamine abusers. J Cereb Blood Flow Metab. 2010;30:950–960. doi: 10.1038/jcbfm.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW. Neural substrates of drug craving and relapse in drug addiction. Ann Med. 1998;30:379–389. doi: 10.3109/07853899809029938. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between D-methamphetamine and D-amphetamine in rats. Psychopharmacology. 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BY. Effect of repeated methamphetamine administrations on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res. 1995;700:99–106. doi: 10.1016/0006-8993(95)00938-m. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, Rawls SM. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. (1996): The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Xue CJ, Li Y, Wavak D. Amphetamine increases glutamate efflux in the rat ventral tegmental area by a mechanism involving glutamate transporters and reactive oxygen species. J Neurochem. 2000;75:1634–1644. doi: 10.1046/j.1471-4159.2000.0751634.x. [DOI] [PubMed] [Google Scholar]
- Xue CJ, Ng JP, Li Y, Wolf ME. Acute and repeated systemic amphetamine administration: effects on extracellular glutamate, aspartate, and serine levels in rat ventral tegmental area and nucleus accumbens. J Neurochem. 1996;67:352–363. doi: 10.1046/j.1471-4159.1996.67010352.x. [DOI] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizoguchi H, Yamada K, Nabeshima T. Relapse of methamphetamine-seeking behavior in C57BL/6J mice demonstrated by a reinstatement procedure involving intravenous self-administration. Behav Brain Res. 2006;168:137–143. doi: 10.1016/j.bbr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Yu LC, Han JS. Involvement of arcuate nucleus of hypothalamus in the descending pathway from nucleus accumbens to periaqueductal grey subserving an antinociceptive effect. Int J Neurosci. 1989;48:71–78. doi: 10.3109/00207458909002152. [DOI] [PubMed] [Google Scholar]


