Introduction
At the beginning of the 21st century, we are experiencing a paradigm change in biology. The dominant view during the last fifty years has considered development to be a function of the unfolding of a genetic program where the environment plays virtually no relevant role. This view has prevailed despite studies from the late 19th century illustrating the phenomenon of environmentally triggered polyphenisms. The arbitrary choice of model organisms which thrive in laboratory facilities where the environment is practically invariable, and the dominance of a genocentric view led to developmental genetics, “a discipline that explicitly treated phenotype as a direct ‘readout’ of the nuclear genome” (Gilbert, 2005). This is a misconception that needs to be corrected.
Thanks to the incorporation of new data, prominent among them epidemiological studies highlighting the developmental plasticity of the human fetus, this rigid view of development is now rapidly being replaced (Barker and Hanson, 2004). Several pathways have been identified that could incorporate environmental cues into the building of a phenotype during early development, namely, i) the neuroendocrine route, whereby the nervous system monitors the environment and transfers signals to the endocrine system, ii) the epigenetic route, whereby environmental agents change the methylation pattern of genes, thereby altering their transcriptional capabilities, and iii) the direct modulation of gene expression, particularly by hormonally-active agents (Gilbert, 2005).
This new emphasis on the role of the environment in development has resulted in the creation of a new discipline, ecological developmental biology (eco-devo) and has prompted scientists to hypothesize that fetal exposure to environmental agents, particularly those with hormonal activity, may cause health effects that manifest during adult life. One of these chemicals, the pesticide DDT, was found to be estrogenic in 1950 (Burlington and Lindeman, 1950). The specter of cancer and other adverse health effects in humans, rather than the protection of wildlife or the environment, motivated the US government to ban the agricultural use of DDT in 1973. The normalization of eggshell development in the bald eagle population after DDT was banned allowed evidence of other widespread effects of DDT exposure to be observed. Hatchling birds showed a diversity of developmental malformations (National Research Council, 1999). These developmental malformations were well documented in the Great Lakes area and they were also observed in other ecosystems (Johnson et al., 1993).
In 1991, the Wingspread Conference held in Wisconsin addressed these new data. The participants proposed that the developmental alterations observed in several wildlife species was due to exposure to multiple chemicals that, through different modes of action, disrupted the endocrine system of developing organisms. They noticed that some of the effects observed in the genital tract of wildlife were comparable to those seen in the daughters and sons of women who had been exposed during pregnancy to a synthetic estrogen, diethylstilbestrol (DES), between the years 1948-71 (Herbst and Bern, 1988; Mittendorf, 1995). They postulated that the DES syndrome was an extreme expression of the plasticity of the fetus to environmental cues provided by hormonally active chemicals such as DDT and polychlorinated biphenyls. They also noticed that in addition to the already banned chemicals, other hormonally active chemicals were present in the environment. The anti-oxidant nonylphenol, which had just been shown to leach from laboratory plasticware (Soto et al., 1991), was one such example. Nonylphenol headed a growing list of chemicals that were subsequently identified as endocrine disruptors including plasticizers, disinfectants, sunscreens, and new pesticides (Colborn et al., 1993; Krishnan et al., 1993; Soto et al., 1994; Schlumpf et al., 2004). The participants of the Wingspread Conference concluded that the developmental abnormalities observed initially and predominantly in birds might foreshadow similar problems beginning to be observed in mammals, including humans (Colborn and Clemens, 1992). A year later, a meta-analysis concluded that the quantity and quality of human sperm had decreased during the last half-century, coincidentally with the introduction of chemicals into the environment (Carlsen et al., 1992).
The consequences of the DES iatrogenic mishap, as well as the gross developmental anomalies observed in wildlife, were the result of exposure to high doses of hormonally active agents. Ecological developmental biology, which considers the environment a main determinant in the construction of the phenotype, provided the conceptual framework to suspect that the phenotype can be affected by small variations in the endocrine milieu of the developing embryo. In fact, even physiological variation of the hormone levels to which fetuses are exposed results in effects that are manifested during adult life. For example, contiguity to male fetuses in the uterus affects the adult behavior, body weight, reproductive senescence (Clemens et al., 1978; Gandelman et al., 1977; vom Saal, 1989, 1992) and the development of the fetal mammary gland in females (Vandenberg et al., 2007). This perspective was a key component in the development of the Wingspread Statement.
Increasing concern about environmental hormones as causal agents of human disease soon started to appear in the literature, resulting in the formulation of the endocrine disruptor hypothesis. This hypothesis states that the increased incidence of malformations of the male genital tract, several neoplasms (uterine leiomyoma, testicular cancer and breast cancer), and the decreased sperm quality observed in European and US populations over the last fifty years was due to fetal exposure to endocrine disruptors (Markey et al., 2003; Skakkebaek et al., 1998). Laboratory animal research and epidemiological studies produced a body of evidence supporting the credibility of this hypothesis. Subsequent research by developmental biologists aimed at testing the endocrine disruptor hypothesis revealed that developmental exposure to these agents produced additional effects to those alluded to above, such as obesity. Based on the correlation between industrial chemical use and increased body weight in industrialized countries, Baillie-Hamilton proposed that the rapid rise in obesity might also be related to the rising levels of industrial chemicals in the environment (Baillee-Hamilton 2002).
One particularly well studied endocrine disruptor is the ubiquitous contaminant bisphenol-A (BPA), a component of plastics and epoxy resins. BPA has structural similarities to DES, and like DES, it is a xenoestrogen. We have chosen BPA as a model for this commentary because the multiple deleterious effects of this chemical in experimental animals resemble recent trends observed in humans. In fact, these similarities prompted the National Institutes of Environmental Health Sciences (NIEHS) to call a group of experts to evaluate the evidence; their findings were published in a series of articles and were summarized in the Chapel Hill Consensus Statement. There they stated that “the wide range of adverse effects of low doses of BPA in laboratory animals exposed both during development and in adulthood is a great cause for concern with regard to the potential for similar adverse effects in humans. Specific examples include the increase in prostate and breast cancer, uro-genital abnormalities in male babies, a decline in semen quality in men, early onset of puberty in girls, metabolic disorders including insulin resistant (type 2) diabetes and obesity, and neurobehavioral problems such as attention deficit hyperactivity disorder (ADHD).” (vom Saal et al., 2007).
Carcinogenesis in the womb: a challenge to the Somatic Mutation Theory
According to the somatic mutation theory, cancer is caused by mutations in the DNA of a single cell (Hahn and Weinberg, 2002). The research emanating from this theory has not provided either an explanation of cancer pathogenesis, reliable tools for cancer prevention, or successful therapies for most cancers. Due to these shortcomings, an old notion that originated in the late 19th century when pathologists described the histological pattern of tumors has resurfaced. It suggested that altered tissue organization was at the core of neoplasia, thus linking carcinogenesis to embryonic development (Sonnenschein and Soto, 2008). A central motif in this theory is the persistence of morphogenic fields throughout adult life; these fields orchestrate histogenesis and organogenesis before birth as well as tissue maintenance and regeneration throughout postnatal life. The tissue organization field theory posits that neoplasia results from a flawed interaction among cells and tissues. In this regard, exposure of stroma to a carcinogenic insult results in the development of carcinomas upon recombination of the exposed stroma with unexposed epithelial cells (Barcellos-Hoff and Ravani, 2000; Maffini et al., 2004). In the same context, carcinogenesis is potentially reversible as has been illustrated by the normalization of neoplastic epithelial cells upon recombination with normal stroma (Maffini et al., 2005).
Guided by the tissue organization field theory, we have hypothesized that in utero exposure to xenoestrogens increases the incidence of mammary cancer in adulthood because these chemicals interfere with mammary gland morphogenesis. Perinatal exposure to low doses of the xenoestrogen BPA (25 and 250 ng BPA/kg body weight) resulted in alterations in the organization of the mouse fetal mammary gland observed during the period of BPA exposure (Vandenberg et al., 2007). In addition, long-lasting effects in the mouse mammary gland became apparent during adult life, long after cessation of exposure (Markey et al., 2001). BPA enhanced sensitivity to estradiol, decreased apoptosis, increased the number of progesterone receptor-positive epithelial cells at puberty, increased lateral branching at 4 months of age and induced intraductal hyperplasias (Munoz de Toro et al., 2005; Vandenberg et al., 2008) . All of these observations in mice suggest that BPA exposure may increase the propensity to develop mammary neoplasia.
Because rats are a better model for mammary gland carcinogenesis than mice, we exposed dams to 2.5, 25, 250, and 1000 ug BPA/kg body weight/day to explore this hypothesis. Female offspring developed ductal hyperplasias at all the doses tested. In addition, carcinomas in situ were observed at postnatal day 50 and 95 in the animals exposed to the two highest doses. In sum, BPA exposure during pregnancy was sufficient to induce preneoplastic and neoplastic lesions in the adult mammary gland in the absence of any additional treatment aimed at increasing tumor incidence (Murray et al., 2007). BPA exposure during lactation followed by exposure to the carcinogen dimethylbenzanthracene (DMBA) resulted in higher mammary tumor multiplicity and reduced tumor latency compared to control animals (exposed solely to DMBA)(Jenkins et al., 2009).
Altogether, the mammary gland data obtained in rodents bring us back to the DES story: recent epidemiological studies have revealed that women exposed in utero to DES have an increased risk to develop breast cancer. This outcome has become apparent as the cohort of women exposed to DES in utero reach the age of prevalence of this neoplasm (Palmer et al., 2006)
Other endocrine disruptors, such as atrazine (which is not estrogenic) (Rayner et al., 2004) and dioxins (which have diverse toxicities, including indirect agonistic and antagonistic estrogenic effects) alter mammary gland development (Fenton et al., 2002). Though these alterations persist during adult life, the effect of these exposures on mammary gland carcinogenesis has yet to be explored.
Evidence that BPA exposure to neonates induces proliferative pre-cancerous lesions in the rat prostate has also been reported. (Ho et al., 2006). Neonatal exposure to 10ug BPA/kg BW/day did not increase the incidence of prostatic intraepithelial neoplasias (PINs). However, the increased propensity of the BPA-exposed animals to develop PINs was revealed when these rats were given a hormonal stimulus (testosterone plus estradiol for 16 weeks) during adulthood.
Endocrine disruption challenges “science as usual”
The multiple effects observed long after cessation of perinatal BPA exposure contradicts traditional reasoning, in which an estrogenic compound like BPA is expected to cause alterations in estrogen target tissues only. These findings merit a detour into epistemology, the science of “how do we know what we know”, which becomes relevant to biologists seeking explanations for the phenomena being observed. There are at least two different types of explanations for reproducible observations: in physics there are laws. In the absence of laws, explanations are of the mechanistic type. According to Bechtel and Abrahamsen, “a mechanism is a structure performing a function by virtue of its component parts, component operations, and their organization. The orchestrated functioning of the mechanism is responsible for one or more phenomena” Reductionists would construct a mechanism as a linear causation chain, i.e. ligand binds to receptor, which regulates (activates or represses) gene expression in a given cell type, which in turn, would explain a phenotype. A reductionist, bottom-up approach may try to build a gene network interactive model. However, how can one translate those intracellular events into altered tissue architecture, i.e. a phenotype?
Morphogenesis entails tissue-based events like reciprocal interactions among cells and the matrix that surrounds them, as well as cellular elongation and movement. These interactions among cells could be classified into two groups: i) those mediated by physical forces and ii) those where biochemical mediators are prominent. It should be noted, however, that these physical and chemical mediators also interact. Perhaps, the best metaphor in this regard is the one implied in the B. Fuller-inspired concept of tensegrity, whereby the organism, from the macro-scale to the micro-scale, is shaped by tensional forces through a balance of push and pull (Ingber, 2008). In this context, physical force “transduces” biochemical signaling, whereas biochemical factors also influence tensional forces. Recent studies have shown, for example, that varying the rigidity of the matrix could direct the same type of stem cell into very different specification paths. A soft matrix induces neuronal differentiation, a rigid one, bone cells (Engler et al., 2006). In more complex systems, the rigidity of the matrix determines the shape of epithelial structures (Paszek and Weaver, 2004) It is also known that cells remodel the matrix that surrounds them. A reductionist approach is ineffective when dealing with the reciprocal and simultaneous causation implicit in cell to cell and cell to matrix interactions, and thus, it is a first limitation for the study of complex phenomena such as endocrine disruption.
A second limitation is due to technically driven research biases. By this we mean that it is easier to work with DNA than with RNA, and with RNAs than with proteins. If we suspect that a chemical would alter chromatin structure, we choose to work on DNA methylation, which is easier to examine than histone modification. Similarly, it is easier to study gene expression than cell-cell interactions.
A third limitation is due to our research focus and expertise which favors the study of a single end point such as obesity, or cancer, or behavior, or reproduction. This is obviously more manageable than attempting to study all these points together. However, these end points may interact, as we know that obesity is a risk factor for certain cancers, as are hormone levels and patterns of carcinogenic exposure. Similarly, hyperactive behaviors may modify the expression of obesity associated with early exposure to a xenoestrogen.
And finally, it is easier to study a single agent at a time than multiple agents; the latter represents the real-life situation, as we are simultaneously exposed to multiple endocrine disruptors). This multi-causality does not stop at the mixture of chemicals we are exposed to. That is, if we explore the prenatal causes of obesity or leanness during adult life, we know that nutrition, gender, stress, xenoestrogens, phytoestrogens, and other endocrine disruptors such as organotins (Grun et al., 2006) nicotine (Toschke et al., 2002), perfluorooctanoic acid (Fenton, in this issue) and hexachlorobenzene (Smink et al., 2008) can all contribute to an outcome.
How do we overcome the limitations of Reductionism? Consider Organicism, the study of emergent phenomena
The complexity of multicellular organisms generates the perception of a discontinuity between low and high level phenomena. For instance, to know how a joint works, we have to understand the mechanics of movement and shock absorption. We cannot deduce it solely from the molecular components of hyaline cartilage. Thus, properties at one level of biological complexity (for instance, tissues) cannot be ascribed directly to their component parts (cells, extracellular matrix); they arise only through the interactions among the parts. Properties are emergent if their presence cannot be explained (predicted) from the properties of individuated parts (Gilbert and Sarkar, 2000).
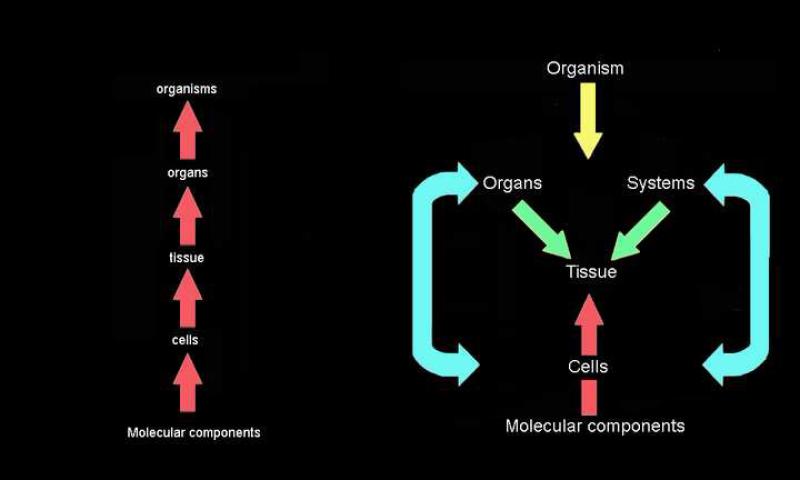
How can emergent phenomena be studied? A strategy might be formulated as follows; first, the level of organization at which the phenomenon of interest is being observed needs to be identified . Second, studies at this level of organization should be initiated. The third step is to move cautiously up and down levels of organization to identify bottom-up and top-down causation (Figure 1). Because we have to deal with reciprocal causality (for example, cell A is releasing an agent a that acts upon a neighboring cell B while simultaneously cell B is releasing an agent b that acts upon cell A), the number of interacting elements escalates and the limit of what can be done using conventional tools is rapidly reached (Soto et al., 2008).
Figure 1.
Comparison of the reductionist and organicist approach to causality. In the reductionist approach, the lower level determines the upper level (left panel) while in the organicist / systemic approach, a given phenomenon should be first studied at the level of organization at which it is observed. Then, both bottom-up and top-down causes should be accounted for. To illustrate the organicist view of development, we choose the tissue level of organization, as most organs start to develop by cell-cell interaction within a tissue compartment (right panel).
Are we technologically capable of studying complex phenomena? Inherent in the scientific thought of the past centuries has been the resolve to create an elegant and simple representation of reality, moving away from complexity. Advances in computational science and applied mathematics resulted in the development of quantitative models which can generate testable predictions derived from computer-based (in silico) simulations. These approaches will likely provide the tools for the study of complex phenomena such as those caused by exposure to endocrine disruptors. Among the recent developments in this field, agent-based simulation is one of the most important computational tools commonly used for modelling morphogenesis (Robertson et al., 2007). Agents are discrete elements, often representing biological cells, which behave according to a fixed set of dynamic rules (Merelli et al., 2007). They typically occupy a position in model space and may interact with neighbouring agents. Different properties of the system need to be represented in the computational mode, from tracking the position of the cells participating in morphogenesis, to describing their interactions with other cells and extracellular matrix. However complex, these models are being developed and most importantly they allow performing simulations via computer run experiments which outcomes can be verified in the live tissue or organ.
A good example of what can be achieved using a systems biology approach is the Virtual Heart developed by Denis Noble and his many collaborators, which include all levels of biological organization, from the gross anatomy of the organ to the molecular levels. “With over 40 years of interaction between simulation and experiment, the models are now sufficiently refined to begin to be of use in drug development.” (Noble, 2007). Gilbert and Sarkar proposed the adoption of this stance for the study of Developmental Biology (Gilbert and Sarkar, 2000). The field of endocrine disruptors will benefit substantially by adopting this organicist/emergentist approach which aims at simultaneously integrating phenomena at the organismal (systemic), organ, tissue, and cell levels of complexity.
In this conference, the effects of administering single compounds including perfluoroctanoic acid, organotins, phthalates, bisphenol A, phyotestrogens, nicotine and DES were discussed with regard to influences on body weight regulation. The data presented coupled with data from other studies of endocrine disruptors suggest that exposure to a single compound can be significantly influenced by its dose level (not necessarily in a linear fashion), time and length of exposure (prenatal and/or neonatal, peripubertal, adulthood), sex of the individual, and gonadal status. Some estrogenic agents, like BPA and DES, promote increased body weight (Howdeshell et al., 1999; Newbold et al., 2008; Rubin et al., 2001) or can decrease body weight (Newbold et al., 2008; Nunez et al., 2001), or lead to increased insulin secretion followed by subsequent development of insulin insensitivity (Alonso-Magdalena P et al., 2006). A diet containing high levels of the phytoestrogens genistein and diadzein from conception through adulthood increased metabolic fitness in males (Cederroth et al., 2007), a rather beneficial effect. Exposure to organotins during gestation (days 12-18) resulted in increased adipose deposition at birth and increased adipose tissue mass, but did not increase body weight at 10 weeks of age (Grun and Blumberg, 2006). The effects of perfluorooctanoic acid on body weight were dependent upon ovarian status (Fenton in this issue) and exposure to nicotine during fetal development results in increased body weight in children (Toschke et al., 2002). For some compounds, shared or overlapping mechanisms of action contribute to the similarity of their effects. PPAR γ has been implicated in the effects of organotins and phthalates and perfluoroctanoic acid on increased adiposity or body weight. Other endocrine disruptors may act through unique pathways in addition to their main or better known endocrine effect. For example, in addition to its estrogenic effects, BPA, interferes with thyroid hormone signaling (Moriyama et al., 2002), and there are examples of BPA action as an estrogen antagonist in the presence of estradiol (Bjerke et al., 1994; Leranth et al., 2008; Zsarnovszky et al., 2005). In addition to this mindboggling complexity, we have to consider that humans and wildlife are exposed to a mixture of endocrine disruptors.
In summary, we have addressed the epistemological problems integral to the centuries-old tradition of reductionist thinking in science, which seeks to extract a representation of reality free of complexity. From the perspective of ecological developmental biology and systems biology, we are encouraged to embrace the complexity inherent in our subject of interest. We would be satisfied if his analysis persuades researchers to heed Whitehead's advice: “Seek simplicity and distrust it (Gilbert and Sarkar, 2000).”
Acknowledgements
This work was supported by NIH grants ES0150182, ES012301 and ES08314. We are grateful to Cheryl Schaeberle and Michael Askenase for their technical and editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. [PubMed] [Google Scholar]
- Barker DJP, Hanson MA. Altered regional blood flow in the fetus: the origins of cardiovascular disease? Acta Paediatricia. 2004;93:1559–1560. [PubMed] [Google Scholar]
- Bechtel W, Abrahamsen A. Explanation: a mechanist alternative. Stud. Hist. Phil. Biol. & Biomed. Sci. 2005;36:421–441. doi: 10.1016/j.shpsc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bern HA, et al. Wingspread consensus statement. In: Colborn T, Clement C, editors. Chemical induced alterations in sexual and functional development: the wildlife/human connection. Priceton Scientific Publishing; Princeton, NJ: 1992. pp. 1–8. [Google Scholar]
- Bjerke DL, Brown TJ, MacLusky NJ, Hochberg RB, Peterson RE. Partial demasculinization and feminization of sex behavior in male rats by in utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin is not associated with alterations in estrogen receptor binding or volumes of sexually differentiated brain nuclei. Toxicol. Appl. Pharmacol. 1994;127:258–267. doi: 10.1006/taap.1994.1160. [DOI] [PubMed] [Google Scholar]
- Burlington H, Lindeman VF. The effect of DDT on testes and secondary sex characters of white leghorn cockerels. Proc. Soc. Exp. Biol. Med. 1950;74:48–51. doi: 10.3181/00379727-74-17805. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keinding N, Skakkebaek NE. Evidence for the decreasing quality of semen during the past 50 years. Br. Med. J. 1992;305:609–612. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Vinciguerra M, Kuhne F, Madani R, Doerge DR, Visser TJ, Foti M, Rohner-Jeanrenaud F, Vassilli JD, Nef S. A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice. Environ. Health Perspect. 2007;115:1467–1473. doi: 10.1289/ehp.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Hormones and Behavior. 1978;10:40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fenton SE, Hamm JT, Birnbaum L, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- Gandelman R, vom Saal FS, Reinisch JM. Contiguity to male foetuses affects morphology and behaviour of female mice. Nature. 1977;266:722–724. doi: 10.1038/266722a0. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Mechanisms for the environmental regulation of gene expression: ecological aspects of animal development. J. Biosci. 2005;30:65–74. doi: 10.1007/BF02705151. [DOI] [PubMed] [Google Scholar]
- Gilbert SF, Sarkar S. Embracing complexity: Organicism for the 21st century. Developmental Dynamics. 2000;219:1–9. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1036>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner D, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006;9:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat. Rev. Cancer. 2002;2:331–342. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Bern HA. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. 1988 [Google Scholar]
- Ho S-M, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol a increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Seminars in Cancer Biology. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. Oral exposure to Bisphenol A increases dimethylbenzanthracene-induced mammary cancer in rats. Environ. Health Perspect. 2009 doi: 10.1289/ehp.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Torri JA, Lippman ME, Dickson RB. The role of cathepsin D in the invasiveness of human breast cancer cells. Cancer Res. 1993;53:873–877. [PubMed] [Google Scholar]
- Krishnan AV, Starhis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Nat. Acad. Sci. USA. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffini MV, Calabro JM, Soto AM, Sonnenschein C. Stromal regulation of neoplastic development: Age-dependent normalization of neoplastic mammary cells by mammary stroma. Am. J. Pathol. 2005;67:1405–1410. doi: 10.1016/S0002-9440(10)61227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotropic assay: a re-evaluation of its validity in assessing the estrogenicity of bisphenol A. Environ. Health Perspect. 2001;109:55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Rubin BS, Soto AM, Sonnenschein C. Endocrine disruptors from Wingspread to environmental developmental biology. J. Steroid Biochem. Molec. Biol. 2003;83:235–244. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- Merelli E, Armano G, Cannata N, Corradini F, d'Inverno M, Doms A, Lord P, Martin A, Milanesi L, Moller S, Schroeder M, Luck M. Agents in bioinformatics, computational and systems biology. Brief Bioinform. 2007;8:45–59. doi: 10.1093/bib/bbl014. [DOI] [PubMed] [Google Scholar]
- Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology. 1995;51:435–445. doi: 10.1002/tera.1420510609. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by Bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Munoz de Toro MM, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM. Perinatal exposure to Bisphenol A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal Bisphenol A exposure. Reproductive Toxicology. 2007;23:383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Hormonally Active Agents in the Environment. 1999 [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int. J. Androl. 2008;31:201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Noble D. From the Hodgkin-Huxley axon to the virtual heart. J. Physiol. 2007;580(pt1):15–22. doi: 10.1113/jphysiol.2006.119370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42:917–922. doi: 10.1016/s0045-6535(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Herbst AL, Noller KL, Hyer M, Hoover RN. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidem. Biomar. 2006;15:1509–1514. doi: 10.1158/1055-9965.EPI-06-0109. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol. Appl. Pharmacol. 2004;195:23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Robertson SH, Smith CK, Langhans AL, McLinden SE, Oberhardt MA, Jakab KR, Dzamba B, DeSimone DW, Papin JA, Peirce SM. Multiscale computational analysis of Xenopus laevis morphogenesis reveals key insights of systems-level behavior. BMC Syst. Biol. 2007;1:46. doi: 10.1186/1752-0509-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol-A affects body weight, patterns of estrous cyclicity and plasma LH levels. Environ. Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpf M, Schmid P, Durrer S, Conscience M, Maerkel K, Henseler M, Gruetter M, Herzog I, Reolon S, Ceccatelli R, Faass O, Stutz E, Jarry H, Wuttke W, Lichtensteiger W. Endocrine activity and developmental toxicity of cosmetic UV filters—an update. Toxicology. 2004;205:113–122. doi: 10.1016/j.tox.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Meyts ER, Jorgensen N, Carlsen E, Petersen PM, Giwercman A, Andersen A, Jensen TK, Andersson A, Muller J. Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS. 1998;106:3–12. doi: 10.1111/j.1699-0463.1998.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Smink A, Ribas-Fito N, Garcia R, Torrent M, Mendez MA, Grimalt JO, Sunyer J. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatricia. 2008;97:1465–1469. doi: 10.1111/j.1651-2227.2008.00937.x. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM. Theories of carcinogenesis: an emerging perspective. Semin. Cancer Biol. 2008;18:372–377. doi: 10.1016/j.semcancer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Chung KL, Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen sensitive cells. Environ. Health Perspect. 1994;102:380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ. Health Perspect. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C, Miquel P-A. On physicalism and Downward Causation in Developmental and Cancer Biology. Acta Biotheoretica. 2008 doi: 10.1007/s10441-008-9052-y. [DOI] [PubMed] [Google Scholar]
- Toschke AM, Koletzko B, Slikker WJ, Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. Eur. J. Pediatr. 2002;161:445–448. doi: 10.1007/s00431-002-0983-z. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reproductive Toxicology. 2008 doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to the xenoestrogen bisphenol-A alters development of the fetal mammary gland. Endocrinology. 2007;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J. Anim. Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reproductive Toxicology. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Montano MM, Wang MH. Sexual differentiation in mammals. 1992;21:17–83. [Google Scholar]
- Zsarnovszky A, Le HH, Wang H-S, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortes: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]