Abstract
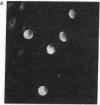
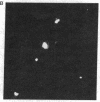
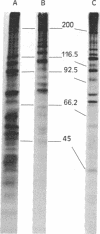
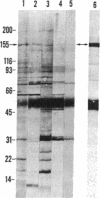
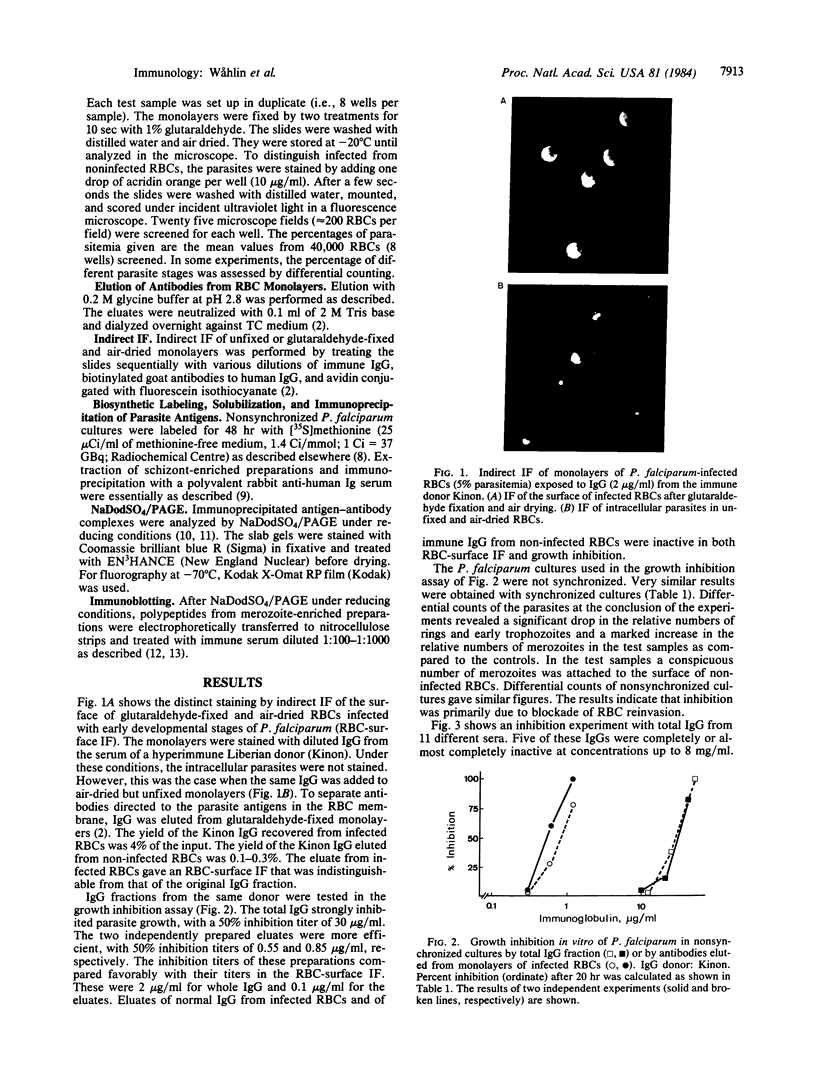
IgG from a donor clinically immune to Plasmodium falciparum malaria strongly inhibited reinvasion in vitro of human erythrocytes by the parasite. When added to monolayers of glutaraldehyde-fixed and air-dried erythrocytes infected with the parasite, this IgG also displayed a characteristic immunofluorescence restricted to the surface of infected erythrocytes. Elution of the IgG adsorbed to such monolayers gave an antibody fraction that was 40 times more efficient in the reinvasion inhibition assay (50% inhibition titer, less than 1 microgram/ml) than the original IgG preparation. The major antibody in this eluate was directed against a parasite-derived antigen of Mr 155,000 (Pf 155) deposited by the parasite in the erythrocyte membrane in the course of invasion. A detailed study of IgG fractions from 11 donors with acute P. falciparum malaria or clinical immunity revealed the existence of an excellent correlation between their capacities to stain the surface of infected erythrocytes, their titers in reinvasion inhibition, and the presence of antibodies to Pf 155 as detected by immunoblotting. No such correlations were seen when the IgG fractions were analyzed for immunofluorescence of intracellular parasites or for the presence of antibodies to other parasite antigens as detected by immunoprecipitation of [35S]methionine-labeled and NaDodSO4/PAGE-separated parasite extracts. The results suggest that Pf 155 has an important role in the process of erythrocyte infection and that host antibodies to this antigen may efficiently interfere with this process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Miller L. H., Johnson J., Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978 Apr;77(1):72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins K., Wahlgren M., Perlmann P. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clin Exp Immunol. 1983 Nov;54(2):313–318. [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Knowles G. Differential effect of immunoglobulin on the in vitro growth of several isolates of Plasmodium falciparum. Infect Immun. 1983 Mar;39(3):1228–1235. doi: 10.1128/iai.39.3.1228-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Mitchell G. F., Heywood P. F. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature. 1982 Jun 17;297(5867):591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Blomberg F., Berzins K., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977 Jul;74(1):181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Dvorak J. A., Miller L. H., Whitehouse W. C., Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975 Feb 28;187(4178):748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- Epstein N., Miller L. H., Kaushel D. C., Udeinya I. J., Rener J., Howard R. J., Asofsky R., Aikawa M., Hess R. L. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J Immunol. 1981 Jul;127(1):212–217. [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Fandeur T., Dubois P., Gysin J., Dedet J. P., da Silva L. P. In vitro and in vivo studies on protective and inhibitory antibodies against Plasmodium falciparum in the Saimiri monkey. J Immunol. 1984 Jan;132(1):432–437. [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen S., Andersen B. J. Immunoadsorbent isolation of antigens from the culture medium of in vitro cultivated Plasmodium falciparum. Acta Pathol Microbiol Scand C. 1981 Apr;89(2):99–103. doi: 10.1111/j.1699-0463.1981.tb02671.x. [DOI] [PubMed] [Google Scholar]
- Jepsen S. Inhibition of in vitro growth of Plasmodium falciparum by purified antimalarial human IgG antibodies. Isolation of target antigens from culture supernatants. Scand J Immunol. 1983 Dec;18(6):567–571. doi: 10.1111/j.1365-3083.1983.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Jungery M., Boyle D., Patel T., Pasvol G., Weatherall D. J. Lectin-like polypeptides of P. falciparum bind to red cell sialoglycoproteins. Nature. 1983 Feb 24;301(5902):704–705. doi: 10.1038/301704a0. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Aikawa M., Dvorak J. A. Malaria (Plasmodium knowlesi) merozoites: immunity and the surface coat. J Immunol. 1975 Apr;114(4):1237–1242. [PubMed] [Google Scholar]
- Miller L. H., Haynes J. D., McAuliffe F. M., Shiroishi T., Durocher J. R., McGinniss M. H. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977 Jul 1;146(1):277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pasvol G., Wainscoat J. S., Weatherall D. J. Erythrocytes deficiency in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. Nature. 1982 May 6;297(5861):64–66. doi: 10.1038/297064a0. [DOI] [PubMed] [Google Scholar]
- Perkins M. E. Surface proteins of Plasmodium falciparum merozoites binding to the erythrocyte receptor, glycophorin. J Exp Med. 1984 Sep 1;160(3):788–798. doi: 10.1084/jem.160.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. Inhibitory effects of erythrocyte membrane proteins on the in vitro invasion of the human malarial parasite (Plasmodium falciparum) into its host cell. J Cell Biol. 1981 Sep;90(3):563–567. doi: 10.1083/jcb.90.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R., Hofer-Warbinek R. Reaction of immune sera with components of the human malarial parasite, Plasmodium falciparum. Am J Trop Med Hyg. 1981 Nov;30(6):1168–1178. doi: 10.4269/ajtmh.1981.30.1168. [DOI] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R. Inhibition of the in vitro growth of Plasmodium falciparum. I. The effects of immune serum and purified immunoglobulin from owl monkeys. J Immunol. 1979 Oct;123(4):1894–1899. [PubMed] [Google Scholar]
- Saul A., Myler P., Schofield L., Kidson C. A high molecular weight antigen in Plasmodium falciparum recognized by inhibitory monoclonal antibodies. Parasite Immunol. 1984 Jan;6(1):39–50. doi: 10.1111/j.1365-3024.1984.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Schofield L., Saul A., Myler P., Kidson C. Antigenic differences among isolates of Plasmodium falciparum demonstrated by monoclonal antibodies. Infect Immun. 1982 Dec;38(3):893–897. doi: 10.1128/iai.38.3.893-897.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Björkman A. Characterization of the humoral immune response in Plasmodium falciparum malaria. I. Estimation of antibodies to P. falciparum or human erythrocytes by means of microELISA. Clin Exp Immunol. 1983 Oct;54(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- Wilson R. J., Phillips R. S. Method to test inhibitory antibodies in human sera to wild populations of Plasmodium falciparum. Nature. 1976 Sep 9;263(5573):132–134. doi: 10.1038/263132a0. [DOI] [PubMed] [Google Scholar]