Abstract
Background
Arm non-use, defined as the difference between what the individual can do when constrained to use the paretic arm and what the individual does do when given a free choice to use either arm, has not yet been quantified in individuals post-stroke.
Objectives
1) to quantify non-use post-stroke and 2) to develop and test a novel, simple, objective, reliable, and valid instrument, the Bilateral Arm Reaching Test (BART), to quantify arm use and non-use post-stroke.
Methods
First, we quantify non-use with the Quality of Movement (QOM) subscale of the Actual Amount of Use Test (AAUT) by subtracting the AAUT QOM score in the spontaneous use condition from the AAUT QOM score in a subsequent constrained use condition. Second, we quantify arm use and non-use with BART by comparing reaching performance to visual targets projected over a 2D horizontal hemi-workspace in a spontaneous-use condition (in which participants are free to use either arm at each trial) to reaching performance in a constrained-use condition.
Results
All participants (N = 24) with chronic stroke and with mild to moderate impairment exhibited non-use with the AAUT QOM. Non-use with BART had excellent test-retest reliability and good external validity.
Conclusions
BART is the first instrument that can be used repeatedly and practically in the clinic to quantify the effects of neuro-rehabilitation on arm use and non-use, and in the laboratory for advancing theoretical knowledge about the recovery of arm use and the development of non-use and ‘learned non-use’ after stroke.
Keywords: Stroke rehabilitation, upper extremity, evaluation, cerebrovascular accident
INTRODUCTION
Most individuals with upper extremity disability resulting from a stroke face difficulties to effectively use their paretic arm and hand in daily activities, resulting in significantly reduced quality of life.1–3 Such “non-use” has been defined as the difference between what the individual can do when constrained to use the paretic arm and what the individual does when given a free choice to use either arm.4 Non-use in individuals with hemiparetic stroke (or with other predominantly unilateral motor neurological disorders) can arise from a number of factors such as pain, limited range of motion, as well as higher effort and attention required for successful use of the impaired hand.5 Non-use has furthermore been hypothesized to have a learned component.6–9 According to this “learned non-use” hypothesis, non-use would develop either after unsuccessful repeated attempts to use the affected arm and hand, or after negative consequences resulting from paretic limb use (such as spilling hot coffee or dropping a retrieved object).
Despite the high clinical and scientific significance of non-use, little work has been conducted to directly quantify non-use in individuals post-stroke. A seminal study by Sterr et al.8 estimated non-use in a relatively heterogeneous group of brain injured adolescents with the Quality of Movement subscale of the Motor Activity Log test (MAL QOM)10 and the Amount of Movement subscale of the Actual Amount of Use Test (AAUT AOU)8,11. Non-use was estimated for each test by the difference between the actual test score and the score obtained when the participants actually perform the tests with the affected hand.
Although the Sterr et al. study demonstrated the feasibility of measuring non-use in hemi-paretic individuals with the MAL QOM and the AAUT AOU, neither instrument fulfills the five criteria for an ideal measurement tool in neurological rehabilitation12: simplicity, objectiveness, test-retest reliability, external validity, and sensitivity. First, neither MAL nor AAUT are simple to administer, and both require adequate training of the tester by experienced therapists. Second, the MAL relies on subjective participants’ ratings of the amount of use and quality of movement of their more affected arm in functional daily activities outside the laboratory. Third, to preserve validity, the modified AAUT cannot be administered repeatedly, and as such, it may lack good test-retest reliability. Fourth, the AAUT AOU has large variability and is relatively insensitive to treatment effect because of the low resolution of scoring.13 In light of these limitations, and in order to advance both theoretical and practical knowledge about the recovery of arm use after stroke, there is therefore a considerable need to develop tools that capture purposeful arm use and non-use with objective activity monitoring.13
The goal of the present study is threefold. First, we investigate the existence of non-use in participants with chronic stroke, as measured from the AAUT QOM. Second, we propose a novel, simple, and quick to administer laboratory-based measurement tool, the Bilateral Arm Reaching Test (BART) to quantify paretic arm use and non-use objectively and repeatedly. Third, we investigate whether measurement of arm non-use with BART in participants with chronic stroke is reliable in test-retest, and whether it exhibits external validity when compared to non-use as estimated from the AAUT QOM.
METHODS
Participants
Twenty-four participants with chronic stroke (18 males, 6 females) were enrolled in this study. The inclusion criteria were: (1) Mini-Mental State Examination score > 25/30; (2) at least 6 months post-stroke; (3) no pain in the paretic arm and hand; (4) right-hand dominant pre-stroke; (5) the ability to reach the farthest target displayed at 30 cm anterior to the midline trunk (the target is the farthest target in front of the body midline presented by BART). For the validity study, an additional criterion was (6) no visual neglect, as measured by the ability to cross all the lines in both left and right workspaces in Albert’s test14. Pre-stroke hand dominance was self-reported.
The upper extremity score of the Fugl-Meyer test (FM-UE)15 was administered to all participants post-stroke by four different testers, all physical therapists with more than two year of clinical experience.
In addition, ten non-disabled (4 males, 6 females) right-handed (according to Edinburgh Handedness Inventory)16 age-matched participants were recruited (age of participants post-stroke: 62.25 ± 2.64 (SE: Standard error), age of non-disabled participants: 58.10 ± 3.76 (SE), t-test: p=0.390). Note that participants in this group were not recruited to serve as controls for comparison of use and non-use with post-stroke participants, but instead to define the average hand use of right-handed age-matched non-disabled participants. Specifically, the average measure of use in non-disabled participants was utilized in BART as a baseline to compute non-use in participants post-stroke. Thus, by definition, participants had zero non-use with BART if they behaved like the average non-disabled participant. Using the average behavior is reasonable, because we showed that there is little between subject variability in hand use between non-disabled participants (see below and Appendix, Figure A).
To test BART test-retest reliability, 19 participants with stroke performed three test sessions at least four days apart. To assess BART validity, 15 participants with stroke performed two test sessions at least four days apart. Validity was assessed with BART data from the second test session. Note that five participants were only enrolled to assess validity and as such performed only two test sessions. Ten participants participated in both reliability and validity, and as such performed three sessions. The study was approved by the Institutional Review Board of the University of Southern California, and all participants read and signed a written informed consent form prior to study enrollment.
Actual Amount of Use Test (AAUT)
The AAUT was administered to the 15 participants post-stroke enrolled in the validity study first in the spontaneous use condition and in the constrained use condition, as in Sterr et al.8. We analyzed only the first 14 items of the original 17 items AAUT, because these items are related to arm use while last three items are related to general activities such as gesturing and posture throughout testing. The AAUT test was administered by three experimenters and rated by one evaluator, a physical therapist with more than two year of clinical experience. We used the AAUT QOM instead of the AAUT AOU to test the external validity of BART for two reasons. First, the cAAUT AOU scores in Sterr et al. were close to maximum for most participants. Second, we recently showed greater variability and insensitivity to treatment effect for the AAUT AOU compared to the AAUT QOM13.
The AAUT QOM scores in the spontaneous use condition, sAAUT, and in the constrained use condition, cAAUT, were calculated and expressed as average scores, from zero to 5. Non-use was computed by nuAAUT = cAAUT − sAAUT.
Bilateral Arm Reaching Test (BART)
Apparatus
Because target distance and location largely influence arm choice in pointing movements16,17, BART displayed one of 100 targets at each trial in pseudorandom order on a 2D hemi-workspace. Targets were white disks of 2 cm in diameter projected on the table from an overhead projector with a preset target presentation schedule. Targets were displayed on 6 arcs, all ranging between 10° and 170° (0 degree is on axis is parallel to the body, to the right). Arcs radii ranged from 10 to 30 cm, with equal 4 cm distance between arcs. The targets were placed every 10 degrees along the arcs. The leftmost and rightmost targets in the upper corners were not presented, making a total of 100 targets (6 arcs *17 position per arcs minus 2 targets). Two Mini-Bird model 500 (5mm) magnetic sensors (Ascension Technology Corporation) were positioned on the tip of the index finger of each hand to measure finger motion and arm choice (sampling 100 Hz).
Testing procedure
Participants were seated with a seat belt to limit upper body movement17. The position of the chair was adjusted to ensure that participants could comfortably reach to the end of the workspace without bending their trunks. The participants were instructed to place both index fingers on the home position (a green disk of 2 cm in diameter), as shown in Figure 1.
Figure 1.
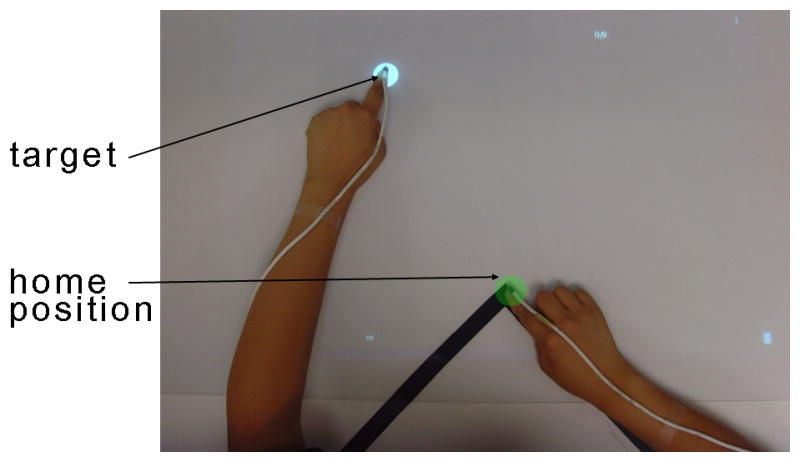
Measuring arm use with the Bilateral Arm Reaching Test (BART). The home position is identified by the green circle and a target by the white circle. For each trial, participants were instructed to reach to the target with their choice of hand using the index finger as quickly and accurately as possible. Magnetic sensors were attached to the tips of index fingers to record choice of hand and performance.
In both spontaneous and constrained use conditions, after a target was presented, participants were instructed to reach the target as quickly and as accurately as possible, to remain in contact with the target until it disappeared, and to return to the home position. After the start position was maintained for 1 sec, the home target disappeared and a target appeared at one of 100 locations. The inter-trial interval was 3 sec. A successful trial was defined when participants reached the target within 1.2 seconds. A pleasant sound was played following successful trials; an unpleasant sound was played following unsuccessful trials. Dragging the arm along the table surface was instructed to be inappropriate and was discouraged whenever observed by the experimenter.
In the spontaneous use condition, participants were instructed to use either hand to reach to each successively displayed target. Targets appeared twice at each position in a pseudorandom order, resulting in 200 trials. Participants were reminded that there was no right or wrong answer in their arm choice of arm, but were instructed to maximize the number of successful trials. In the constrained use condition, participants post-stroke were instructed to reach each target with the index finger of the paretic arm. Targets appeared once at each position in a pseudorandom order, resulting in 100 trials. Note that non-disabled participants only performed the spontaneous condition, as we noticed in our beta version that all non-disabled individuals could reach all targets in the hemi-workspace within 1.2 sec (and thus obtain a maximum score in the constrained condition).
BART Dependent Measures
Overview
By definition, non-use is computed by subtracting what post-stroke participants do from what they can do. In BART, direct subtraction of successful movements made by the paretic in the spontaneous condition from those made in the constrained condition would not work, however, for the following reason. Non-disabled participants can reach targets in both left and right workspaces with their dominant right hand within 1.2 sec with no failures. However, when given a choice, they use their right hand for the right workspace and their left hand for the left workspace. Computation of non-use by simple subtraction of the “spontaneous reach area” from the “constrained area” would yield large non-use for non-disabled participants, which is not appropriate. We therefore assumed that the average non-disabled participant has zero non-use. To compute non-use, we collected non-disabled participant choice behavior in the spontaneous BART condition, and used the average behavior as a mask to the performance data of the participant post-stroke. In this way, when paretic arm use in the spontaneous condition is equal to the “masked” performance, there is zero non-use. When arm use in the spontaneous condition is less than the “masked” performance, there is positive non-use.
Spontaneous use (sBART)
To compute arm use in participants post-stroke, we quantified the successful trials made by the affected arm (for age-matched controls, we quantified the successful trials made by the dominant arm). For each target, we computed the probability of successful reach with the paretic arm Pfree(success, handChoice = affectedArm| target). A second order logistic regression model interpolated and smoothed these probabilities to obtain a probabilistic use map over the 2D workspace. The logistic regression model has an extended input feature space, [x2,y2,xy,x,y,1], where x and y are the coordinates of 2D workspace. The choice was established based on our data showing that the indifference line (i.e., line of 50% choice probability) can be very curved in participants post-stroke (e.g., Figure 2d); a simple first order logistic regression model cannot account for such curvature. sBART was then computed by integrating the volume beneath the probability surface given by the logistic regression model of use, using 1000 by 1000 grid over the workspace.
Figure 2.
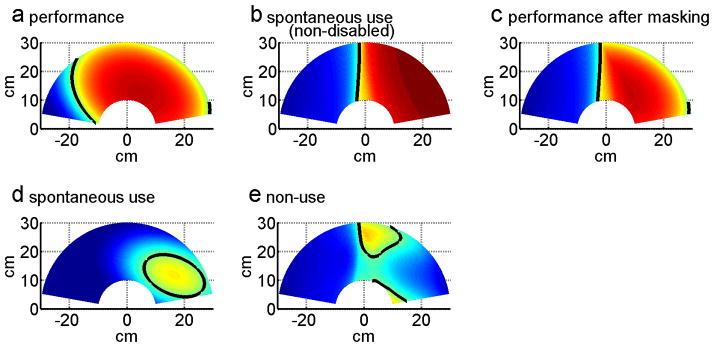
Computing non-use with BART in one session over the 2D reaching workspace for right-affected post-stroke participant ID2, in session 3. (a) Constrained use (performance) probability for ID2. (b) Average spontaneous use probability for right handed non-disabled participants. (c) Constrained use (performance) probability after masking with average healthy subject data of panel (b). (d) Spontaneous use probability. (e) Non-use probability for ID2. Color coding: red = 100% use the paretic arm (right arm for healthy controls), blue = 0% use of the paretic arm. The indifference line, indicated by the thick black line, corresponds to the 50% decision boundary. Note that the non-use probability maps of in (e) is solely for illustrative purpose; it was obtained by subtracting, for each target, the probability of successful reach with the affected arm in the spontaneous condition from the probability of successful reach with the affected arm in the constraint condition.
Performance after masking (cBART)
As discussed above, because the workspace covered in the constrained use condition can be equal to the whole workspace in participants post-stroke with mild impairments, we cannot directly subtract sBART from the integrated performance over the whole hemi-workspace in the constrained use condition. We therefore masked the successful reach area in the constrained use condition with the average arm use of the non-disabled participants from the spontaneous use condition. Specifically, we first defined the probability of reaching each target successfully with the affected arm (see Figure 2 for steps of the computation):
| (1) |
where Pconst(success| target) was computed from a second order logistic regression model (as above) with the successful trial data in the constrained use condition (Figure 2a), and Pfree _ healthy (handChoice=affectedArm| target) was computed from a logistic regression model with the successful trial data from the spontaneous condition for non-disabled controls (see Figure 2b). Pperf (success, handChoice=affectedArm | target) was thus a probability of successful reach in the constrained condition, masked by the spontaneous choice data in non-disabled age-matched controls (Figure 2c). cBART was then computed by integrating the volume beneath the probability surface given by:
Non-Use (nuBART): Finally, nuBART was then computed by subtracting sBART from cBART, and taking possible negative values to 0, in line with the definition of non-use and similarly to non-use as estimated with the AAUT. Thus,
| (2) |
where the function |x|+ returns x if x>0 and 0 otherwise.
Statistical Analyses
The number of trials per session in the spontaneous use condition (200) was determined from a sensitivity analysis of the non-disabled participants’ choice data: We computed sBART for increasing number of trials from 50 to 400, and observed that sBART converged at between 150 trials and 200 trials for all participants (data not shown).
For each variable, sBART, cBART, and nuBART, we performed test-retest reliability, and external validity computations. Reliability was assessed with intraclass correlation coefficients (ICCs) for three sessions and with Pearson or Spearman correlations for session-to-session measurements. External validity was tested with Pearson or Spearman correlations. External validity for sBART, cBART, and nuAAUT were tested by correlations with sAAUT, cAAUT, and nuAAUT respectively. In addition, we tested for possible correlations between nuBART and months since stroke and age, and tested the effect of the side of paresis.
For all analyses, the level of statistical significance was set at P < 0.05. When the data were not normal (as tested with Shapiro-Wilk test), non-parametric statistics were used (as indicated in results). Data analyses were performed using the SPSS 13.0 and MATLAB 7.5. All average results are reported as average ± standard errors (SE).
RESULTS
Demographic and stroke characteristic data
Table 1 summarizes the demographic data for the 24 participants post-stroke. There was no difference in age between groups (62.25 ± 2.64 post-stroke, 58.10 ± 3.76 controls P = .390). In the stroke group, there was no difference between affected sides (P = .186). Time from stroke onset was 79.46 ± 12.19 months (range 11 to 275 months). The FM-UE was 49.21 ± 2.18 (range 22 to 63). The 15 participants in the validity study had a score of 0 on Albert’s test, indicating no neglect.
Table 1.
Demographic data of the stroke group
| Mean | SE | Minimum | Maximum | |
|---|---|---|---|---|
| Age(yrs) | 62.25 | 2.64 | 35 | 83 |
| Time from Onset (month) | 79.46 | 12.19 | 11 | 275 |
| FM-UE Motor Score (66 max) | 49.21 | 2.18 | 22 | 63 |
| FM-Cor (6 max) | 4.21 | 0.24 | 2 | 6 |
| Gender | 18 Male, 6 Female | |||
| Affected Hand | 13 Right, 11 Left | |||
| Reliability/Validity | 19 Reliability, 15 Validity | |||
FM=Fugl-Meyer, FM-Cor=coordination subscale of the FM-UE, SE=Standard Error
Measuring use, performance, and non-use with the AAUT test
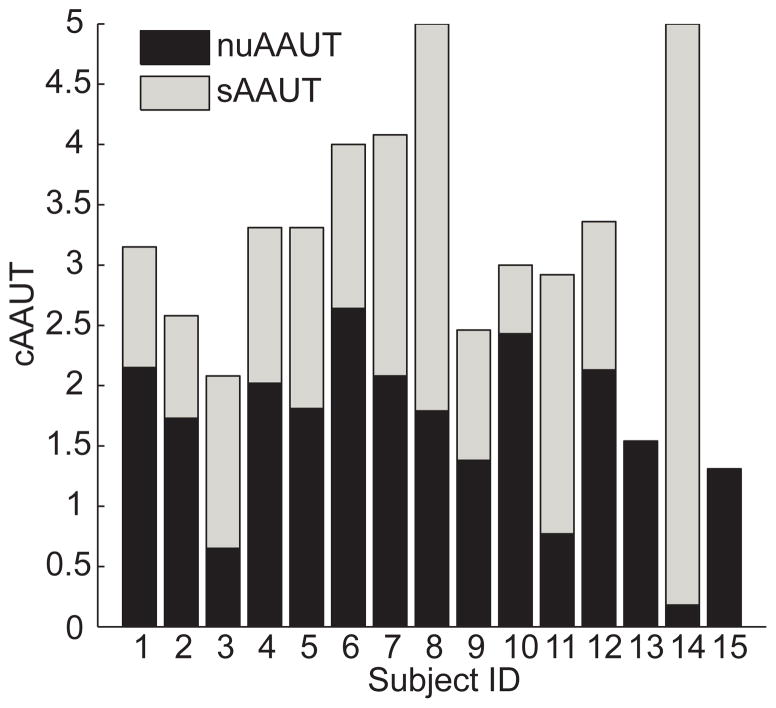
For those 15 post-stroke participants enrolled for the validity study, the sAAUT QOM was 1.50 ± 0.32, and cAAUT QOM was 3.17 ± 0.28. Non-use, nuAAUT was normally distributed (P = .209), and significantly greater than zero (1.67 ± 0.18 P < .0001, one sample t-test). Thus, all participants did exhibit some degree of non-use overall, although the range of non-use observed was large (range: 0.18 – 2.64; Figure 3).
Figure 3.
Arm use and non-use in participants post-stroke as estimated from AAUT QOM. The total height of each bar is cAAUT, the score in the constrained use condition. Because nuAAUT = cAAUT − sAAUT, cAAUT decomposes into sAAUT (grey), performance in the spontaneous use condition, and nuAAUT (black), estimated arm non-use.
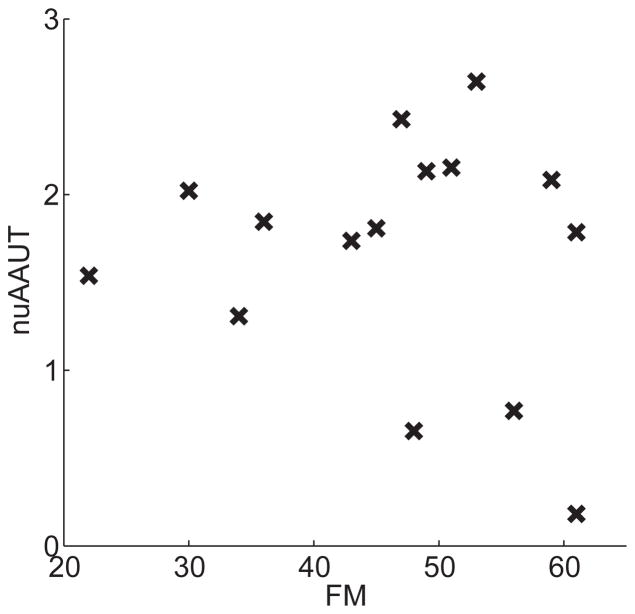
The cAAUT correlated with FM-UE (r = 0.758, P = .001). nuAAUT did not correlate with the FM-UE, as expected (r = −0.136, P = .629) – see Appendix, Figure B. In addition, nuAAUT did not correlate with stroke duration (P > .5). There was no difference in nuAAUT between left and right hand paresis (P > .1, Mann–Whitney U test). There was a trend for a positive correlation between nuAAUT and age (r = 0.473 and P = .075, Pearson).
Measuring use, performance, and non-use with BART
Non-Disabled Participants
All non-disabled participants successfully reached all targets within the 1.2-second time constraint (Figure 2b). The mean indifference line was slightly shifted leftward of the midline. Consequently, mean sBART of non-disabled participants was greater than 0.5, with sBART = 0.60 ± 0.10, indicating a 10% handedness bias on average.
As discussed above, out results highly depend on this “average non-disabled” use behavior, because non-use is computed with these average use data. We therefore verified with a bootstrap analysis (1000 samples) that the indifference line varied only for a few degrees on the right and left of the average indifference line, suggesting that arm choice showed little variability among the right-handed non-disabled age-matched participants (Appendix, Figure A).
Participants Post-Stroke
Figure 4 shows examples of use and non-use probability maps (non-use maps are shown for illustrative purpose only, since non-use was computed by subtracting sBART from cBART, in line with the definition and with non-use as estimated with the AAUT) for three participants: a participant with little non-use (ID10); a participant with large non-use, albeit mild impairment (ID1); and a participant with moderate non-use and impairment (ID3). In participants with low sBART values, the indifference line was not often straight, and could form an island instead of a straight boundary on the 2D map (see for example, Figure 2d and 4, ID3, spontaneous use).
Figure 4.
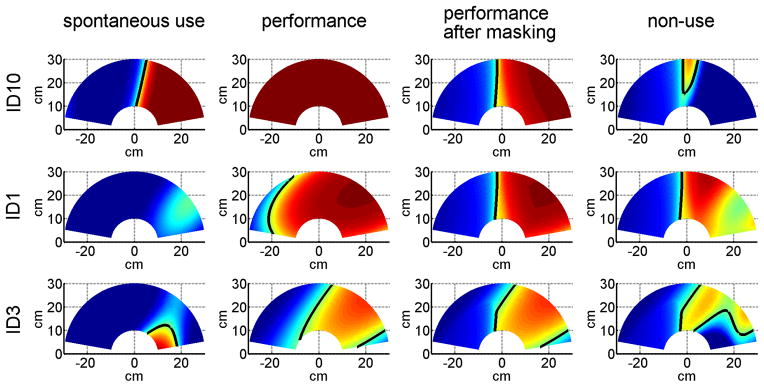
Examples of use and non-use with BART in one session over the 2D reaching workspace for three right affected participants post-stroke. Each row represents a different participant: a participant with little non-use (ID10; session 2, FM 63, FM_cor 5, sBART = 0.64, cBART = 0.57, nuBART = 0.113), a participant with large non-use, albeit mild impairment (ID1; session 2, FM 57, FM_cor 4, sBART = 0.10, cBART = 0.54, nuBART = 0.433), and a participant with moderate non-use (ID3; session 2 c, FM 49, FM_cor 3, sBART = 0.18, cBART = 0.340, nuBART = 0.239). Maps from left to right for each row: spontaneous use, performance, performance after masking with healthy subject choice data, and non-use. As in Figure 2, non-use maps are shown for illustrative purpose only.
For those 24 post-stroke participants enrolled for the study overall, sBART was 0.28 ± 0.04, and cBART was 0.41 ± 0.03. The nuBART was significantly greater than zero (0.17 ± 0.04, P < 0.001, One Sample Wilcoxon Signed Ranks Test), although not normally distributed (P <0. 01 Shapiro-Wilk test).
There were however large differences in use, performance, and non-use across participants post-stroke. Eight participants out of 24, 2 with left- and 6 with right-hemiplegia, exhibited no arm use, with sBART = 0. Three of these participants showed maximal performance cBART – these three participants thus exhibited maximal non-use with BART. The other 16 participants use their arm to some extent, as sBART was positive (0.42 ± 0.03, P < .001, Mann-Whitney). Eight of these participants, all with left hemiplegia, exhibited non-disabled-like patterns of use (sBART 0.4 for left hemiplegia, 0.6 for right hemiplegia).
Thirteen participants out of 24, 6 with left- and 7 with right-hemiplegia, had non-disabled-like performance, as measured by cBART. Among the other eleven participants, cBART was 0.26 ± 0.03 for the 5 left hemiplegia participants and 0.40 ± 0.08 for the 6 right hemiplegia participants (note that because of the masking procedure, average maximum cBART for left hemiplegia was 0.4 and for right hemiplegia was 0.6, see above). We verified that these eleven participants with lower performance had a lower coordination subscale of the FM-UE (FM_cor) (median FM_cor of 3, range 2–5) than the 13 participants with non-disabled-like performance (median FM_cor of 5, range 4–6, P < .05, Mann-Whitney).
Eleven participants out of 24, 9 with left-, and 2 with right-hemiplegia showed no non-use, with nuBART = 0. The thirteen participants with positive ‘non-use’ had a median nuBART = 0.235 (different from 0, P = .0001, Mann-Whitney). Most participants with right-hemiplegia exhibited at least some degree of non-use (2 out of 13), in contrast to participants with left-hemiplegia for whom only 2 out of 11 exhibited greater than zero non-use.
Finally, there were no significant correlations between nuBART with months since stroke (P > .4), and age (P > .5).
BART Test-retest Reliability
For the 19 participants post-stroke who participated in the reliability study, sBART was 0.25 ± 0.04 for the first session, 0.28 ± 0.05 for the second session and 0.29 ± 0.04 for the third session. sBART, cBART, and nuBART had good test-retest reliability across the three sessions: sBART intraclass correlation coefficient (ICC) was 0.840, (P < .0001), cBART ICC was 0.807, (P < .0001), and nuBART ICC was 0.786, (P < .0001; note however that these ICC results should be considered with caution because sBART and nuBART were not distributed normally). The correlations of sBART, cBART, and nuBART between sessions 1 and 2 were good (sBART: r = 0.668, P = .002, Spearman correlation; cBART: r = 0.803, P <.0001, Pearson correlation; nuBART: r = 0.711, P = .001, Spearman correlation), and increased between session 2 and 3 (sBART: r = 0.854, P < 0.0001, Spearman correlation; cBART: r = 0.883, P < .0001, Pearson correlation; nuBART: r = 0.950, P < .0001, Spearman correlation). This demonstrates excellent reliability of BART between session 2 and 3, but lower reliability between session 1 and 2. As a result, we considered the first session as a familiarization session. In the validity study, we therefore only analyzed the data from session 2.
Unlike non-disabled participants who could always reach to all the targets within 1.2 sec, reach success in the sBART condition was lower for participants with stroke, although success rates improved somewhat over the sessions (first session success rate = 87.26 ± 2.64%, second session 91.97 ± 1.96% and third session, 91.02 ± 2.19%. repeated ANOVA, P < .037). Success rates were greater in session 2 than in session 1 (paired t-test P = .012), but not between session 2 and 3 (paired t-test P > 0.5).
External Validity of BART use sBART, performance cBART, and non-use nuBART
Here, we validate sBART, cBART, and nuBART with equivalent AAUT QOM measures for 15 participants who participated in the validity study, using BART measurements from the second session. Correlation between sBART and sAAUT QOM was good (r = 0.679, P = .005, Spearman). Correlation between cBART and cAAUT QOM just reached significance (cAAUT QOM r = 0.515, P = .05, Spearman). Finally, correlation between nuBART and nuAAUT was good (r = 0.683, P = .005, Spearman). Figure 5 shows nuAAUT as a function of nuBART. Note that nuBART was zero for six participants and very close to zero (0.008) for another participant.
Figure 5.
External validity of nuBART shown by plotting nuAAUT as a function of nuBART for 15 participants post-stroke in the validity study (correlation between nuBART and nuAAUT r = 0.683, P = .005, Spearman).
DISCUSSION
The present study makes three important and novel contributions. First, we have, to our knowledge, for the first time directly quantified paretic arm non-use in individuals with chronic stroke. For this purpose, we subtracted average use obtained in the spontaneous use condition of the AAUT QOM from average performance obtained in the constrained use condition of the AAUT QOM. Second, we have developed a novel laboratory-based measurement tool, the Bilateral Arm Reaching Test (BART), to measure arm non-use objectively. BART is simple to administrate, requires minimal instructions to the participant and personnel training, and is objective. Although we have developed and studied BART especially for individuals post-stroke, we believe that BART can reliably quantify arm use and performance in participants with other (lateralized) neurological conditions such as Parkinson’s disease, Cerebral Palsy, focal dystonia, or even non-neurological conditions such as scapular pain. Third, we showed that non-use measured with BART has excellent test-retest reliability and good external validity with non-use measured with the AAUT QOM.
We found no correlation between either measure of non-use, nuAAUT and nuBART, with time since stroke and age. However, we found with BART that non-use depends on the arm affected. Most participants with affected right (dominant) arm exhibited non-use; in contrast, most left arm participants did not exhibit any non-use. This last result is in line with previously reported differences in use between affected left and right hand18. It also indirectly support the learned non-use hypothesis, because our (right-handed) left hemiplegic participants presumably have few opportunities to experience negative consequences following use of their paretic arm in daily activities. This result needs to be independently reproduced however because we found no such difference between arms with the nuAAUT.
As a measure of arm use, BART has several advantages compared to existing instruments such as the MAL and AAUT. First, it provides an objective and quantitative measure of voluntary paretic arm use in daily life post stroke. In contrast, MAL scoring is based on participants’ recall and is subjective. Second, BART exhibits excellent reliability for repeated measures, making it ideal for examining the effectiveness of rehabilitation on non-use. In contrast, the AAUT, is best used only once, or at most only infrequently at it was in the EXCITE trial 19, because the spontaneous use condition must be covert. Third, BART is simple and timesaving. Unlike the MAL, which takes at least an hour to administer, a single BART session takes less than 15 minutes.
There are nonetheless several limitations of the present study. First, the amount of non-use quantified with the AAUT may be due at least in part to a learning effect in AAUT since the constrained use condition is given soon after the spontaneous use condition. We indeed found a small improvement on the tasks that the participants could perform (score > 0) in the constrained AAUT condition (mean score 3.61 ± 0.11 on these tasks) compared to the spontaneous AAUT condition (3.26 ± 0.13 on these tasks; Paired t-test: P < 0.0001). Thus, a small learning effect cannot be excluded, however, this value (0.35 in average) is small compared to the average nuAAUT = 1.67 in our participants. Second, because of the difficulty in recruiting a large group of left-hand dominant participants before stroke, we only developed BART for participants who were right hand dominant before stroke. Third, we did not control for compensatory arm movements such as excessive shoulder elevation and abduction during reaching20, and we observed that several participants were using such compensatory strategies. Preventing such movements may further reduce arm use. Fourth, an important limitation of our current BART system is the time allowed to complete the reaching movements, which was set to a single value of 1.2 sec. In our beta version of BART, we noticed that without a time limit in the constrained condition, participants post-stroke reached almost all targets all the times. Because data from the beta version revealed that median movement time was 845ms, we hypothesized that 1.2 second could discriminate performance of post-stroke individuals with mild to moderate impairments from non-disabled controls. However, with this 1.2 sec time limit, 13 out of 24 participants post-stroke still showed control-like performance as measured by cBART. This suggests that 1.2 sec is too long for most of these 13 participants. In contrast, eight participants chose not to use the paretic arm at all in the second session with BART, as measured by sBART. This suggests that 1.2 sec is too short for at least of sub-group of our participants. Thus, in future work, we will need to parameterize BART to detect non-use across a large proportion of patients with variable initial characteristics. Fifth, the pointing task in BART mimics only one aspect of upper extremity use (e.g. reaching), but does not include other actions that might be part of daily use, such as stabilizing, supporting, grasping, tapping, etc. This may be the reason why we found a somewhat lower than expected correlation between the AAUT and BART in the spontaneous condition. In future work, improved systems to automatically assess arm and hand use could, for instance, present tools that allow for grasping at different spatial locations. Task-based rehabilitation robot, i.e.21,22, could be modified for this purpose. Finally, while administering BART requires very little expertise from the tester (unlike the MAL and the AAUT), the hardware (mini-bird magnetic sensors and projector mounted above table) and software (Matlab for data analysis) needed in the current implementation makes it a research tool only. Cheaper versions will need to be developed for use in the clinic. In addition, the current method to compute use and non-use is somewhat complicated, notably with the 2nd order logistic model fit. While we could have used a simpler method without model fitting and smoothing, and only based on counts at each target, the proposed method leads the way for the spatial assessment and treatment of non-use. In the current manuscript, to validate BART, we computed an overall non-use value. However, inspection of the maps of figures 2 and 4 show that we can compute target-by-target, performance, use, and non-use values. Thus, as an example of a strategy to reduce non-use, reaching movements with the hemiparetic arm to targets in areas of greater non-use could be rewarded to a greater extent than reaching to other targets.
Besides the Sterr et al. study8 discussed above, we are aware of only two other studies that have attempted to measure use and non-use in individuals post-stroke. First, Johnson et al.23 evaluated non-use in five participants post-stroke by measuring errors during a steering task in affected, unaffected, and bimanual arms conditions. Second, Brown 24 measured arm use (but not non-use) in 13 participants with stroke with a device similar to our BART device with 5 actual reaching tasks involving proximal and/or distal hand movements.
In addition to its usefulness as a measurement tool to capture use and non-use of the paretic limb after stroke, BART may be useful to elaborate our understanding of motor control and decision making underlying reaching choice, and notably the factors that affect changes in these choices, including changes in performance due to motor therapy. We have previously proposed and have begun to test an “optimal therapy threshold” hypothesis,25–27 according to which a minimum amount of therapy is needed to reach a minimum arm use level such that use and performance continue to improve following therapy. BART can be helpful in determining such a threshold with repeated measurements of use and non-use before, during, and after therapy.
Acknowledgments
We thank Dr. Young Geun Choi for help with computer programming, Dr. James Gordon for his inputs during development of the task, and Neerav Parikh for help with data collection. This work was in part supported by NIH grants P20 RR020700-01 and R03 HD050591-02 and R01 HD065438-01A2. CEH is in part supported by the WCU program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R32-10142). RO is in part supported by the Funding Program for Next Generation World-Leading Researchers, Japan and SRPBS, MEXT, Japan.
Appendix
Figure A.
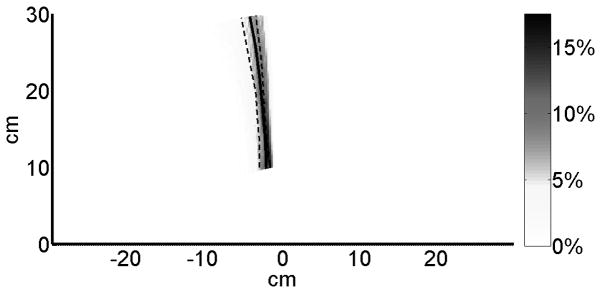
Variability in average indifference lines for non-disabled participants. We obtained a distribution of indifference lines with 1000 bootstrapping samples (draws with replacements) of the non-disabled participants. The original indifference line is shown in solid black and the lower-quartile (25% percentile of decision lines) and upper quartile (75% percentile of decision lines) are shown in dashed black. Yellow color represents higher probabilities of indifference lines.
Figure B.
Relation between residual recovery (FM) and the magnitude of non-use (nuAAUT) in 15 participants with stroke in the validity study. Correlation was not significant (P = 0.629, Pearson).
References
- 1.Mayo NE, Wood-Dauphinee S, Cote R, et al. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83:1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 2.Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 4.Andrew K, Stewart J. Stroke recovery: he can but does he? Rheumatol Rehabil. 1979;18:43–48. doi: 10.1093/rheumatology/18.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Sunderland A, Tuke A. Neuroplasticity, learning and recovery after stroke: a critical evaluation of constraint-induced therapy. Neuropsychol Rehabil. 2005;15:81–96. doi: 10.1080/09602010443000047. [DOI] [PubMed] [Google Scholar]
- 6.Taub E, Uswatte G, Mark VW, et al. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- 7.Taub E, Uswatte G. Constraint-induced movement therapy: bridging from the primate laboratory to the stroke rehabilitation laboratory. J Rehabil Med. 2003:34–40. doi: 10.1080/16501960310010124. [DOI] [PubMed] [Google Scholar]
- 8.Sterr A, Freivogel S, Schmalohr D. Neurobehavioral aspects of recovery: assessment of the learned nonuse phenomenon in hemiparetic adolescents. Arch Phys Med Rehabil. 2002;83:1726–1731. doi: 10.1053/apmr.2002.35660. [DOI] [PubMed] [Google Scholar]
- 9.Taub E, Crago JE, Burgio LD, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61:281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uswatte G, Taub E, Morris D, et al. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 11.Uswatte G, Taub E. Implications of the learned nonuse formulation for measuring rehabilitation outcomes: lessons from constraintinduced movement therapy. Rehabil Psychol. 2005;50:34–42. [Google Scholar]
- 12.Wade DT. Measurement in Neurological Rehabilitation. Oxford Medical Publications; 1992. [PubMed] [Google Scholar]
- 13.Chen S, Wolf SL, Zhang Q, et al. Minimal Detectable Change of the Actual Amount of Use Test and the Motor Activity Log: The EXCITE Trial. Neurorehabil Neural Repair. 2012 doi: 10.1177/1545968311425048. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fullerton KJ, McSherry D, Stout RW. Albert’s test: a neglected test of perceptual neglect. Lancet. 1986;1:430–432. doi: 10.1016/s0140-6736(86)92381-0. [DOI] [PubMed] [Google Scholar]
- 15.Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 16.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 17.Roby-Brami A, Feydy A, Combeaud M, et al. Motor compensation and recovery for reaching in stroke patients. Acta Neurol Scand. 2003;107:369–381. doi: 10.1034/j.1600-0404.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 18.Rinehart JK, Singleton RD, Adair JC, et al. Arm use after left or right hemiparesis is influenced by hand preference. Stroke. 2009;40:545–550. doi: 10.1161/STROKEAHA.108.528497. [DOI] [PubMed] [Google Scholar]
- 19.Winstein CJ, Miller JP, Blanton S, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–152. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 20.Steenbergen B, Van Thiel E, Hulstijn W, et al. The coordination of reaching and grasping in spastic hemiparesis. Human Movement Science. 2000;19:75–105. [Google Scholar]
- 21.Choi Y, Gordon J, Kim D, et al. An Adaptive Automated Robotic Task-Practice System for Rehabilitation of Arm Functions After Stroke. IEEE Transactions on Robotics. 2009;25:556–568. [Google Scholar]
- 22.Choi Y, Gordon J, Park H, et al. Feasibility of the adaptive and automatic presentation of tasks (ADAPT) system for rehabilitation of upper extremity function post-stroke. J Neuroeng Rehabil. 2011;8:42. doi: 10.1186/1743-0003-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson M, Paranjape R, Strachota E, et al. Quantifying learned non-use after stroke using unilateral and bilateral steering tasks. IEEE Int Conf Rehabil Robot. 2011;2011:1–7. doi: 10.1109/ICORR.2011.5975457. [DOI] [PubMed] [Google Scholar]
- 24.Brown E. Thesis. Department of Kinesiology, University of Waterloo; 2011. Hand preference after stroke: The development and initial evaluation of a new performance-based measure. [Google Scholar]
- 25.Han CE, Arbib MA, Schweighofer N. Stroke rehabilitation reaches a threshold. PLoS Comput Biol. 2008;4:e1000133. doi: 10.1371/journal.pcbi.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweighofer N, Han CE, Wolf SL, et al. A Functional Threshold for Long-Term Use of Hand and Arm Function Can Be Determined: Predictions From a Computational Model and Supporting Data From the Extremity Constraint-Induced Therapy Evaluation (EXCITE) Trial. Phys Ther. 2009;89:1327–1336. doi: 10.2522/ptj.20080402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hidaka Y, Han CE, Wolf SL, et al. Use it and improve it or lose it: Interactions between arm function and use in humans post-stroke. PLoS Comput Biol. 2012 doi: 10.1371/journal.pcbi.1002343. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]