Abstract
This invited review covers research areas of central importance for orthopaedic and maxillofacial bone tissue repair, including normal fracture healing and healing problems, biomaterial scaffolds for tissue engineering, mesenchymal and foetal stem cells, effects of sex steroids on mesenchymal stem cells, use of platelet-rich plasma for tissue repair, osteogenesis and its molecular markers. A variety of cells in addition to stem cells, as well as advances in materials science to meet specific requirements for bone and soft tissue regeneration by addition of bioactive molecules, are discussed.
Keywords: bone regeneration, stem cells, biomaterials, polymers, regenerative medicine
Introduction
New approaches to clinical problems based on translational medicine start with basic research and progress ‘hand-in-hand’ with clinical observations. Scientists are increasingly aware that this ‘bench-to-bedside’ approach to translational research is really a two-way street which can strengthen and accelerate critical points of the research process.
Bone defects due to trauma and to pathological and physiological bone resorption represent a major challenge and are a global health problem. The need for bone regeneration in cranial, oral and maxillo-facial and orthopaedic surgery is one of the central clinical issues in regenerative and rehabilitation medicine. It is difficult to convey the enormous social and psychological handicap of persons with bone defects and the significant reduction in their quality of life. In addition to trauma, bone healing problems may be related to age, sex and infection as exemplified by diagnoses such as osteoporosis, osteopenia and severe dental problems related to loss of teeth. The aim of this review is to describe the current state of the art in our understanding of bone healing and bone regeneration.
Bone fracture healing and healing problems
Bone repair after fracture is a special process where sequential cellular and molecular events take place to generate new bone, rather than a fibrous scar like other connective tissues. The precise series of ordered events required to produce new bone are modulated by systemic and local factors, and disruption of these orderly events may cause healing problems. Thus, a clear understanding of the sequence of events and their regulation is needed to decide when and how an intervention is required to promote healing and to avoid complications.
Since early histological descriptions of fracture healing in man [1], the general pattern of indirect fracture healing, based on endochondral ossification, has included the chronological phases of haematoma, inflammation, angiogenesis, chondrogenesis to osteogenesis and finally bone remodelling [2]. Direct healing based on membranous ossification, with no periosteal reaction or visible callus formation, is seldom seen. The well-established characteristics of the above mentioned phases require different processes of cell migration and differentiation, extracellular matrix formation and organization towards calcification, as well as both local and systemic modulation. Apart from the classical histological phases of fracture healing, much remains to be understood about the regulation of these processes both at the molecular and the cellular level.
Formation of a haematoma related to blood vessel damage is accompanied by an inflammatory response [3], where pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-6, and particularly tumour necrosis factor-α, initiate the bone healing cascade and push it towards endochondral bone formation and remodelling [4]. Secondarily, apart from ischemia, growth and differentiation factors and particularly the transforming growth factor-β (TGF-β) superfamily, including bone morphogenetic protein (BMPs), as well as platelet-derived growth factors (PDGF), fibroblast growth factors (FGFs) and insulin-like growth factors (IGFs), orchestrate crucial events for chondro-osteogenesis, including chemotaxis, mesenchymal and osteoprogenitor cell proliferation and differentiation, and extracellular matrix ossification [5]. Finally, angiogenesis, a key aspect of fracture healing, is also regulated at the molecular level. An angiopoietin pathway has been described in the early stages of the healing process [6], as has a vascular endothelial growth factor (VEGF)-dependent pathway related to endochondral bone formation, where BMPs stimulate the expression of VEGF by osteoblasts and osteoblast-like cells [7]. Inhibitory molecules are also needed to control growth factors (GFs), and various BMP antagonists [8] are released into the extracellular compartment (noggin, sclerostatin, follistatin, etc.). Other inhibitory mechanisms include receptor inhibition of some members of the TGF-β superfamily that has been related to a pseudo-receptor defined as BAMBI (BMP and activin membrane bound inhibitor) [9], and intracellular inhibition by the activation of I-Smads [10, 11], among other mechanisms. Many aspects of this bone healing cascade of molecules, cells and events have been identified, but the complex interactions and processes are still only partially understood.
Interestingly, adult bone healing reproduces normal development during embryogenesis, except for the associated inflammation, despite the limited number of osteoprogenitor cells available in the adult, and the strong influence of external and internal mechanics. This fact offers researchers an invaluable clue towards biological events in the developmental processes of skeletogenesis [12]. Bone formation during embryogenesis is initiated by mesenchymal stem cells (MSC) aggregation and condensation, which then progresses to endochondral ossification through cartilage formation, or to membranous ossification through osteoblast differentiation. Although MSC are scarce in adult bone, both committed osteoprogenitor cells from the periostium and undifferentiated multipotent MSC from bone marrow are involved in callus formation, which is important in the structural progress of fracture healing. In addition to the classical triad of bone healing (cells, extracellular matrix and osteoinductive factors), a fourth major factor (the biomechanical properties of the callus) has been stressed recently [13, 14]. Mechanical influences on biological processes, known as mechanobiology, significantly affect all phases of bone formation. Not only may major external forces disrupt the healing process, but mechanical loading influences on endochondral ossification are also important as compression enhances bone apposition [13], empirically defined since the 19th century as Wolff’s law. However, earlier phases of osteogenesis show increased cell proliferation if cyclic motion with associated shear stresses occurs, although intramembranous ossification may be permitted in areas with low stress and strain. Mechanical signalling at the cellular level may modulate molecular changes in cytoskeleton, integrins and ion channel activities with consequences to the differentiation and gene expression of cells involved in the healing process. Transduction mechanisms range from direct mechanical stimulation of cells [15, 16], to fluid shear stresses and various matrix effects that indirectly affect cells [17]. Many of these experimental studies were developed after clinical studies showed the beneficial influence of interfragmentary motion (of about 0.6 mm) in the early stages of the healing process [18], and the role of mechanical stimulus in general as an influence on the rate of healing.
Despite this well developed natural mechanism of fracture healing, alteration of local and systemic factors may lead to impaired healing. Non-union (or pseudoarthrosis), due to the false articulation generated in the area of the unhealed fracture, and delayed union, are the main problems in bone repair and are significant health care issues. Systemic factors such as use of non-steroidal anti-inflammatory drugs (which inhibit cyclooxygenase and therefore prostaglandins required in the inflammation phase), age (with decreased expression of mediators, hormonal changes, impaired osteoblast function), or smoking [19], and local factors both influence fracture healing. Mechanical stability is required for callus formation, and surgical techniques focusing on stability greatly improve the chances of fracture healing, as excess motion impairs it. Angiogenesis and appropriate tissue differentiation, key factors required for callus formation, are also improved with fracture stability [20]. However, the rate of differentiation of osteoprogenitors is modulated by the mechanical environment, thus osteogenic differentiation may be promoted or impaired by movement. When tensile strains occur in the interfragmentary gap, they lead to ‘cleavage’ of the callus, and hydrostatic pressure and tensile strain at the ends of both fracture fragments may cause fibrocartilage formation, leading to pseudoarthrosis instead of bone healing, as modelled by Loboa et al.[21]. Despite all the advances in surgical treatment of fractures, the management of these healing problems is a challenge that would benefit significantly from a deeper knowledge of normal bone healing and the potential therapeutics derived from this knowledge.
Biomaterial scaffolds and tissue engineering in bone formation
Rebuilding human anatomy has long been the goal of reconstructive medicine. Currently, various strategies are in use to stimulate healing of bone defects. Despite the fact that material science technology has resulted in clear improvements in the field of bone regeneration, no material fulfilling all requirements of a bone substitute has been developed, and dealing with large bone defects/ injuries still represents a major challenge.
Tissue engineering has emerged as an interdisciplinary field with tremendous potential to develop and use new knowledge-based materials that can be used in the fast-growing market of advanced regenerative medicine and dentistry. In this context biomaterials play a crucial role in the development of tissue-engineered organs. Degradable biomaterials can be used to fabricate a scaffold which is well vascularized, integrated with the host skeleton to place cells in close proximity with each other, and to stimulate the formation of new living bone, connected to the body’s biological systems. The fabrication of materials to provide appropriate scaffolding conducive to cell adhesion and maintenance of cell function is crucial.
Innovation in the structures of polymers, cell adaptation and the mechanics of fundamental molecular biology offer the possibility of developing biodegradable substances that may be injected or shaped in tissues that will allow restoration of form and function while the added material will disappear through programmed decomposition. By controlling the hydrophilicity and the surface nano/microstructure, the degradable polymers can be good candidates as scaffolds in tissue engineering and it may also be possible to encourage and optimize the growth of cells within the artificial structure. Thus cell-biomaterial based bone tissue engineering is a promising concept for reconstruction/regeneration of bone defects. However, much work remains before this approach can be routinely applied in the clinical setting.
Bone is one of the hardest tissues of the human body and is the main constituent of the skeleton; it supports fleshy structures, protects vital organs such as those contained in the cranial and thoracic cavities and contains the bone marrow, where the blood cells are formed. Furthermore, bones form a system of levers that multiply the forces generated during skeletal muscle contraction, transforming them into bodily movements such as walking and chewing. Bone is also highly vascularized and serves as a mineral reservoir for homeostasis of the calcium blood level [22].
Bone tissue develops either by intramembranous or endochondral ossification and the embryonic development starts as early as the fourth to sixth week. Membranous or direct ossification occurs in parts of the skull or craniofacial skeleton, clavicles and scapulae when mesenchymal cells proliferate and gradually change their shape, which occurs within a layer of connective tissue. Endochondral or indirect ossification takes place within a cartilaginous model in the long bones and the rest of the skeleton, which in addition to the growth of the length also increase in width via appositional bone growth. This cartilage model is gradually destroyed and replaced by bone formed by incoming cells from adjacent periosteal connective tissues. In both processes the bone tissue appears first as primary or immature tissue and grows rapidly from the first postnatal year to the end of adolescence. In the adult skeleton bone tissue is either arranged in a trabecular pattern or in a compact pattern [23]. A tentative third mode of bone formation has been described in distraction osteogenesis studies. Transchondroid ossification produces bone via chondroid bone. It is speculated that hypertrophic and/or early stage chondrocytes undergo differentiation into osteoblast-like cells, which lay done bone without capillary invasion.
As the development of bone is very complicated, birth bone defects can occur in any bone although the bones of the skull and face, spine, hips, legs and feet are affected most often resulting in abnormal appearance and function [24]. The most common defects of the skull and face are cleft lip, cleft palate and jaw deformities. Most of these defects can be repaired surgically. Often, the surgery is complex and involves reconstructing deformed or absent body parts [25–27].
Bone defects due to trauma, and to pathological and physiological bone resorption still represent a major challenge. The need for bone regeneration in cranial, oral maxillo-facial and orthopaedic surgery is a major clinical issue. Most fractures heal well using standard treatments, mostly without any scar tissue formation. However, bone defects due to tumour resections, unhealed fractures, major trauma and bone resorption of edentulous jaws or of tooth-supporting alveolar bone are candidates for bone reconstruction and cause significant handicap without adequate treatment. In neurosurgery, spinal fusion is performed in many patients suffering from intervertebral disc degeneration, and this procedure also requires bone grafting.
Plastic and reconstructive surgeons conventionally fill and repair bony defects using autologous bone transplantation as the gold standard [28]. Current clinical treatments to repair bony defects can be problematic and often yield poor healing due to the anatomy and physiology of bone tissue, as well as the limitations of knowledge of the process. Because of the major problems associated with autograft transplantation, such as insufficient tissue, donor-site injury and surgical risks as such as bleeding, infection and chronic pain, alternative approaches are needed. Skeletal defects may require volumes of bone often not available. The donor site for bone harvesting is usually the iliac crest, which requires a second surgical intervention and has some surgical morbidity. Allografts can be used, especially in prosthetic reconstruction, but may still not solve many problems of bone deficiency.
Because of the disadvantages associated with both autografts and allografts, scientists have long searched for biocompatible materials that could be substituted for the transplanted bone. Although most of the available synthetic bone substitutes have some of the positive properties of an autograft, to date no single synthetic material offers all the benefits of the patient’s own bone. For instance, calcium phosphate bioceramics do not possess sufficient osteogenic properties to allow reconstruction of large defects [29, 30]. Thus despite the commercial availability of many bone substitute materials for clinical application, the use of alloplastic materials and autologous bone grafting remains the preferred approach to treatment of bone defects [31]. Thus, in bone tissue engineering, it may be of importance to primarily specify the area and the function for regeneration or implantation of bone. In those cases, orthopaedics, neurosurgery and maxillofacial surgery and implant dentistry may have different treatment modalities and may ask for materials to be used either in a solid or an injectable phase for the different targets.
Bone tissue engineering
In view of the above limitations and the increasing demand for bone grafting procedures, surgeons are looking for a better approach. Tissue engineering combines bone marrow cells or MSC, synthetic scaffolds and molecular signals (growth or differentiating factors) to form hybrid constructs. In a classical approach, bone tissue engineering consists of harvesting bone marrow from a patient, isolating MSC by adherence to tissue culture plastic, expanding and differentiating MSC in culture and then seeding them onto a suitable synthetic scaffold prior to implantation into the same patient [32]. It has been shown that from a small volume (0.1–3 ml) aspirate, alveolar bone marrow stromal cells (BMSC) expand by 70%[33].
Although this raises few ethical issues, harvesting of cells from bone marrow is still an invasive procedure, and in addition stem cell numbers may decrease significantly with the age of the individual. The search for more readily accessible sources of pluripotent cells has led to investigation of other tissues, including mobilized peripheral blood, umbilical cord blood and more recently, periodontal ligament and deciduous and permanent teeth. Dental pulp tissue is also a readily accessible source of pulp-derived MSC (PDSC) and can be easily isolated. PDSC express the endothelial and smooth muscle marker STRO-1 [34] and display a pericyte phenotype, with expression of the pericyte-associated antigen 3G5 [34]. It is therefore assumed, but not confirmed, that the perivascular region in the pulp is the niche for PDSC and that pericytes give rise to dental pulp stem cells. Isolated dental pulp stem cells have been shown to be plastic-adherent and express the MSC markers STRO-1, CD90, CD29, CD44, CD166, CD105, CD106, CD146, CD13 and are also negative for CD14 and CD34 [35, 36]. In vitro, PDSC are capable of self-renewal, display plasticity and mutilineage potential (adipocytes, chondrocytes, osteoblasts, neural cell progenitors and myotubes) and can therefore be defined as stem cells [37].
To date, these approaches have been evaluated in both animal experiments and clinical studies involving low numbers of patients, with inconclusive outcome and low bone regeneration efficacy [38–40]. Overall, the clinical outcome have been disappointing, especially with respect to restoration of large bone defects. The limited success of clinical studies to date may be attributable to a number of issues, related primarily to translation of laboratory procedures to clinical application. Translation of laboratory processes into clinically effective, reproducible, safe, economically viable and competitive products is generally acknowledged to be a complicated and challenging phase in the development of new clinical techniques [32]. In the context of bone tissue engineering, the difficulties reflect the multidisciplinary nature of the concept.
Biomaterial scaffolds
Bone tissue engineering utilizes scaffolds to deliver biofactors including cells, genes and proteins to generate bone and assessment of blood vessel formation and maturation into the construct. The scaffold itself must fulfil three primary functions to ensure successful treatment of bone defects. First, the scaffold must provide the correct anatomic geometry to define and maintain the space for tissue regeneration. Second, the scaffold must provide temporary mechanical load bearing within the tissue defect and third, the scaffold should enhance the regenerative capability of the chosen biofactor; a balance to a regenerative capacity. For load-bearing purposes, achieving stiffness and strength equivalent to bone tissue requires minimally porous scaffolds. Conversely, enhanced delivery of biofactors requires more highly connected porous scaffolds to allow cell migration, vascularization and connective tissue formation within scaffolds. In clinical applications, it is obvious that scaffolds, matching complex anatomic defects require computational methods and control of anatomic shape and architecture.
Many different materials have been proposed as synthetic bone substitutes. Hydroxyapatite (HA), Ca10(PO4)6(OH)2, is regarded as one of the most bioactive bone substitute ceramics because of its superior osteoconductivity. This applies to HA of synthetic or biological origin, β-tricalcium phosphate (β-TCP) and biphasic calcium phosphate. Calcium phosphate biomaterials are currently used, for example, for bone repair, substitution, augmentation and regeneration. As synthetic bone substitutes, the main advantages of the ceramics are excellent biocompatibility and better stability than polymers and metals in the living body. Synthetic octacalcium phosphate has been shown to be a good precursor of biological apatite in bone and tooth and has shown better bone regenerative and biodegradable characteristics than other calcium phosphate bone substitute materials [41]. A disadvantage is that it is sometimes difficult to achieve close apposition of the material to the surrounding bone, especially in complicated defects and this is essential for complete and successful bone regeneration. Another general disadvantage of the ceramics is inherent brittleness. This can be overcome by blending ceramics with, for example, polyesters to form a composite that combines the mechanical strength and osteoconductivity of calcium phosphates with the high affinity for cells and good biodegradability of polyesters.
Development of composite materials which consist of a biodegradable matrix incorporating bioactive rigid particles combines the reinforcement activity provided by HA-based particles with the tailored degradation kinetics of resorbable polymers [42]. Many studies have examined the use of bioceramic particles such as silica, HA or other calcium phosphates in combination with biodegradable polymers (i.e. poly(ɛ-caprolactone) [PCL], poly(lactide) [PLA]) to produce bone substitutes (Fig. 1).
Fig 1.
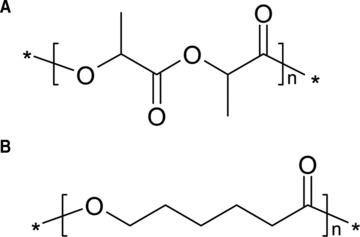
Molecular structure of (A) PLA and (B) PLC.
The positive results of studies using these systems might be attributable to structural similarity to the mineral phase of bone, and osteoconductive and bone-binding properties [43–45]. For example, compared to PCL scaffolds, composite scaffolds containing PCL and TCP, designed for load-bearing applications, exhibit improved hydrophilicity, compressive modulus and strength. Human alveolar osteoblasts, grown on the composite, exhibit higher seeding efficiency, better proliferation and earlier expression of bone matrix related proteins than cells grown on PCL [46]. The addition of calcium phosphate particles to this system generates needle-like, Ca-deficient HA crystals, which improves the compressive strength of the construct. This material thus meets many of the major requirements of scaffolding material for bone-tissue engineering [47].
Mechanical properties can also be improved by cross-linking. For example, the mechanical properties of cross-linked poly(propylene fumarate) (Fig. 2) and CaSO4/β-TCP) composite are similar to those of cancellous bone substitutes, with compressive strengths of 5 MPa and a compressive modulus of 50 MPa during degradation [48]. Composite bone substitutes are also available in injectable form, an obvious advantage in the clinical setting. For example, HA-atelocollagen composites have proved suitable precursors for osseous regeneration [49]. However, despite many positive outcome, the results of most studies to date show that the incorporation of a ceramic phase improves bioactivity, but the mechanical properties of the composites are inadequate [50]. Another observed disadvantage is that the HA particles within a PCL matrix tend to migrate to the surface and form clusters, creating inhomogeneous scaffolds [51].
Fig 2.
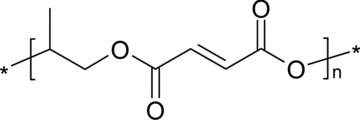
Molecular structure of poly(propylene fumarate).
Another fundamental component of bone tissue engineering is the addition of GFs. BMP-2, a member of a class of proteins and TGF-βs are regarded as the most important regulators of bone repair and regeneration. Addition of GFs to the scaffolds is intended to create an osteogenic microenvironment and various strategies are commonly used for controlled delivery of GFs, such as hydrogels, direct loading, electrostatic interaction and covalent binding.
Synthetic scaffolds
There are a wide variety of synthetic polymers that have been investigated for biomaterial and tissue engineering applications. Scaffolds comprised solely of degradable polymers have also been successfully used to aid bone regeneration in vivo[52]. The biggest advantage of polymers is that properties such as hydrophilicity, degradation rate and mechanical properties can be optimized. These properties can be manipulated in various ways, most commonly by copolymerization or the introduction of different architecture. Control of the rate and extent of degradability of a polymeric biomaterial is critical. Many factors influence degradability, such as chemical structure, copolymer composition, architecture, molecular weight, morphology, surface area and medium character.
Among the various families of degradable polymers, aliphatic polyesters are prominent, because hydrolytic and/or enzymatic chain cleavage yields ω-hydroxyacids. Aliphatic polyesters can be synthesized through either polycondensation of functional acids and alcohols, or ring-opening polymerization of cyclic esters. PLLA belongs to the group of poly(α-hydroxy acids) but PLLA is of limited application because it is hydrophobic, with no reactive-chain group. It is difficult to chemically attach active molecules like drugs and recognition agents onto these and other polyesters: the high hydrophobicity sometimes interferes with the intended medical application.
A scaffold made of resorbable material is intended primarily as a temporary support during the regeneration phase. Ideally, once the damaged tissue has been sufficiently regenerated, the scaffolding should start to degrade and lose its mechanical properties. The material needs good mechanical properties to be able to withstand complex forces and for this reason crystalline materials are often used. A potential disadvantage of crystalline materials is the risk of unfavourable tissue responses: amorphous polymers are preferable, but their mechanical properties may be inadequate.
In this context, biodegradable block copolymers show promise as biomaterials because their amphiphilic behaviour, mechanical and physical properties can be manipulated by adjusting the ratio of the constituting block or adding new blocks of desired properties. Research in this area dates back many years and there are numerous publications about the properties of block copolymers. Poly(lactide-co-glycolide) has shown good results in several research studies; however, its clinical utility is limited due to its relatively poor mechanical properties. Copolymerization of PLLA and poly (ethylene glycol) offers several means of modifying the properties to optimize them for medical application. It has been shown that adjusting the block lengths of both components can modulate the crystallinity and that hydrophilicity is better that that of PLA homopolymers [53]. Another interesting system is copolymerization with dioxanone. Poly(1,5-dioxepan-2-one) (Fig. 3) is an amorphous polymer: the soft, amorphous phase provides elasticity and desirable degradation characteristics, whereas copolymerization with lactide gives a rigid crystalline phase, which acts as a physical cross-linker and therefore improves the mechanical properties. It has also been shown that the hydrophilicity of the polymer changes when different monomer compositions are used [54, 55]. In a series of experiments using poly(1,5-dioxepan-2-one) (DXO)-containing polymers different in vitro cell culture systems have indicated that these polymers are promising as scaffolds for bone tissue engineering [56–59].
Fig 3.
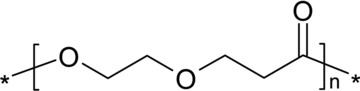
Molecular structure of poly(1,5-dioxepan-2-one) (DXO).
Copolymerization can also be used to solve one of disadvantages of many aliphatic polyesters, i.e. the absence of reactive sites. Multiple sites that allow selective attachment of substances such as bioactive molecules have been synthesized by copolymerization [60]. Synthetic bone substitutes, composite grafts and polymers all have enormous potential and optimal results can be achieved by careful design.
Micro- and nanostructural properties of scaffolds
Besides the choice of appropriate composition, both micro- and nanostructural properties of the scaffolding materials are of utmost importance. Surface properties, both chemical and topographical, significantly affect the potential for cellular adhesion and proliferation, determining not only cell survival, signalling, growth and reorganization but also cellular phenotype and gene expression profile. Moreover, scaffolds must have open pores that allow cell in-growth and even cell distribution throughout the porous structure. The porosity should also facilitate neovascularization from the surrounding host tissue. Furthermore, the scaffolds should have adequate microporosity in order to allow capillary in-growth. However, the degree of porosity influences other important properties of the scaffolds such as degradation characteristics and mechanical stability, which need to be considered in the context of the mechanical demands of the particular tissue to be replaced.
Conclusion
Despite major advances in material science technology, a material fulfilling all requirements of a bone and cartilage substitute has yet to be developed. For the repair of bone defects, tissue engineering should combine cells capable of osteogenic activity with an appropriate scaffolding material, in order to stimulate physiological bone regeneration and repair processes.
MSC derived from bone marrow are multipotential: depending on culture conditions, such cells have the potential to differentiate into many different cell types, including osteoblasts and chondrocytes. Thus, MSC comprise a readily available and abundant source of cells for tissue engineering applications. The lack of immunogenicity of MSC has opened up the potential of using these cells in tissue repair.
The goal of these strategies is to exploit the body’s natural ability to repair injured bone with new bone cells, and to remodel the new bone tissue in response to local stress factors. The primary function of an optimal biomaterial scaffold is to support the area undergoing reconstruction, providing adequate initial mechanical strength. It should also stimulate new bone formation in the region, and then gradually degrade without causing a pronounced inflammatory response, thus allowing the new bone to remodel and assume the mechanical support function.
Selection of the most appropriate scaffolding material is crucial in a tissue-engineered construct. Preferable to ceramics are biodegradable scaffolds based on polyesters and hydrogels, which can be chemically modified with a controlled degradation time and readily processed into three dimensional, porous structures, with desired pore morphology. Tissue engineering has thus emerged as an interdisciplinary field with tremendous potential to develop and apply new, knowledge-based materials in advanced regenerative medicine and dentistry. In this context, biomaterials play a crucial role in the development of tissue-engineered organs. Degradable biomaterials can be used to fabricate a three-dimensional construct or scaffold which is well vascularized, integrated with the host skeleton to maintain cells in close proximity to one another, and to stimulate the formation of new living bone, functioning as part of the body’s biological systems. Thus the fabrication of materials which provide appropriate scaffolding conducive to cell adhesion and maintenance of cell function is of fundamental importance to successful bone regeneration.
To fulfil the prerequisites of biocompatibility, as well as to meet the increasing demands of functional and tailor-made surfaces of the future, much research effort is focused on surface modification, especially the design and development of functional and nano-structured surfaces. Whenever a polymeric biomaterial is used, the surface chemistry and structure are of fundamental importance, especially in the early stages after implantation. Biological interactions between the device and the host tissue occur mainly on the surface of the scaffold. For this reason, there is a strong demand for viable techniques for covalent surface modification of polymers, in order to enhance surface wettability and thus circumvent an important limitation of current polymeric biomaterials: their hydrophobicity. Surface modification also offers the potential to functionalize the surface, so that bioactive moieties may be attached and the biocompatibility improved. Depending on the nature of the substrate, there are several different techniques by which the surfaces of both synthetic and natural materials can be covalently modified.
Mesenchymal stem cells and osteogenesis
Bone tissue
Bone tissue is composed of bone matrix and bone cells. Bone matrix is primarily built of type I collagen (90%), with the remaining 10% being composed of a large number of non-collagenous proteins (e.g. osteocalcin [OC], osteonectin, bone sialoproteins and various proteoglycans). Non-collagenous proteins participate in the process of matrix maturation, mineralization and may regulate the functional activity of bone cells. Two main types of bone cells have been identified. Osteoblasts (bone-forming cells) and osteoclasts (bone resorbing cells) that together with their precursor cells and associated cells (e.g. endothelial cells, nerve cells) are organized in specialized units called bone multicellular units (analogous to the organization of kidney cells into nephrons) [61]. The main function of the bone multicellular units in the adult skeleton is to mediate a bone ‘rejuvenation’ mechanism called ‘bone remodelling’ aimed at the maintenance of the integrity of the skeleton by removing old bone of high mineral density and high prevalence of fatigue micro-fractures through repetitive cycles of bone resorption and bone formation [61, 62]. During bone formation phase, the osteoblasts are recruited from MSC present in bone marrow [63]. On the other hand, osteoclasts are derived from haematopoietic stem cells through committed osteoclast progenitors that fuse to form mature multinucleated cells [64]. Understanding the mechanisms that control the differentiation of osteoblastic cells from MSC is thus of one of the fundamental areas of research of bone biology. The ability of MSC to differentiate into non-osteoblastic cells has been recognized recently and here we attempt to provide an update regarding the trans-differentiation potential of MSC with regard to the relationship between osteoblast and adipocyte differentiation.
Origin of osteoblasts
One current dogma of bone biology is that mature osteoblasts are differentiated from precursor cells present in the bone marrow. Based on the pioneer work of Friedenstein and coworkers [65, 66], it has been recognized that the non-hematopoietic compartment of bone marrow (known as bone marrow stroma) contains a group of fibroblast-like stem cells with osteogenic differentiation potential [referred to as MSC, bone marrow stromal cells (BMSC), or skeletal stem cells]. The exact location of these cells in vivo is not known but recent work suggests that MSC are located in the perivascular spaces as sub-endothelial cells surrounding the vascular sinusoids in the bone marrow [67].
Isolation and characterization of bone marrow derived MSC
MSC have been isolated from the low-density mononuclear fraction of bone marrow aspirates by their selective capacity for adherence to plastic surfaces compared to haematopoietic cells [68–71]. However, MSC cultures established by this method are heterogeneous and contain both stem cells and progenitor cell populations. This has been demonstrated by clonal analysis of MSC where ∼30% of the clones represented true stem cells [72, 73]. Stemness of MSC is defined as the ability of the clonal cells to form ectopic bone and bone marrow organ upon in vivo implantation with an osteo-conductive carrier such as HA/TCP in immune deficient SCID mice [74]. Thus, describing the whole plastic-adherent MSC population as ‘stem cells’ is not appropriate.
The human MSC (hMSC) phenotype has been defined through expression of many CD markers. hMSC are negative for haematopoietic surface markers (CD34, CD45, CD14) and positive for STRO-1, CD29, CD73, CD90, CD105, CD106, CD166, CD146 and CD44 [74–76]. Several attempts have been made to isolate a homogeneous population of the true stem cells among MSC based on the expression of one or more of these specific surface markers. STRO-1 alone or in combination with CD106 (VCAM-1) or CD146 (MUC18) [77], CD271 (low-affinity nerve GF receptor) [78], CD18 (β2 integrin) [79] or the embryonic stem cell marker SSEA-4 [80] have been tried. A global plasma membrane proteomic signature has been established in order to identify novel surface markers for ‘stemness’[75]. However, there is still a need to identify both sensitive and specific markers that allow prospective isolation of the true multipotent MSC population.
Interestingly, MSC with similar biological characteristics to those derived from bone marrow have been isolated from other sources including peripheral blood [81], umbilical cord blood [82], synovial membrane [83], deciduous teeth [84] and amniotic fluid [85]. These various MSC populations share some common properties and surface phenotypes but differ in their differentiation potential and their gene expression profile in ways that reflect their tissue of origin [86].
In vitro differentiation of MSC into osteoblast lineage cells
MSC are capable of differentiation under appropriate in vitro conditions to mesoderm-type cells, e.g. osteoblasts, adipocytes and chondrocytes [74, 87]. Differentiation is induced through addition of ‘cocktails’ of morphogens and chemicals important for differentiation of a particular cell type. For example, for osteoblast differentiation a mixture of dexamethasone, calcitriol, ascorbic acid and β-glycerophosphate is usually added. In vitro differentiation is verified by demonstrating the induction of osteoblast-specific gene expression and proteins. Similar procedures are used for demonstrating differentiation to other cell types. For example, the ability of hMSC to differentiate into adipocytes has been reported by several investigators using a cocktail of dexamethasone, isobutyl methyl xanthine, insulin and rosiglitazone (a peroxisome proliferator-activated receptor γ2 (PPAR-γ2) agonist) [71, 88, 89].
In vivo differentiation of MSC into bone
There is increasing recognition that in vitro osteoblast differentiation assays have limitations and thus there is a need to verify osteoblast differentiation potential of MSC based on an in vivo assay. Friedenstein employed ‘diffusion chambers’ to determine the differentiation potential of MSC [66]. Ectopic implantation of MSC with a carrier (HA/TCP) in an open system (subcutaneous implantation) in SCID mice has evolved as the ‘gold standard’ assay for osteoblast differentiation and stemness characteristics of MSC [87, 90, 91]. Under these conditions, MSC can form bone and a bone marrow microenvironment supporting haematopoesis, whereas osteoprogenitor cells can only form bone [91]. This assay has also been employed to demonstrate the ability of the multipotential MSC cell to exhibit self-renewal and maintenance of stemness capacity during serial implantations [92].
The importance of testing the in vivo phenotype of cultured MSC is shown by the recent demonstration of the limited value of standard in vitro criteria for identification of hMSC clones with in vivo bone-forming capacity [93]. In this study, using DNA microarray analysis, a predictive molecular signature was identified for in vivo bone formation of MSC that can be employed to select for an MSC population with high in vivo osteogenic capacity for bone regeneration [93].
Factors and pathways controlling osteoblast differentiation of hMSC
Several approaches have been employed in order to identify factors and pathways important for lineage-specific differentiation of hMSC. Using a genetic approach, several lineage-specific transcription factors have been identified and demonstrated to induce MSC differentiation. For example, core-binding factor 1(CBFA1/Runx2; Runx = Runt-related factors) [94], osterix [95] (Osx) and lipoprotein related receptor 5 (Lrp5) [96] overexpression lead to osteoblast differentiation, whereas PPAR-γ2[97] and Sox9 [98] induce adipocyte or chondrocyte lineages, respectively. Alternatively, a ‘micro-environmental’ approach has been employed where MSC are exposed to different mixtures of GFs, hormones and extracellular matrix components to induce their differentiation. These experiments identified several factors that induce osteoblast differentiation, e.g. BMPs [99], Wnt [100] or that inhibit osteoblast differentiation, e.g. Dlk1/Pref-1 [101] and Noggin [102].
We have employed novel proteomic approaches to identify the intracellular signalling pathways that determine the osteoblast differentiation fate of MSC [103]. A newly developed quantitative proteomic method called stable isotope labelling in cell culture has also been employed to compare the phosphotyrosine signalling pathway initiated by epidermal growth factor (EGF) which resulted in osteoblast differentiation, with that of PDGF which did not. Interestingly, more than 90% of the signalling proteins were utilized by EGF and PDGF, whereas the phosphatidylinositol 3-kinase (PI3K) pathway was exclusively activated by PDGF, implicating PI3K as a possible control point. Indeed, chemical inhibition of PI3K in PDGF-stimulated cells rescued the osteoblast differentiation phenotype [103]. These studies demonstrate the ability of state-of-the-art quantitative proteomic approaches to identify targets for pharmacological intervention in order to control MSC differentiation.
Defining the relationship between osteoblast and adipocyte differentiation from MSC
Recent studies have suggested that bone mass and fat mass are strongly associated processes mediated by the same hormonal factors, including insulin [104], growth hormone [105] and recently leptin [106].
The majority of clinical conditions associated with bone loss, including aging, osteoporosis, anti-diabetic drugs (thiazolidinediones) treatment and increased cortisol production, are known to be accompanied by increasing marrow adipocytes, due to a shift in the differentiation decision of MSC to favour the adipocyte lineage over the osteoblast lineage [107, 108]. Several lines of evidence support this relationship between osteoblastogensis and adipogenesis. At a cellular level, an inverse relationship and phenotypic plasticity exists between osteoblast and adipocyte differentiation of bone marrow MSC. Several transcriptional factors and pathways have been demonstrated to control the balance between osteoblast and adipocyte differentiation of MSC, for example, transcriptional coactivator with PDZ-binding motif [109], PPAR-γ2[110], ΔFosB [111], canonical Wnt-β-catenin and non-canonical Wnt signalling pathways [112]. We have also reported that Wnt co-receptor, Lrp5 with active mutation (T253I) and Wnt activation control the differentiation of hMSC into osteoblast versus adipocyte in patients with high bone mass due to this mutation [96].
On the other hand, bone marrow adipocytes secret several inflammatory factors, including leptin, adipsin, adiponectin and resistin, that act in a paracrine manner to suppress osteoblast function and differentiation [113, 114]. Very recently, Lee et al.[115] provided the first evidence that bone can act as an endocrine organ to regulate glucose and fat metabolism through the secretion of OC (osteoblast-specific protein), which acts as a pro-hormone to decrease fat mass and promotes expression of adiponectin, an insulin-sensitizing adipokine [115, 116]. This novel finding established a cross-talk between bone formation and energy metabolism through the secretion of systemic factors. In this context, we have recently identified dlk1/FA1 protein (δ like 1/foetal antigen 1) as a novel pre-adipocyte derived factor that has regulatory effects on both osteoblast and adipocyte differentiation of hMSC [101]. dlk1/FA1 is an imprinted paternally expressed gene that encodes for a trans-membrane protein. It has six EGF-like repeats in its extracellular domain, which is similar to other members of the Notch/Delta/Serrate family. Also, we showed that the extracellular domain of the molecule FA1 which circulates in blood and tissue fluids, acts in an endocrine fashion to control bone and fat mass in vivo[117]. Using DNA microarray technology, we found that dlk1/FA1 modulates the expression of several pro-inflammatory cytokines by MSC and thus may influence hMSC differentiation by controlling the composition of the microenvironment ‘niche’[118]. Thus, identifying novel secreted factors that regulate osteoblast/adipocyte cross-talk is an important research area that can potentially lead to identification of therapeutic targets for enhancing bone formation.
MSC and sex hormones
Sex hormones, especially oestrogens, have a critical role in development and maintenance of healthy skeleton [119, 120], but it is clear that sex steroids influence not only mature differentiated bone cells, but also the behaviour of stem and different stage progenitor cells [121]. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of hMSCs [122].
hMSC express oestrogen receptor (ER)-α and possibly also several ER-β isoforms [122–125]. Interestingly, osteogenic differentiation seems to increase the mRNA expression of all ERs present in MSC with the exception of ER-β4 isoform that seems to be mainly expressed in undifferentiated MSC [122]. Such changes in ER profile during differentiation of MSC suggest different effects of and sensitivity to oestrogens depending of the differentiation lineage and stage. ER gene polymorphism may also account to certain interindividual variablity in responses to estradiol stimulations [125].
17β-estradiol enhances dose dependent osteogenesis from hMSC as shown by up-regulation of OC and increased calcium deposition [124]. In other work, where MSC from both male and female donors were stimulated with estradiol and testosterone, estradiol also increased calcium deposition, but alkaline phosphatase (ALP) activity increased only in cells isolated from male donors [125]. Testosterone, on the other hand, had no effect on calcium deposition in either sex. Resveratrol, a polyphenolic phytoestrogen, has been shown to enhance osteoblastic maturation of hMSC, possibly through extracellular signal-regulated kinase 1/2 (ERK1/2) pathway [126, 127].
More recent studies have revealed that hMSC can express several key enzymes needed for intracrine conversion of dehydroepiandrosterone to oestrogens or testosterone [122, 128, 129]. This suggests the interesting possibility that hMSC may themselves in certain conditions make the needed sex hormones or adjust their levels using serum dehydroepiandrosterone as a precursor, and participate therefore in an intra-, auto- or paracrine manner in their own differentiation processes and in overall more complex local sex hormone regulation in bone [130].
Effect of aging on osteoblastogenesis
One of the most consistent histomorphometric findings in bone biopsies obtained from elderly persons is the presence of decreased mean wall thickness in both trabecular and cortical bone indicating decreased osteoblastic bone-forming capacity during bone remodelling [131]. Mean wall thickness is dependent on both the number of recruited osteoblasts at the beginning of bone formation phase and the activity of individual osteoblasts. Recruitment of an adequate number of osteoblasts is dependent on the availability of stem and precursor cells and their proper response to growth, differentiation and chemotactic signals in the bone microenvironment. Matrix production and mineralization functions of mature osteoblasts are dependent on the proper response of osteoblasts to hormones, GFs and cytokines as well as the availability of nutrients and ions necessary for accomplishing these processes. Age-related impairment of osteoblast functions can thus be the result of any mechanism interfering with these processes. It has been shown that the number of MSC does not change with aging or in osteoporosis [132] and that MSC exhibit a limited lifespan in vitro, with all the characteristics of the in vitro replicative senescence phenotype [133]. Interestingly, an age-related decline in the maximal lifespan of hMSC has been observed, from 41 ± 10 population doublings in young donors to 24 ± 11 population doublings in old donors [133]. In addition to impairment of cell proliferation of MSC with aging, it was also shown that the senescent microenvironment affects MSC functions. As a surrogate condition for senescent microenvironment experienced by hMSC, the effects of sera from young and old persons on MSC function were tested, and it was found that aged-sera inhibited osteoblast differentiation and functions of MSC [134]. Thus, intrinsic aging of MSC and the negative effects of the senescent microenvironment are possible mechanisms for the observed defective osteoblast function and bone formation in vivo in aged human beings. The senescent phenotype of MSC can be ‘rescued’ through overexpression of human telomerase reverse transcriptase gene [135]. The telomerized hMSC exhibit enhanced cell proliferation and in vivo bone formation capacity [135]. Telomerization also enhances gene expression of some osteoblastic genes [136]. Other approaches to rejuvenation of senescent MSC need to be explored for their potential use in therapy.
Conclusion
During the last decade, enormous amounts of data have been gathered related to the mechanisms of osteoblast differentiation from stem cells. It is expected that this knowledge will be translated into novel approaches to enhance bone regeneration and bone formation, a much needed task to manage the major health care problem of bone fractures and osteoporosis.
Embryonic, foetal and adult stem cells in osteogenesis
Cellular therapy is becoming a useful addition to medical therapies for repairing, restoring or ameliorating function of tissues. Several cell types and tissues have been proposed as starting material including autologous cells, adult stem cells (including specific tissue populations and also bone marrow and adipose MSC), embryonic stem cells, foetal cells and tissues from placental and amniotic fluid [137–143]. Some cell choices are more adaptable to cellular therapy in patients [144]. Tissue from animals and human beings at all stages of development must be evaluated for the advantages and disadvantages of each cell type (Fig. 4). The terminology of embryonic, foetal and adult stem cells is complicated. Legally, the term ‘embryo’ denotes the earliest stages following fertilization of an ovum by a sperm. Zygote would include early stage cleavage embryos produced by cell division up to 50–60 cell-stages (each cell of which is a blastomere) and the blastocyte for the 60 cell stage to the point of implantation at about 2 weeks after fertilization. Pathology would classify the embryonic stage as up to 9 weeks of gestation and thereafter as the foetal stage from 9 weeks to until birth. Different cell lines can be established from each of these tissues, but with different complications encountered in tissue culture techniques needed for each. Embryonic stem cells are harvested from pre-implantation embryos from the inner-cell mass before the first 2 weeks of development. These cells are frequently obtained from extra embryos developed by in vitro fertilization techniques to aid couples with fertility problems. There has been a moratorium on use of embryonic stem cells since 1975 with new laws in 1993 permitting use under certain circumstances. Because these particular cells have created an ethical debate, other researchers have begun using embryonic or foetal cells derived from voluntary interruption of pregnancy between 5 and 8 weeks [145]. Cell lines are normally developed from the genital ridge of the foetus but specific tissues can be isolated from immature tissue with the necessity to add GFs to assure particular cellular differentiation. Most foetal cell research has developed from research using specific tissues at the latter end of the first trimester (11–14 weeks) following voluntary interruption of pregnancies. As this tissue (>9 weeks) is considered an organ donation in most countries, it bypasses the major problems that have been raised by embryonic stem cells. Cell lines at this stage are tissue specific and therefore cells are differentiated and have specific functions [142, 146–148].
Fig 4.
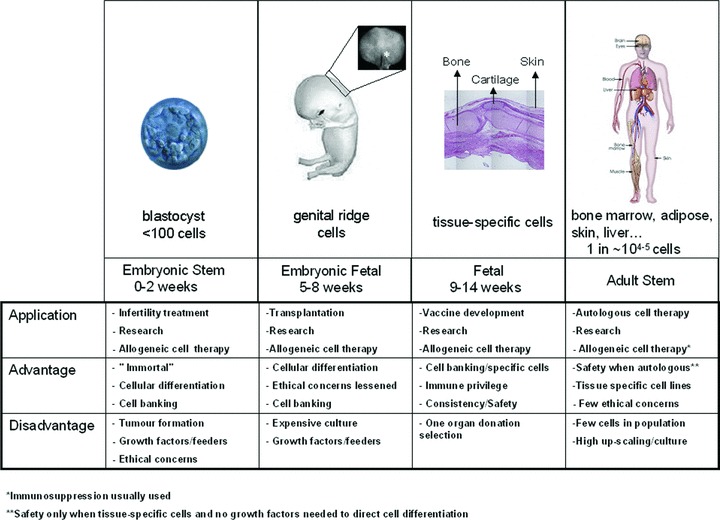
Development stage, cell type and source of cells currently used in cellular therapy. Cellular source can come from human and animals at different stages of development including embryonic, embryonic-foetal, foetal and adult involving different beginning tissue sources ranging from zygotes to specific tissues (bone marrow, adipose, amniotic fluid, skin, liver, bone, cartilage etc.). Embryonic stem cells are derived from embryos at early stage where less than 100 cells are present, followed by foetal stem cells that are taken from the genital ridge section from 5 to 8 weeks of gestation (drawing modified from http://commons.wikimedia.org/wiki/Image:10_weeks_pregnant.jpg donated by wouter.vengeer@tribal.nl with a free license). Tissue-specific foetal cells are taken following 9 weeks of gestation usually up to 14–16 weeks from normal tissue. Adult stem cells can be isolated from most tissue sources but are rare with only 1 in every 104 to 105 of total cell volume.
Cell-based therapies for bone
The first cell-based strategy used for repair of bone tissue was autologous connective tissue progenitors harvested from the iliac crest and immediately transplanted to sites for skeletal repair in the same patient [149, 150]. This autograft procedure can be accomplished without any outside manipulation of the tissue (i.e. cell separation, culture or expansion) and is thus considered part of a surgical procedure and is not regulated as a complex cell therapy. If extensive manipulation has to be done, then the procedure falls into a specific regulatory process which requires a certain infrastructure for cell culture. Although the auto-grafting strategy is used by many surgeons due to the ‘supplemental’ biological activity and low risk involved with use of autologous bone marrow, only a very small fraction of the cells actually survive and only 1 of 103 or 104 cells are potentially active [151]. The success of marrow grafting is therefore entirely dependent on transfer of sufficient numbers of progenitor cells, and therefore this approach may be least applicable in those situations where it is most needed, because ageing or disease are accompanied by a reduction of healthy marrow elements and especially osteogenic progenitors. Alternative cell sources are therefore of great interest, in particular normal allogeneic sources, which would provide ‘ready-for-use’ products for bone repair strategies.
Other adult stem cells, frequently referred to as MSC, have raised hopes for new treatments because they a have high self-renewal capacity and can generate multiple cell lineages. They can be isolated not only from bone marrow but also from many tissues such as amniotic fluid, adipose tissue, brain, skin, heart, kidneys and liver. Although widely distributed, adult stem cells represent only a small fraction of a tissue cell population (many times only 1 in every 104 to 105 cells), thus requiring extensive in vitro separation and expansion steps [152]. Stem cell cultures are technically demanding. Maintenance and expansion of stem cells in an undifferentiated state requires the addition of many specific GFs [153]. Culture of these cells without feeder layers (which are usually formed by animal cells), is difficult and feeder layers are responsible for some aspects of inconsistent colony cell growth. The necessity of using exogenous GFs as well as animal products is a limiting factor for the scale up of stem cell cultures for clinical applications [138].
Unlike stem cells, foetal cells are differentiated cells with high expansion and regeneration and low immunogenic properties [154]. They can be isolated from foetal tissues, which follow embryonic stage after 9 weeks of development. Foetal cells have extensive expansion capabilities and cell culture requirements are minimal compared to stem or mesenchymal cell types. As the foetal cells are already differentiated and do not need to be directed or altered, the vast number of additional GFs normally necessary are not needed for cell culture and expansion [146]. Cell choice is of utmost importance and each element of processing necessitates special attention in order to produce a safe, consistent and successful cell therapy for clinical trials [155].
Specific features of bone cells needed to be advantageous for clinical use
As whole bone marrow transplantation may not be optimal biologically, the expansion of bone marrow osteoprogenitors has been evaluated. The procedure is laborious as their isolation and characterization has been difficult due to their low numbers in the original tissue and lack of specific reactive antibodies to assure separation of active cellular components. Patient treatment is only possible following 4–6 weeks of expansion in cell culture. Demonstration of cell function is of particular importance so that separated cells are shown to be capable of matrix deposition, mineralization, ossification, nodule formation and bone formation in vivo without fibrous tissue formation. Although these cells have been shown to fulfil the required needs, it is labour intensive to use these procedures for each individual patient.
More recently, femurs from foetal tissue of ∼8 weeks were evaluated for isolation of bone progenitors and thus these cell populations would be formally considered as foetal stem cells (5–8 weeks) [156]. Mirmalek-Sani et al. have shown that these cells histologically resemble cartilage and this was confirmed by the high plasticity seen with these cells when compared to studies using foetal bone cell populations derived from 12- to 14 week gestation femurs. In vivo mineralization of 8 week stem cells is seen with osteoinductive media but with 12–14 week foetal bone cells such media are not necessary because spontaneous minerialization is seen without external GFs [157]. Spontaneous behaviour of cells is thus important to eliminate the use of multiple GFs and increase biosafety while reducing the cost of cell production. It is advantageous to use differentiated cells with lower plasticity as they depend less than undifferentiated cells on the many environmental factors such as nutrition, mechanical loading, inflammation, angiogenesis and interaction with endogenous cells. In addition, foetal bone cells from 12 to 14 weeks have been extensively evaluated in vivo for immunological privilege, safety and osteoinduction.
Montjovent et al.[158] have demonstrated that a composite made of poly-L-lactic acid and β-TCP particles used as scaffolds to seed foetal bone cells can offer suitable conditions for osteoblasts to achieve full differentiation due to the high osteogenic potential of these cells. Furthermore, in vivo studies using dedicated foetal bone cell banks and a solid matrix have shown significant promotion of bone growth using two different model systems [158]. Safety studies in a large animal model using sheep have also shown that 12 week foetal bone cells derived from a dedicated cell bank are immunologically accepted, do not form fibrous tissue and promote biological activity with bone growth (personal communication by Applegate and Pioletti).
Development of therapeutic biological agents
Organ donation, whole cell bioprocessing and procedures adaptable to good manufacturing processes (GMP) make it possible to develop extensive master cell banks and working cell banks to facilitate thorough testing (Fig. 5) [159]. Once master cell banks can be produced, working cell banks can be produced to establish individual batches for treatment of large numbers patients (thousands from one cell bank). Further, these cell banks can be tested completely for safety regarding sterility, pathogens and adventitious agents and tumorigenicity. Once safety can be assured, efficient cell presentation with biocompatible delivery systems can be assessed for specific tissues. For delivery systems, biocompatible biomaterials need to be available in order to provide an extracellular matrix environment for cell differentiation, delivery and release. Cells and materials need to be tested together to not only assure biocompatibility but also their interactions, cellular stability, possible degraded by-products of combination and degradation or absorption. Ease of applicability of the final product will be of importance for clinical use.
Fig 5.
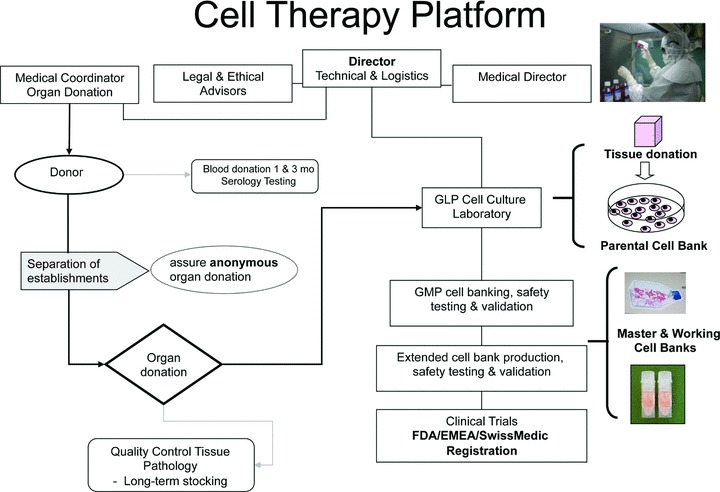
Organization of a cell therapy platform. Director of technical and logistics coordinates the program with essential legal and ethical advisors and a medical director for interpretation of medical quality assurance (serology and pathology reports). The separation of hospitals for the organ donation and all other aspects of the platform including serology, pathology and the GLP cell culture laboratory can assure complete anonymous and coded organ donations. Importantly, the Director of Technical and Logistics is not involved in any manner in the organ donation process as required by law. Final approval for use of validated cell banks for human therapy is coordinated and approved with both Hospital Ethics Committees and National regulatory agencies (i.e. European Medicines Association, Food and Drug Administration, SwissMedic [32]).
All cellular products must be in compliance with GMP guidelines with respect to medicinal products and investigational medicinal products for human use. The European Union (EU) regulation on advanced therapy medicinal products was adopted by all European Member States on December 30, 2008 and the United States Food and Drug Administration (FDA) recently also proposed regulations on human cells, tissues and cellular and tissue-based products [160–164]. The main scope of these regulations is to establish clear classification criteria for many new cell-based medicinal products. For the EU, it makes reference to the 2004/23/EC directive on donation, procurement and testing of human cells and tissues and also with directive 2002/98/EC on human blood and blood components. These directives dictate that human cells used for therapeutic purposes must be in compliance with the quality requirements they describe and that all advanced therapy medicinal products must be prepared under GMP conditions [161, 165, 166]. Key elements for cellular-based products include identity, purity, sterility, stability, safety and efficacy are recommended. In all, these new regulations impose strict criteria for the production and the environment used for the production of cell-based products to be used in clinical trials and treatments [167, 168].
Clinical application concerns
Risk assessment of final cellular products for human use is of utmost concern. Self-renewal of undifferentiated cells represents potential for tumour formation. Certain techniques such as cellular cloning or encapsulation of cellular products are alternatives to assure safety. Many cell-based therapies will not consist of a uniform cell population. Associated accessory cells raise additional questions for potential risk as well as their physiological role after administration. Cellular death of transplanted cell populations, ectopic tissue formation or migration from the site of administration could be a problem when cellular therapies are used within anatomically sensitive areas such as the central nervous system, joints or myocardium. Pre-clinical animal models for specific pathologies or tissue repair are an important step and special care must be taken in the interpretation of pertinent safety and biological activity. A thorough appreciation of relevant advantages and limitations must be made regarding an animal model choice.
Conclusions
Cell-based therapies are being developed and introduced for all types of tissue repair including skin, bone, cartilage, muscle and spine. They offer promise for repairing and/or replacing damaged tissue and restoring lost functionality. One of the major challenges for assuring that more patients will benefit from cell-based therapies in the future will be the optimization of the choice of cell type as well as methods for their isolation and expansion. Today, it is still unknown what type of osteogenic cell will be the most suitable for engineering bone tissue. MSC, foetal-stem and foetal cells, bone marrow stromal cells, periosteal cells and osteoblasts have been successfully used for generation of bone tissue. Isolation and expansion efficiency, stability of osteoblastic phenotype, in vivo bone formation capacity and long-term safety are essential requirements that have to be met by any type of osteogenic cell for successful clinical application.
Equally important is the delivery system for the cell of choice and their interaction with scaffolds to assure biocompatibility. The development of master cell banks from the cells of choice provides a major advantage for the creation of a therapeutic biological agent. Careful selection of donors and extensive screening of both the donor and cultured cells avoids transmissible viral, fungal or bacterial disease and therefore can provide a safe and secure utilization of cells for therapeutic purposes. Clear regulatory oversight of cellular use, particularly for organ donation, embryonic and foetal cells will be necessary. Overall, cooperative interdisciplinary efforts and cooperation to form successful translational medicine platforms in universities and hospitals will serve to ensure further patient safety.
Platelet-rich plasma (PRP), growth factors and osteogenesis
PRP is a concentrate of platelets in a small volume of plasma from freshly drawn whole blood activated with a mixture of thrombin and calcium [169]. PRP is used to facilitate wound and bone healing, thanks to the molecules released by platelets during activation. A number of these substances affect osteogenesis, such as TGF-β1, PDGF-BB, VEGF-A and IGF-I (Table 1). These molecules are more concentrated in PRP than in a normal clot as a result of the higher platelet number. After release, they are trapped within the fibrin mesh, then slowly pass into the microenvironment and bind to receptors on the cell membrane. The molecules released by platelets regulate key processes involved in tissue repair, including chemotaxis, cell proliferation, differentiation and extracellular matrix synthesis (Table 1). The fibrin mesh itself can act as a conductive matrix or ‘scaffold’ for cell adhesion [170].
Table 1.
The role in bone remodelling of the GFs and other molecules released by platelets
| Molecule | Role in bone remodelling |
|---|---|
| TGF-β | Mesenchymal stromal cell proliferation; osteoblast precursor recruitment; osteoblast and chondrocyte differentiation (but inhibition of terminal differentiation); bone matrix production; recruitment of osteoclast precursors but inhibition of terminal differentiation and induction of apoptosis |
| PDGF | Osteoprogenitor migration, proliferation and differentiation; osteoclastogenesis |
| VEGF | Conversion of cartilage into bone; osteoblast proliferation and differentiation |
| IGF | Osteoblast proliferation; bone matrix synthesis; bone resorption |
| EGF | Osteoblast recruitment and proliferation |
| Fibronectin | Osteoblast migration and adhesion |
| Vitronectin | Osteoblast migration and adhesion |
| Prostaglandin E2 | Bone resorption |
The combination of GFs in PRP is in an optimal level and ratio, and seems to be more efficient than the single rhGF, owing to their synergistic effects [87]. The technique for obtaining GF from PRP is relatively simple and less expensive than rhGF. Because of its autologous origin, PRP does not hold any risk of immunological reactions and transmissible diseases. The drawbacks of PRP are the high variability of GF concentrations, due both to individual factors and to different preparation methods [171]. We found a high variability in PDGF-BB and TGF-β1 levels assayed in the supernatant of thrombin-activated PRP, on lysates of platelet concentrates, on serum and platelet poor plasma from 11 healthy individuals and from 11 patients with osteonecrosis (Table 2).
Table 2.
Mean and standard deviation of TGF-β1 and PDGF-BB in healthy individuals and in patients with osteonecrosis
| Sample | TGF-β1 ng/ml | PDGF-BB ng/ml | ||
|---|---|---|---|---|
| Healthy individuals | Osteonecrosis | Healthy individuals | Osteonecrosis | |
| Thrombin-activated PRP supernatant | 31.4 ± 38 | 78.8 ± 70.8 | 6.7 ± 3.2 | 9.6 ± 4.4 |
| Lysate | 99.1 ± 68.2 | 120.5 ± 83 | 10.8 ± 6.3 | 12.4 ± 3.9 |
| Serum | 25.9 ± 15.1 | 37.6 ± 18 | 2.3 ± 0.5 | 4.8 ± 3.2 |
| PPP | 2.6 ± 1.2 | 2.4 ± 1.5 | 0.7 ± 0.3 | 0.9 ± 0.7 |
The original Marx protocol obtained PRP by the addition of calcium and thrombin to a platelet concentrate [169]. Recently, other protocols for PRP preparation have been proposed, which differ for anticoagulant [172], leucocyte depletion and platelet activators [173, 174]. Leucocyte depletion avoids the release of pro-inflammatory cytokines [175]. However, some authors suggest that leucocytes represent an additional source of GF and have an important role in host immune defence [176]. Myeloperoxidase contained in neutrophils and monocytes generates reactive oxygen species, that act as potent bactericidal and may be helpful to prevent post-surgical infections and in the prophylaxis and treatment of infection-related delayed healing and non-union [177].
PRP effects in vitro on the cells involved in bone repair
PRP effects on osteoblasts
The use of PRP was proposed in order to provide a microenvironment for the orchestration of the sequential process of bone regeneration involving migration, proliferation and differentiation of osteogenic cells. The mitogenic effect of PRP was demonstrated by in vitro studies on hMSC [178, 179] and human trabecular osteoblasts [180]. Among the GF released by platelets, the highest contribution to the proliferation of osteoblasts was from PDGF and TGF-β1[181].
In vivo platelet effects are likely restricted to the early stages of bone regeneration until the blood clot is replaced by granulation tissue, which is rich in osteogenic progenitor cells and blood vessels. Similarly, in vitro PRP increased MSC proliferation but inhibited differentiation [182]. PRP seems to have opposite effects than BMPs. PRP stimulated migration and proliferation of the osteogenic cell line MC3T3-E1, whereas BMP did not have any effect on migration and induced only a moderate increase of proliferation. However, PRP suppressed ALP activity in response to BMP. Together, these findings indicate that activated platelets can influence the microenvironment in such a way that osteogenic differentiation is suppressed. Once platelet GF have lost their activity, osteogenic cells can regain their responsiveness to BMP [183].
A PRP enriched in fibrin better stimulated proliferation and mineralization of rat osteoblasts than soluble PRP, probably due to a more gradual release of GF from the fibrin component [184]. Additional signals derived from the microenvironment modulate the effects of PRP. hMSC proliferation was increased by the combination of platelet concentrates and demineralized bone matrix, but osteoblastic differentiation decreased [185]. On the contrary, the authors observed that the combination of PRP and freeze-dried bone allografts accelerated MSC differentiation, as demonstrated by the significantly higher osterix expression at 15 days, and sustained autonomous production of GF by the cells themselves, as shown by the higher level of FGF-2 in the conditioned culture medium after 11 days [186].
In bone engineering, PRP represents a source of GF to be added to MSC seeded on an artificial scaffold, which could modulate the effects of PRP owing to its physical and chemical properties. PRP favoured the adhesion and proliferation of MSC on three-dimensional ceramic scaffolds with high specific surface area, such as calcium-deficient HA, in comparison with scaffolds with lower specific surface area, such as β-TCP [187]. The effect of PRP lysates on the osteogenic differentiation of goat MSC on HA and silica-coated HA was lower than that of fibrin glue, which represents an extracellular matrix with arginine–glycine–aspartic acid motifs that can bind to integrins favouring cell adhesion [188]. However, in clinical use platelet gel preparation generally involves fibrin clotting; therefore platelet gel has the advantages of both platelet concentrates and fibrin mesh.
PRP effects on osteoclasts
Activated platelets affect osteoclasts through the release of modulators of osteoclast differentiation, such as TGF-β1, IGF-I, PDGF [189] and prostaglandin E2. VEGF favoured osteoclast differentiation in the presence of macrophage colony-stimulating factor [190].
Conflicting results have been reported about the effect of PRP on osteoclasts. In vitro, PRP was shown to favour osteoclast differentiation of mouse bone marrow cells, particularly the generation of tartrate-resistant acid phosphatase (TRACP)+ multinucleated cells, the formation of bone pits and the expression of mRNA for cathepsin K and matrix metalloproteinase-9 [191, 192]. However, other authors found that PRP decreased the generation of TRACP+ multinucleated cells from rat bone marrow and enhanced the secretion of osteoprotegerin from osteoblasts [193]. From research on the osteoclast precursors of human peripheral blood, 10% PRP interfered with the complete differentiation process and osteoclast activation, as shown by the decrease of bone collagen type I degradation. At higher dosage PRP affected osteoclast formation also at an early stage of differentiation so that it particularly impaired the fusion of precursors and the formation of TRACP+ multinucleated cells [194]. These results suggest a possible inhibitory effect of the substances released by PRP on osteoclast activation.
In vivo, dogs with mandibular defects treated with autologous bone grafts and PRP initially showed an increase of osteoclast number, but after 2 months there was no significant difference between PRP- and non-PRP-added grafts [195].
On the basis of these results, we suggest that PRP can have opposing effects on osteoclast generation and differentiation through the simultaneous interaction of several released molecules with osteoclast precursors. TGF-β1 could be a possible inhibitor of terminal differentiation of osteoclasts, because TGF-β1 has been shown to favour the recruitment of haematopoietic precursors [196] but to inhibit bone resorption [197].
PRP effects on endothelial cells
Angiogenesis is crucial for good bone healing. Newly formed vessels mediate delivery of cell precursors, secrete GF and transport nutrients and oxygen [198]. Endothelial cells synthesize molecules which affect bone cells, such as cytokines, chemokines, prostanoids, GF, adhesion molecules, matrix constituents [199], endothelin-1 [200] and BMP-2 [201].
The GF released by platelets, such as VEGF, TGF-β, PDGF and EGF [202], not only have pro-angiogenic properties [203–205], but also induce the endothelial cells to express a pro-osteogenic phenotype. PRP induced human umbilical endothelial cells (HUVEC) to express PDGF-β, which in turn has chemotactic effects [206], recruiting MSC to the site of bone repair. MSC migration was higher in HUVEC incubated with PRP in comparison with HUVEC incubated with low serum medium or with PRP [207]. PRP favours monocyte adhesion to the vessel wall, through the stimulation of endothelial cell expression of intercellular adhesion molecule 1 [207], which is important because the monocyte/macrophage response is essential for bone repair.
PRP effects in vivo on experimental animals
The results of research on osteogenic activity of PRP in animals are conflicting. In combination with bovine cancellous bone, PRP increased bone formation in a non-critical-size bone defect in rabbit cranium [208]. However, other authors noticed that the use of bone graft material in combination with PRP was not superior to that of bone allograft alone [209–211]. These different results may depend on the animal species used, the method of gel preparation, type of bone defect, outcome parameters measured, the presence of MSC and of scaffolds or bone substitutes. An important reason for the discordant results from different authors is the fact that critical-size bone defects were not assayed. When critical-size bone defects were tested, the combination of PRP, bone substitutes and MSC induced the greatest improvement in healing compared to a single component assayed alone [212]. Another variable seems to be the ratio of bone graft to PRP [213].
When PRP was used in combination with artificial scaffolds, the physical and chemical properties of the scaffold material should be considered because they can affect the performance of PRP [187]. When PRP was used in association with β-TCP [214] or triphasic ceramic- (calcium silicate, HA and TCP) coated HA [215], more intense bone regeneration was observed.
Even though it is generally assumed that PRP might support osteogenesis in the presence of precursor cells, sometimes the addition of PRP to MSC did not further increase the rate of bone formation in comparison to MSC alone. In goats no effect was shown when PRP was added to MSC seeded on scaffolds made of the combination of HA and β-TCP implanted at an orthotopic or ectopic location [216]. MSC added to the critical size defect were likely already induced towards osteogenesis and PRP could not significantly increase this. Moreover, osteotomy might recruit osteoblast precursors from the bone marrow [217].
The effect of PRP combined with BMSC was also tested in experimental dental implant models. In a dog mandible model of bone defects, the combination of canine MSC and PRP favoured well-formed mature bone formation and neo-vascularization, comparable with that induced by autologous cancellous bone, whereas PRP alone performed poorly [218]. In other experiments, the combination of MSC-PRP-fibrin gel, used as grafting material for alveolar augmentation in adult dogs, lead to the highest degree of direct bone-implant contact [219].
From the analysis of the results of in vivo research, it can be concluded that PRP promotes bone healing [220], particularly in the early phase [221] and in combination with MSC and bone grafts [222, 223] or an artificial scaffold. In the later course of bone healing, the PRP effects were less evident [224, 225]. However, a beneficial effect of PRP on bone healing was not demonstrated in every animal model and with every bone substitute, probably owing to the differences in the protocol design and the method for measuring bone formation [226].
The clinical use of PRP for bone repair
In orthopaedics, PRP is generally considered a tool for promoting bone repair, even though there have been discordant or inconsistent results [227]. PRP, sometimes combined with MSC, has been proposed for the treatment of non-unions [228], in femoral and tibial lengthenings [229], in distraction osteogenesis, in spinal fusions, in foot and ankle surgery and in joint arthroplasties. PRP is also used in odontostomatology and maxillo-facial surgery, to improve bone regeneration.
Non-union
The scientific basis for the use of PRP in non-unions and pseudoarthrosis is the significant reduction of GF which has been observed at the non-union sites, in comparison with the site of fresh fractures [230]. In non-unions, both PRP administration during surgery together with bone allograft and percutaneous administration of PRP have been proposed. PRP with autologous bone graft gave resolution of non-unions of the foot and ankle at a mean of 60 days [230]. With percutaneous administration healing was improved only if the treatment was performed within 11 months after the initial surgery [231]. In a prospective study, PRP, injected into atrophic non-unions, treated with percutaneous stabilization, failed to improve consolidation [232]. Therefore, at present there is no clinical evidence of benefit of PRP in non-unions in comparison with other treatments. An important issue is the option to treat with PRP or recombinant BMP. The preliminary results of a recent prospective randomized clinical study on 120 patients, where PRP was compared with rhBMP-7 for the treatment of persistent long bone non-unions, supported the view that the application of rhBMP-7 was superior to PRP [233].
Distraction osteogenesis
In distraction osteogenesis of the femur or tibia, for patients with achondroplasia, hypochondroplasia or congenital pseudoarthrosis, PRP in combination with MSC resulted in acceleration of new bone regeneration [234].
Spinal fusion
In a prospective study on lumbar interbody fusion, PRP with an allogeneic bone graft gave a clinical and radiological outcome at 12 and 24 months comparable to an autologous graft [235]. However, other researchers failed to show an enhancement in lumbar fusion using autologous bone graft with or without PRP [236].
Foot and ankle surgery
In total ankle arthroplasty PRP improved the syndesmosis union rate [237, 238]. PRP may help to achieve an acceptable time for fusion also in high-risk patients with diabetes, immunosuppression, malnutrition, alcohol abuse, osteomyelitis and history of impaired osseous healing or multiple same site surgeries [239].
Total knee arthroplasty
The primary indication for use of PRP in total knee arthroplasty is to promote wound healing and to decrease blood loss. A study of 71 patients treated with PRP fibrin sealant, which was sprayed in the knee just prior to closure, showed not only higher post-operative haemoglobin levels, but also a significantly greater knee range of motion compared with the untreated group after 6 weeks [240].
Odontostomatology and maxillofacial surgery
PRP has been largely applied to promote bone regeneration in odontostomatology and maxillofacial surgery. MSC and PRP applied simultaneously with titanium implants provided stable and predictable results in terms of implant success [241]. Implants of β-TCP seeded with MSC and PRP for maxillary sinus floor augmentation were clinically stable 12 months after loading [242]. Recently, PRP associated with cancellous marrow grafting has been proposed in selected cases of osteonecrosis of the jaws [243]. A systematic review of human controlled clinical trials designed to treat maxillofacial bony defects with the application of PRP found evidence for beneficial effects in the treatment of periodontal defects, but minimal effects in sinus elevation [244].
There are few randomized, controlled studies of the clinical effects of PRP, and the apparent discrepancies reported in clinical effects and the diverse success rates among the different orthopaedic and maxillofacial applications could be a function of the different PRP preparation methods, poor characterization of the platelet concentrate used, the presence or absence of fibrin glue, the presence or absence of leucocytes, the presence or absence of MSC, the presence or absence of bone allografts or scaffolds (and what scaffold) and differences in the primary outcome measured. In addition, the power of different studies varies considerably. Although there are a number of studies in orthopaedic patients, the majority of them are anecdotal and only a few are well-designed controlled clinical trials. A recent systematic literature review on the use of PRP in orthopaedic surgery, after the exclusion of the applications in periodontal and maxillofacial surgery, identified 39 articles concerning clinical trials [245]. Only one was a randomized controlled study [246], which demonstrated that PRP, particularly if combined with MSC, increased the osteogenic potential of lyophilized bone chips. The strength of this clinical research was the randomization and the comparison among different treatments (freeze-dried bone allograft alone or in combination with PRP with or without MSC); its weakness was the lack of the evaluation of PRP effect in alone and of a clear primary outcome. More well-designed, prospective, rigorous clinical trials using consistent techniques may lead to more conclusive general recommendations [247].
Conclusion
PRP has been evaluated through in vitro, in vivo and clinical models of bone regeneration, with conflicting results. PRP use appears to be safe and feasible and may have a beneficial therapeutic effect, particularly in the early phases of bone formation. In vitro data have demonstrated that PRP, in addition to the stimulation of cell growth, induces target cells to synthesize GF and other substances able to sustain bone healing. The best in vivo and clinical results are obtained when PRP is added to MSC and osteogenic scaffolds or bone allografts. Further basic science and clinical research is needed to define the clinical indications, the methods of preparation and administration and the primary outcome with PRP.
Molecular control of osteogenesis
The timeline of molecular events that regulate osteogenesis has been described by Li et al., who analysed the ‘fracture healing transcriptoma’ and the ‘fracture healing proteoma’ by comparing non-fractured and post-fractured rat femurs during early and late stages of bone repair [248, 249]. In early phases after the bone injury, they observed an up-regulation of genes related to cell-cycle and cell-to-cell signalling, supporting the assumption that both cell division and cell communication are essential to initiate fracture healing. Many of the genes controlling cell growth and survival are constantly up-regulated, whereas those functionally associated with differentiation of osteogenic precursors and bone matrix formation undergo temporary modulation over time. In particular, expression of IGF-I and IGF-II, PDGF, FGFR, fibronectin, matrix metalloproteinases, glypican, byglican, osteomodulin, osteonectin, tenascin C, cartilage collagen (types VI and XI) and bone collagen (types I, V, VI and XII) increase until the immature osteoid synthesized by osteoblast progenitors is histologically detectable. The start of endochondral ossification is marked by a number of molecular pathways, but p38/MAPK (mitogen-activated protein kinase) signalling plays a pivotal role in advancing the process to this phase. Even though recent studies clearly demonstrated that native MSC are phenotypically and functionally different from cultured MSC [250], the time course and extent of gene expression in in vitro expanded osteogenic precursors is not significantly different from that which has been observed in vivo[251]. The gene expression profile of hMSC undergoing osteogenic induction has been analysed at different time-points until MSC were able to form mineral nodules [252]. As expected, in the early stage of differentiation most of the up-regulated genes are related to cell proliferation, whereas in later stages the expression of genes with a biological function relevant to osteogenesis, such as GF-signalling pathways, bone related genes and adhesion molecules, gradually increases (Fig. 6). In recent years the most important pathways which are known to play important roles in driving the osteogenic differentiation of MSC have been identified by in vitro and in vivo studies [253–258].
Fig 6.
Schematic representation of the principal signalling networks and transcription factors regulating osteoblast differentiation and osteogenesis (see text for details). Lines with arrowheads indicate a positive action, lines with bars indicate an inhibition and dashed lines indicate that function has to be proven. The lineage commitment and the differentiation of cultured MSC are signed by the acquisition of specific osteoblast functions. In boxes are shown genes that are significantly up-regulated during osteogenic differentiation of MSC. The ‘Gene Ontology Annotation’ of genes listed here is associated to GF-signalling pathways, cell communication and skeletal development. The up-regulated genes have been grouped according to the differentiation status of cultured MSC: ‘Pre-osteoblasts’ are MSC which are able to produce ALP (cytochemical activity, ×10) and generate ‘Colony Forming Units’; ‘Differentiated osteoblasts’ are cells which have the competence to deposit mineral nodules in vitro (von Kossa staining, ×10). Genes are reported with the official symbol, as shown in ‘Gene’ database (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene).
TGF-β signalling
The TGF-β superfamily is a large family of growth and differentiation factors including 34 members at least [259]. BMPs are members of the TGF-β superfamily and have a strong impact on osteoprogenitor differentiation, although it has been shown that osteogenesis is initiated normally in BMP-deficient mice [250]. In addition, BMPs are able to modulate bone formation, e.g. BMP-2, BMP-7, BMP-6 and BMP-9 show positive effects on the osteogenesis, in contrast to the inhibitory activity of BMP-3. The BMP/TGF-β superfamily signalling is transduced through two types of serine/threonine kinase receptors: type I receptors are BMPR-IA or ALK-3, BMPR-IB or ALK-6 and type IA activin receptor (ActR-IA or ALK-2); type II receptors are BMPR-II, ActR-II and ActR-IIB. Whereas BMPR-IA, BMPR-IB and BMPR-II are specific to BMPs, ActR-IA, II and IIB are also signalling receptors for activins. Type II receptors bind ligands in the absence of type I receptors, but they require their respective type I receptors for signalling, whereas type I receptors require their respective type II receptors for ligand binding [260]. The ligand-receptor complex acts through Smad proteins which are transcriptional modulators. There are three classes of Smads: (i) receptor-regulated Smads (R-Smad), that can be activated by BMP (BMPR-Smad 1, 5 and 8), or TGF-β (TGFR-Smad 2 and 3); (ii) common partner BMP and TGF-β mediator Smads (Co-Smads), such as Smad 4; (iii) inhibitory Smads, such as Smad 6 and 7 [261]. Phosphorylated R-Smads bind to the common partner Smad 4, and the complex translocates into the nucleus and regulates transcription of target genes by interacting with various transcription factors (see afterwards). Although the Smads are critical mediators in the TGF-β signalling pathway, BMP-2 can activate Smad-independent pathways, including MAPK that have distinct roles in regulating ALP and OC expression in osteoblastic cells [262].
FGF signalling
The FGF gene family is composed of 23 members that variously bind to seven FGF tyrosine kinase receptor isoforms (Fgfr) [263]. The FGF-family polypeptides play a critical role in regulating endochondral and intramembranous ossification. Fgfr1 is expressed in hypertrophic chondrocytes, and Fgfr1 signalling has stage-specific effects on osteoblast maturation, because it acts either to stimulate differentiation in osteoprogenitor cell or to arrest the maturation of differentiated osteoblasts [264]. FGFs 2, 9 and 18 probably bind Fgfr1 in osteoblasts. FGF9 and FGF18 are expressed in the perichondrium/periosteum, and seem to play a predominant role during the embryonic skeletogenesis [255]. FGF2 is expressed in periosteal cells and osteoblasts, and its activity should be more relevant during postnatal stages, as proven by the decreased ability to mineralize in vitro which has been observed in MSC cultures from FGF2-null mice [265]. Fgfr2 is expressed in reserve chondrocytes and appears to be down-regulated in proliferating chondrocytes. Fgf2r shows tissue-specific alternative splicing and the mesenchymal variants are the Fgfr2c forms, which are activated by FGF2, 4, 6, 8 and 9. FGF18 acts as a physiological ligand for Fgfr3, which regulates cell growth and differentiation of proliferating chondrocytes, whereas in differentiated osteoblasts it regulates bone density and cortical thickness [266].
IGF signalling
The IGF family consists of two secreted GFs, namely IGF-I and IGF-II, two receptors (IGF1R and IGF2R) and six high-affinity binding proteins. Both IGFs are expressed by osteoblasts and show similar biological activities, even though IGF-I is more potent than IGF-II. They stimulate osteoblast function and bone matrix deposition favouring the synthesis of collagens and non-collagenous proteins [267]. IGF-I does not influence the differentiation of MSC into osteoblasts and cell proliferation, which instead is stimulated by IGF-II [5]. Actions of both IGFs are mediated by the IGF-I receptor, a ligand-activated tyrosine protein kinase that uses a series of intracellular adaptor molecules, including the insulin receptor substrate proteins IRS1 and IRS2, to engage downstream signalling pathways [268]. IGF-I utilizes both the P13K, which induces the activation of Akt, and the MAPK pathway, which activates p38, Jun-N-terminal kinases and ERK1/2. The use of each pathway is dependent on culture conditions and the stage of cell differentiation [267]. Studies in experimental animals have concluded that action of IGF is essential for normal bone formation, growth and maintenance.
PDGF signalling
PDGF is an extracellular factor which exists as three biologically active isoforms composed of PDGF-A and PDGF-B polypeptide chain. Binding of PDGF requires and results in the dimerization of the two distinct receptors (α-PDGFR and β-PDGFR) that form three distinct receptor patterns (αα, αβ, ββ). MSCs express α and β receptors. Although PDGF signalling has been reported to play an important role in the control of various cell functions of skeletal cells, the network of PDGF signalling for MSCs has not been fully clarified. PDGF-BB, but not PDGF-AA, inhibited osteogenic differentiation accompanied by decreased ALP activity and mRNA levels The depletion of PDGFR-β gene in MSC decrease the mitogenic and migratory responses and enhanced osteogenic differentiation, as evaluated by increased ALP activity and mRNA levels, OC, BMP-2, Runx2 and Osx [269]. More recent data show that the pharmacological inhibition of PDGFR decreases the MSC proliferation induced by PDGF-BB, but does not affect the osteoblastic differentiation, as evaluated by analysing the expression of other osteogenic marker genes and the mineralized matrix production [270].
MAPK signalling pathway
MAPK pathway is activated by a variety of GFs with a role in osteogenesis, including FGF, PDGF, TGF-β and IGFs. The extracellular stimuli lead to the activation of a signalling cascade composed of MAP kinase, MAP kinase kinase (MKK or MAP2K) and MAP kinase kinase kinase (MKKK or MAP3K) [271]. The physiological functions of this complex cascade are summarized in Table 3.
Table 3.
Families of MAPKs
| Family | Name | Function |
|---|---|---|
| Extracellular signal-regulated kinases | ERK1, ERK2 | The ERKs (also known as classical MAPK) signalling pathways are activated in response to GFs, and regulate cell proliferation and cell differentiation |
| ERK5 (MAPK7) | Activated both by GFs and by stress stimuli; participates in cell proliferation | |
| ERK3 (MAPK6) ERK4 (MAPK4) | Atypical MAPKs. ERK3/4 are mostly cytoplasmic proteins which bind, translocate and activate the MK5 (PRAK, MAP2K5). ERK3 is known as an unstable unlike ERK4 which is relatively stable | |
| ERK7/8 (MAPK15) | New members of MAPKs structurally related to ERK3/4 | |
| c-Jun N-terminal kinases (JNKs) | MAPK8, MAPK9, MAPK10 | Stress-activated protein kinases |
| p38 isoforms | MAPK11, MAPK12 (ERK6), MAPK13, MAPK14 | Both JNK and p38 signalling pathways are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock and osmotic shock, and are involved in cell differentiation and apoptosis |
Wnt signalling pathway
Wnts (for wingless) forms a large family of secreted molecules, which are ligands for the membrane-spanning frizzled (FZD) receptors involved in various aspects of cellular biology, including cell growth, differentiation, function and death. Wnts can transduce their signals through two different pathways, namely ‘canonical’ and ‘non-canonical’ Wnt signalling. The first one involves the formation of a complex among Wnt proteins, FZD and low density Lrp5 or Lrp6 receptors. There is considerable evidence indicating that Wnt canonical pathway plays a critical role in bone formation resulting in the expression of osteoblast-specific markers [272, 273]. The binding of Wnt proteins to the FZD/Lrp5/6 complex generates a signal, which inhibits the activity of glycogen synthase kinase 3 (GSK-3). GSK-3 inactivity blocks the phosphorylation of β-catenin, and prevents its degradation in the cytoplasm by the ubiquitin-pathway, leading to translocation of β-catenin into the nucleus where it cooperates with transcription factors of the T-cell factor/lymphoid enhancer factor family [274]. The β-catenin activity seems to be a requirement to direct the bipotential osteochondroprogenitor towards a specific lineage. Indeed, the β-catenin/T-cell factor 1 complex enhances expression and activity of the osteogenic promoter Runx2, whereas low levels of β-catenin direct the mesenchymal precursors toward chondrogenesis [275].
Hedgehog signalling
Hedgehog signalling (Hh) family members provide positional information during the skeletogenesis, and initiate or maintain cellular differentiation programs regulating the formation of cartilage and bone. Indian hedgehog (Ihh) is produced by pre-hypertrophic chondrocytes and its signalling appears to act directly on osteoblast progenitors located in the perichondrium [276, 277]. The pathway is triggered by binding Hh protein to its receptor Patched thus activating the seven-transmembrane protein smoothened (Smo). The Hh signal transduction cascade activates the transcription factor GliA, a member of the Kruppel family of zinc finger proteins, which is translocated into the nucleus, regulates stem cell proliferation and activates gene targets. Several pieces of data show that Ihh signalling and Wnt signalling cooperate in controlling various aspects of skeletal development. Interestingly, Smo receptors have considerable amino acid sequence similarity with the Wnt receptor FZD, and GSK-3β is able to inhibit the Hh signalling [250]. Experimental data prove that Ihh signalling acts upstream of Wnt signalling and its activity seems to be limited to the early stage of osteoblast commitment [278]. In fact, when Smo activity is removed in Osx+ osteoprogenitors, normal osteoblasts are generated and the endochondral skeleton at birth is indistinguishable from wild-type [279].
Notch signalling
Notch signalling is well known for determining cell fate. Because Notch receptors and their ligands (δ1, 3, 4 and Jagged 1, 2) are transmembrane proteins, a cell–cell interaction is required for activating signalling cascade [280]. Notch 1 and Notch 2 are generally expressed in osteoblasts, whereas Notch 3 and Notch 4 have been found in subsets of the osteogenic lineage [281]. Experimental data support a dimorphic function of Notch signalling, which is able to up-regulate bone related genes, to favour the proliferation of immature osteoblasts, and to induce a severe osteosclerosis, but also to repress the BMP-induced osteoblast maturation by inhibiting the Runx2 transactivation function [282].
Ephrin signalling
There are two classes of ephrins: the B class (B1 to B3) are ligands for EphB1–6 tyrosine kinase receptors, whereas the A class (A1 to A5) are ligands for GPI-anchored EphA receptors (A1 to A10). Ephrins have the capacity of bidirectional signalling. Thus, when a cell expressing an Eph receptor contacts a cell expressing an ephrin ligand, signals are transduced into both cells by forward and reverse signalling, respectively [283]. The reverse signalling from EphB4 in osteoblasts to ephrinB2 in osteoclast progenitors leads to the inhibition of osteoclast differentiation, whereas the forward signalling through EphB4 induces osteogenic transcription factors [284]. However, ephrinA2–EphA2 interaction facilitates the initiation phase of bone remodelling by enhancing osteoclast differentiation and suppressing osteoblast differentiation [285].
Transcription factors regulating osteoblast differentiation
The signalling pathways described above lead to the activation of transcription factors that exert positive and negative regulatory effects on the expression of genes controlling the acquisition of the osteoblast phenotype [255, 286]. Runx2 is a member of the Runx family of transcription factors abundantly expressed in calcified cartilage and bone, and it is considered as a master regulatory switch essential for osteoblast differentiation [287]. Runx2 may be expressed in early osteoprogenitors, but is also required for osteoblast function beyond differentiation. Targeted disruption of Runx2 results in the complete inhibition of bone formation, revealing that Runx2 is essential for both endochondral and intramembranous bone formation. Runx2 acts in nuclear microenvironments as a scaffolding protein that controls gene expression in response to physiological signals. Many transcription factors interact with Runx2: some provide costimulatory signals, whereas others repress Runx2 function by affecting its DNA binding activity and/or transactivation potential [255]. The second transcriptional regulator for the final stages of bone tissue formation is the human homologue of the mouse Osx i.e. specificity protein-7. This is a zinc finger transcription factor which contains specific domains responsible for the activation of OC and Col1a1 genes. Osx-null osteoblast precursors in the periosteum of membranous bones express chondrocyte markers, such as Sox9 and Col2a1. This suggests that Runx2-expressing preosteoblasts are still bipotential cells. Nevertheless, Osx-null osteoblast precursors cannot differentiate into osteoblasts and deposit bone matrix, so that endochondral or intramembranous bone formation do not occur [95]. These data prove that Osx acts downstream of Runx2 to induce osteoblastic differentiation in osteochondroprogenitor cells. Osx may be induced by additional signalling pathways acting in parallel to, or independent of, Runx2. It has been shown that Runx2 is required but not sufficient for the BMP-2-mediated Osx induction, because MAPK signalling pathways serve as points of convergence to mediate the BMP-2 effect on Osx expression. Cooperation between Osx and nuclear factor of activated T cells activates the Col1a1 and OC promoters and accelerates osteoblast differentiation and bone formation in a Runx2-independent manner [288]. Another important transcription factor controlling osteoblast differentiation is ATF4 (activating transcription factor 4) or cAMP response elements binding protein 2. ATF4 is required for terminal differentiation of osteoblasts, and it interacts with Runx2 to regulate bone sialoprotein and OC expression. The crucial role of ATF4 has been shown by blocking its activity and obtaining a severe decrease in bone formation [289].
Conclusion
The sequential phases of osteoblast commitment and differentiation are regulated by a variety of complex activities, including hormones, GF, mechanical stimuli, cell–cell and cell–matrix interaction, that act via interconnected signalling networks, resulting in the activation of specific transcription factors and, in turn, their target genes.
Summary
Indirect fracture healing is usually clinically uncomplicated, is based on endochondral ossification, and proceeds by stages from haematoma through inflammation, angiogenesis, chondrogenesis and osteogenesis, to bone remodelling. Non-union, delayed union and various congenital or acquired bone defects can cause problems. Instead of traditional internal or external fracture fixation or osteosynthetic devices, regenerative medicine attempts to recruit, guide endogenous or to implant various exogenous mesenchymal or foetal stem cells to support active healing or to replace missing bone. These attempts often include composites of biodegradable polymers, e.g. PCL, poly(propylene fumarate) or PLA, with silica, HA or other calcium phosphate filler particles. Scaffold/matrix-cell interactions will be better utilized once the biological effects of aging, hormonal, intracrine, transcriptional and the legal regulation of adult and foetal stem cells are more clearly understood. PRP provides an inexpensive autologous source of potent biofactors, which together with other signals contribute to osteogenesis via TGF-β, FGF, IGF, PDGF, MAPK, Wnt, hedgehog, Notch and ephrin signalling via transcriptional regulation, which also provide the tools to monitor regeneration and healing.
The European Science Foundation was established in 1974 to create a common European platform for cross-border cooperation in all aspects of scientific research. With its emphasis on a multidisciplinary and pan-European approach, the Foundation provides the leadership necessary to open new frontiers in European science. Today the frontiers of regenerative medicine are rapidly expanding. Regenerative medicine has already provided new insight into cellular proliferation, effects of humoural and matrix signalling on cells, angiogenesis and tissue remodelling as well as basic insights into cell biology. Nevertheless, regenerative medicine is in its infancy and to facilitate the process of growth, 14 EU countries funded by European Science Foundation have joined forces in an attempt to improve networking and clarify where the frontiers and future needs are in this complex multidisciplinary field. Proof of principle experiments and randomized clinical trials remain in the future for regenerative medicine. In the most visionary view, scientists hope to see the human body undergo repeated and well timed maintenance of failing body parts through cycles of healing cell therapies.
Acknowledgments
D.P. and L.A. were financially supported by several grants from the Swiss National Science Foundation and in part from the Foundation S.A.N.T.E and Sandoz Family Foundation. M.K., B.M.A. and the study were support by grants from the Danish Medical Research Council, the Novo Nordisk Foundation, the Vellux Foundation and Danish Center for Stem Cell Research (DASC) and by a local grant from the University Hospital of Odense, Denmark. E.G.B. was financially supported by The EU by means of the 7th Frame Program in the REBORNE project (‘Regenerating Bone defects using New biomedical Engineering approaches’, EC-GA n°241879, HEALTH-2009–1.4.2). N.B., E.C. and D.G. were supported by grants from Italian Ministry of the Health for research activities of the Rizzoli Orthopaedic Institute, and from ‘Regione Emilia-Romagna’ for the research program on ‘Regenerative Medicine in Osteo-articular Diseases’. Y.T.K. and T.S. were financially supported by the EU MATERA project ‘Bioactive Nanocomposite Constructs for Regeneration of Articular Cartilage’, EU MNT ERA Net project ‘New Generation Titania’, Danish Council for Strategic Research ‘Individualized Musculoskeletal Medicine’, Sigrid Jusélius Foundation, Finska Läkaresällskapet, evo grants, Helsinki University Central Hospital and ORTON Orthopedic Hospital of the Orton Foundation. A.F.W., K.M., K.A. were financially supported by The EU support, the 7th Frame Program, VascuBone project (‘Construction kit for tailor-made vascularized boneimplants’, EC-GA n° 242175 HEALTH-2009–1.4.2) and by The Research Council of Norway ([FRIMED, 17734/V50], [156744], [Stem Cell 180383/V40]).
Conflict of interest
The authors report that there are no conflicts of interest.
References
- 1.Urist MR, Johnson RW., Jr Calcification and ossification. IV The healing of fractures in man under clinical conditions. J Bone Joint Surg Am. 1943;25:375–426. [Google Scholar]
- 2.Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36:S5–7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn TA, Majeska RJ, Rush EB, et al. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–81. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003a;18:1584–92. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 5.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules. Injury. 2007;38:S11–25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003b;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 7.Deckers MM, van Bezooijen RL, van der Horst G, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–53. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura Y, Nomura S, Kawasaki S, et al. Colocalization of noggin and bone morphogenetic protein-4 during fracture healing. J Bone Miner Res. 2001;16:876–84. doi: 10.1359/jbmr.2001.16.5.876. [DOI] [PubMed] [Google Scholar]
- 9.Onichtchouk D, Chen YG, Dosch R, et al. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–5. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 10.Nakao A, Afrakhte M, Morén A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–5. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 11.Bai S, Shi X, Yang X, Cao X. Smad6 as a transcriptional corepressor. J Biol Chem. 2000;275:8267–70. doi: 10.1074/jbc.275.12.8267. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation. Mech Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 13.Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355:S41–55. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- 14.Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38:S3–6. doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang H, Wang W, Tahernia AD, et al. Mechanical strain-induced proliferation of osteoblastic cells parallels increased TGF-beta 1 mRNA. Biochem Biophys Res Commun. 1996;229:449–53. doi: 10.1006/bbrc.1996.1824. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki K, Takagi M, Konttinen Y, et al. Upregulation of matrix metalloproteinase (MMP)-1 and its activator MMP-3 of human osteoblasts by uniaxial cyclic stimulation. J Biomed Mater Res B Appl Biomater. 2007;80B:491–8. doi: 10.1002/jbm.b.30622. [DOI] [PubMed] [Google Scholar]
- 17.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–26. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 18.Kershaw CJ, Cunningham JL, Kenwright J. Tibial external fixation, weight bearing, and fracture movement. Clin Orthop Relat Res. 1993;293:28–36. [PubMed] [Google Scholar]
- 19.Kwong FN, Harris MB. Recent developments in the biology of fracture repair. J Am Acad Orthop Surg. 2008;16:619–25. doi: 10.5435/00124635-200811000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Claes L, Eckert-Hübner K, Augat P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J Orthop Res. 2002;20:1099–105. doi: 10.1016/S0736-0266(02)00044-X. [DOI] [PubMed] [Google Scholar]
- 21.Loboa EG, Beaupre GS, Carter DR. Mechanobiology of initial pseudarthrosis formation with oblique fractures. J Orthop Res. 2001;19:1067–72. doi: 10.1016/S0736-0266(01)00028-6. [DOI] [PubMed] [Google Scholar]
- 22.Gartner LP, Hiatt JL. Color textbook of histology. 3rd ed. Philadelphia, PA: Saunders Elsevier; 2007. pp. 19103–2899. [Google Scholar]
- 23.Langman J. Medical embryology. human development-normal and abnormal. 3rd ed. Baltimore: The Williams & Wilkins Company; 1975. [Google Scholar]
- 24.ten Cate AR. Oral histology. Development, structure and function. St. Louis, MO: The C.V. Mosby Company; 1980. [Google Scholar]
- 25.Glenny AM, Hooper L, Shaw WC, et al. Feeding interventions for growth and development in infants with cleft lip, cleft palate or cleft lip and palate. Review. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003315.pub2. CD003315. [DOI] [PubMed] [Google Scholar]
- 26.Mossey PA, Little J, Munger RG, et al. Cleft lip and palate. Review. Lancet. 2009;9703:1773–85. doi: 10.1016/S0140-6736(09)60695-4. [DOI] [PubMed] [Google Scholar]
- 27.Pena WA, Vargervik K, Sharma A, Oberoi S. The role of endosseous implants in the management of alveolar clefts. Review. Pediatr Dent. 2009;31:329–33. [PubMed] [Google Scholar]
- 28.Esposito M, Grusovin MG, Coulthard P, et al. The efficacy of various bone augmentation procedures for dental implants: a cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2006;21:696–710. [PubMed] [Google Scholar]
- 29.Kelly CM, Wilkins RM, Gitelis S, et al. The use of a surgical grade calcium sulfate as a bone graftsubstitute: results of a multicenter trial. Clin Orthop Relat Res. 2001;382:42–50. doi: 10.1097/00003086-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Schindler OS, Cannon SR, Briggs TWR, et al. Composite ceramic bone graft substitute in the treatment of locally aggressive benign bone tumours. J Orthop Surg. 2008;16:66–74. doi: 10.1177/230949900801600116. [DOI] [PubMed] [Google Scholar]
- 31.Schieker M, Seitz H, Drosse I, et al. Biomaterials as scaffold for bone tissue engineering. Eur J Trauma. 2006;2:114–24. [Google Scholar]
- 32.Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara T, Suardita K, Ishii M, et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20:399–409. doi: 10.1359/JBMR.041117. [DOI] [PubMed] [Google Scholar]
- 34.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;8:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 35.Shi S, Bartold PM, Miura M, et al. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda E, Hirose M, Kotobuki N, et al. Osteogenic differentiation of human dental papilla mesenchymal cells. Biochem Biophys Res Commun. 2006;342:1257–62. doi: 10.1016/j.bbrc.2006.02.101. [DOI] [PubMed] [Google Scholar]
- 37.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 38.Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–5. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 39.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 40.Khan Y, Yaszemski MJ, Mikos AG, et al. Tissue engineering of bone: material and matrix consideration. J Bone Joint Surg Am. 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki O, Imaizumi H, Kamakura S, et al. Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr Med Chem. 2008;15:305–13. doi: 10.2174/092986708783497283. [DOI] [PubMed] [Google Scholar]
- 42.Guarino V, Causa F, Ambrosio L. Bioactive scaffolds for connective tissue regeneration. Exp Rev Med Dev. 2007;4:406–18. doi: 10.1586/17434440.4.3.405. [DOI] [PubMed] [Google Scholar]
- 43.Coombes AGA, Rizzi SC, Williamson M, et al. Precipitation casting of polycaprolactone for applica- tions in tissue engineering and drug delivery. Biomaterials. 2004;25:315–25. doi: 10.1016/s0142-9612(03)00535-0. [DOI] [PubMed] [Google Scholar]
- 44.Shikinami Y, Okazaki K, Saito M, et al. Bioactive and bioresorbable cellular cubic-composite scaffolds for use in bone reconstruction. J R Soc Interface. 2006;3:805–21. doi: 10.1098/rsif.2006.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mondrinos MJ, Dembzinsky R, Lu L, et al. Porogen-based solid freeform fabrication of polycaprolactone–calcium phosphate scaffold for tissue engineering. Biomaterials. 2006;27:4399–408. doi: 10.1016/j.biomaterials.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Hutmacher DW, Varawan S-L, et al. In vitro bone engineering based on polycaprolactone and polycaprolactone-tricalcium phosphate composites. Polym Int. 2007;56:333–42. [Google Scholar]
- 47.Guarino V, Ambrosio L. The synergic effect of polylactide fiber and calcium phosphate particle reinforcement in poly ɛ-caprolactone-based composite scaffolds. Acta Biomaterialia. 2008;4:1778–87. doi: 10.1016/j.actbio.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Cai Z-Y, Yang D-A, Zhang N, et al. Poly(propylene fumarate)/(calcium sulphate/β-tricalcium phosphate) composites: preparation, characterization and in vitro degradation. Acta Biomaterialia. 2009;5:628–35. doi: 10.1016/j.actbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Irina M, Pelin, Maier StelianS, et al. Preparation and characterization of a hydroxyapatite–collagen composite as component for injectable bone substitute. Mater Sci Engin. 2009;29:2188–94. [Google Scholar]
- 50.Azevedo MC, Reis RL, Claase MB, et al. Development and properties of polycaprolactone/hydroxyapatite composite biomaterials. J Mater Sci Mat Med. 2003;14:103–7. doi: 10.1023/a:1022051326282. [DOI] [PubMed] [Google Scholar]
- 51.Guarino V, Causa F, Netti PA, et al. The role of hydroxyapatite as solid signal on performance of PCL porous scaffolds for bone tissue regeneration. J Biomed Mater Res B. 2008;86:548–57. doi: 10.1002/jbm.b.31055. [DOI] [PubMed] [Google Scholar]
- 52.Kroeze RJ, Helder MN, Govaert LE, et al. Biodegradable polymers in bone tissue engineering. Materials. 2009;2:833–56. [Google Scholar]
- 53.Li F, Li S, Ghzaoui AE, et al. Synthesis and gelation properties of PEG-PLA-PEG triblock copolymers obtained by coupling monohydroxylated PEG-PLA with adipoyl chloride. Langmuir. 2007a;27:2778–83. doi: 10.1021/la0629025. [DOI] [PubMed] [Google Scholar]
- 54.Ryner M, Albertsson A-C. Resorbable and highly elastic block copolymers from 1,5-dioxepan-2-one and L-lactide with controlled tensile properties and hydrophilicity. Biomacromolecules. 2002;3:601–8. doi: 10.1021/bm015658z. [DOI] [PubMed] [Google Scholar]
- 55.Ryner M, Valdre A, Albertsson A-C. Star-shaped and photo-crosslinked poly(1,5-dioxepan-2-one) – synthesis and characterization. J Polym Sci A: Polym Sci. 2002;40:2049–54. [Google Scholar]
- 56.Idris S, Arvidson K, Plikk P, et al. Polyester copolymer scaffolds enhance expression of bone markers in osteoblast-like cells. J Biomed Mater Res A. 2010;94:631–9. doi: 10.1002/jbm.a.32726. [DOI] [PubMed] [Google Scholar]
- 57.Dånmark S, Finne-Wistrand A, Albertsson A-C, et al. Osteogenic differentiation in rat bone rarrow derived stromal cells on refined biodegradable polymer scaffolds. J Bioact Compat Pol. 2010;25:207–23. [Google Scholar]
- 58.Schander K, Arvidson K, Mustafa M, et al. Response of Bone and Periodontal Ligament Cells to Biodegradable Polymer Scaffolds in vitro. J Bioact Compat Pol. 2010;25:584–602. [Google Scholar]
- 59.Idris S, Danmark S, Finne-Wistrand A, et al. Biocompatibility of Polyester Scaffolds with Fibroblasts and Osteoblast-like Cells for Bone Tissue Engineering. J Bioact Compat Pol. 2010;25:567–83. [Google Scholar]
- 60.Wang S, Cui W, Bei J. Bulk and surface modifications of polylactide. Anal Bioanal Chem. 2005;381:547–56. doi: 10.1007/s00216-004-2771-2. [DOI] [PubMed] [Google Scholar]
- 61.Frost HM. Why should many skeletal scientists and clinicians learn the Utah paradigm of skeletal physiology. J Musculoskel Neuron Interact. 2001;2:121–30. [PubMed] [Google Scholar]
- 62.Parfitt AM. Bone forming cells in clinical conditions. In: Hall BK, editor. Bone, the osteoblast and osteocytes. London, UK: The Telford Press; 1991. pp. 351–426. [Google Scholar]
- 63.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–55. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 64.Blair HC, Robinson LJ, Zaidi M. Osteoclast signalling pathways. Biochem Biophys Res Commun. 2005;328:728–38. doi: 10.1016/j.bbrc.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 65.Friedenstein AJ, Ivanov-Smolenski AA, Chajlakjan RK, et al. Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants. Exp Hematol. 1978;6:440–4. [PubMed] [Google Scholar]
- 66.Friedenstein AJ. Osteogenic stem cells in the bone marrow. Bone Miner. 1991;7:243–72. [Google Scholar]
- 67.Benayahu D, Akavia UD, Shur I. Differentiation of bone marrow stroma-derived mesenchymal cells. Curr Med Chem. 2007;14:173–9. doi: 10.2174/092986707779313363. [DOI] [PubMed] [Google Scholar]
- 68.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea- pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 69.Luria EA, Panasyuk AF, Friedenstein AY. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11:345–9. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 70.Kassem M, Mosekilde L, Eriksen EF. 1,25-dihydroxyvitamin D3 potentiates fluoride-stimulated collagen type I production in cultures of human bone marrow stromal osteoblast-like cells. J Bone Miner Res. 1993;8:1453–8. doi: 10.1002/jbmr.5650081207. [DOI] [PubMed] [Google Scholar]
- 71.Rickard DJ, Kassem M, Hefferan TE, et al. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–24. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 72.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 73.Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43:32–9. doi: 10.1016/j.bone.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Abdallah BM, Haack-Sorensen M, Burns JS, et al. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005;326:527–38. doi: 10.1016/j.bbrc.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 75.Foster LJ, Zeemann PA, Li C, et al. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23:1367–77. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- 76.Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 77.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003a;116:1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 78.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–91. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 79.Miura Y, Miura M, Gronthos S, et al. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc Natl Acad Sci USA. 2005;102:14022–7. doi: 10.1073/pnas.0409397102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gang EJ, Bosnakovski D, Figueiredo CA, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 81.Kuznetsov SA, Mankani MH, Gronthos S, et al. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–40. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosada C, Justesen J, Melsvik D, et al. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72:135–42. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- 83.De Bari C, Dell’Accio F, Tylzanowski P, et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 84.Miura M, Gronthos S, Zhao MR, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 86.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–16. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 88.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 89.Justesen J, Stenderup K, Eriksen EF, et al. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- 90.Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–16. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 91.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 92.Piersanti S, Sacchetti B, Funari A, et al. Lentiviral transduction of human postnatal skeletal (stromal, mesenchymal) stem cells: in vivo transplantation and gene silencing. Calcif Tissue Int. 2006;78:372–84. doi: 10.1007/s00223-006-0001-y. [DOI] [PubMed] [Google Scholar]
- 93.Larsen KH, Frederiksen CM, Burns JS, et al. Identifying a molecular phenotype for bone marrow stromal cells with in vivo bone-forming capacity. J Bone Miner Res. 2010;25:796–808. doi: 10.1359/jbmr.091018. [DOI] [PubMed] [Google Scholar]
- 94.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 95.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 96.Qiu W, Andersen TE, Bollerslev J, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22:1720–31. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 97.Lecka-Czernik B, Moerman EJ, Grant DF, et al. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–84. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 98.Ng LJ, Wheatley S, Muscat GE, et al. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–21. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 99.Luu HH, Song WG, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–77. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 100.Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 101.Abdallah BM, Jensen CH, Gutierrez G, et al. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–52. doi: 10.1359/JBMR.040118. [DOI] [PubMed] [Google Scholar]
- 102.Rifas L. The role of noggin in human mesenchymal stem cell differentiation. J Cell Biochem. 2007;100:824–34. doi: 10.1002/jcb.21132. [DOI] [PubMed] [Google Scholar]
- 103.Kratchmarova I, Blagoev B, Haack-Sorensen M, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–7. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 104.Barrett-Connor E, Kritz-Silverstein D. Does hyperinsulinemia preserve bone. Diabetes Care. 1996;19:1388–92. doi: 10.2337/diacare.19.12.1388. [DOI] [PubMed] [Google Scholar]
- 105.Mukherjee A, Murray RD, Shalet SM. Impact of growth hormone status on body composition and the skeleton. Horm Res. 2004;62:35–41. doi: 10.1159/000080497. [DOI] [PubMed] [Google Scholar]
- 106.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 107.Chan GK, Duque G. Age-related bone loss: old bone, new facts. Gerontology. 2002;48:62–71. doi: 10.1159/000048929. [DOI] [PubMed] [Google Scholar]
- 108.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–96. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 109.Hong JH, Hwang ES, McManus MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–8. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 110.Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sabatakos G, Sims NA, Chen J, et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–90. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 112.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–7. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 113.Gimble JM, Zvonic S, Floyd ZE, et al. Playing with bone and fat. J Cell Biochem. 2006;98:251–66. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 114.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone. Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 115.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferron M, Hinoi E, Karsenty G, et al. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdallah BM, Ding M, Jensen CH, et al. Dlk1/FA1 is a novel endocrine regulator of bone and fat mass and its serum level is modulated by growth hormone. Endocrinology. 2007a;148:3111–21. doi: 10.1210/en.2007-0171. [DOI] [PubMed] [Google Scholar]
- 118.Abdallah BM, Boissy P, Tan Q, et al. dlk1/FA1 regulates the function of human bone marrow mesenchymal stem cells by modulating gene expression of pro-inflammatory cytokines and immune response-related factors. J Biol Chem. 2007b;282:7339–51. doi: 10.1074/jbc.M607530200. [DOI] [PubMed] [Google Scholar]
- 119.Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419–47. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- 120.Douchi T, Oki T, Nakamura S, et al. The effect of body composition on bone density in pre- and postmenopausal women. Maturitas. 1997;27:55–60. doi: 10.1016/s0378-5122(97)01112-2. [DOI] [PubMed] [Google Scholar]
- 121.Ray R, Novotny NM, Crisostomo PR, et al. Sex steroids and stem cell function. Mol Med. 2008;14:493–501. doi: 10.2119/2008-00004.Ray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heim M, Frank O, Kampmann G, et al. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology. 2004;145:848–59. doi: 10.1210/en.2003-1014. [DOI] [PubMed] [Google Scholar]
- 123.Ramalho AC, Jullienne A, Couttet P, et al. Effect of oestradiol on cytokine production in immortalized human marrow stromal cell lines. Cytokine. 2001;16:126–30. doi: 10.1006/cyto.2001.0956. [DOI] [PubMed] [Google Scholar]
- 124.Hong L, Colpan A, Peptan IA. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006;12:2747–53. doi: 10.1089/ten.2006.12.2747. [DOI] [PubMed] [Google Scholar]
- 125.Leskelä HV, Olkku A, Lehtonen S, et al. Estrogen receptor alpha genotype confers interindividual variability of response to estrogen and testosterone in mesenchymal-stem-cell-derived osteoblasts. Bone. 2006;39:1026–34. doi: 10.1016/j.bone.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 126.Boissy P, Andersen TL, Abdallah BM, et al. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65:9943–52. doi: 10.1158/0008-5472.CAN-05-0651. [DOI] [PubMed] [Google Scholar]
- 127.Dai Z, Li Y, Quarles LD, et al. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14:806–14. doi: 10.1016/j.phymed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 128.Pino AM, Rodriguez JM, Rios S, et al. Aromatase activity of human mesenchymal stem cells is stimulated by early differentiation, vitamin D and leptin. J Endocrinol. 2006;191:715–25. doi: 10.1677/joe.1.07026. [DOI] [PubMed] [Google Scholar]
- 129.Sillat T, Pöllänen R, Lopes JR, et al. Intracrine androgenic apparatus in human bone marrow stromal cells. J Cell Mol Med. 2009;13:3296–302. doi: 10.1111/j.1582-4934.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Compston J. Local biosynthesis of sex steroids in bone. J Clin Endocrinol Metab. 2002;87:5398–400. doi: 10.1210/jc.2002-021420. [DOI] [PubMed] [Google Scholar]
- 131.Brockstedt H, Kassem M, Eriksen EF, et al. Age- and sex-related changes in iliac cortical bone mass and remodelling. Bone. 1993;14:681–91. doi: 10.1016/8756-3282(93)90092-o. [DOI] [PubMed] [Google Scholar]
- 132.Stenderup K, Justesen J, Eriksen EF, et al. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16:1120–9. doi: 10.1359/jbmr.2001.16.6.1120. [DOI] [PubMed] [Google Scholar]
- 133.Stenderup K, Justesen J, Clausen C, et al. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 134.Abdallah BM, Haack-Sorensen M, Fink T, et al. Inhibition of osteoblast differentiation but not adipocyte differentiation of mesenchymal stem cells by sera obtained from aged females. Bone. 2006;39:181–8. doi: 10.1016/j.bone.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 135.Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–6. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 136.Gronthos S, Chen SQ, Wang CY, et al. Telomerase accelerates osteogenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J Bone Miner Res. 2003b;18:716–22. doi: 10.1359/jbmr.2003.18.4.716. [DOI] [PubMed] [Google Scholar]
- 137.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–21. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 138.Guillot PV, Cui W, Fisk NM, et al. Stem cell differentiation and expansion for clinical applications of tissue engineering. J Cell Mol Med. 2007;11:935–44. doi: 10.1111/j.1582-4934.2007.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaviani A, Perry TE, Dzakovic A, et al. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg. 2001;36:1662–5. doi: 10.1053/jpsu.2001.27945. [DOI] [PubMed] [Google Scholar]
- 140.Kaviani A, Perry TE, Barnes CM, et al. The placenta as a cell source in fetal tissue engineering. J Pediatr Surg. 2002;37:995–9. doi: 10.1053/jpsu.2002.33828. [DOI] [PubMed] [Google Scholar]
- 141.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 142.Quintin A, Schizas C, Scaletta C, et al. Isolation and in vitro chondrogenic potential of human foetal spine cells. J Cell Mol Med. 2009;13:2559–69. doi: 10.1111/j.1582-4934.2008.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tobita M, Uysal AC, Ogawa R, et al. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14:945–53. doi: 10.1089/ten.tea.2007.0048. [DOI] [PubMed] [Google Scholar]
- 144.Hohlfeld J, de Buys Roessingh A, Hirt-Burri N, et al. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366:840–2. doi: 10.1016/S0140-6736(05)67107-3. [DOI] [PubMed] [Google Scholar]
- 145.Kent J, Pfeffer N. Regulating the collection and use of fetal stem cells. BMJ. 2006;332:866–7. doi: 10.1136/bmj.332.7546.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Applegate LA, Scaletta C, Hirt-Burri N, et al. Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Skin Pharmacol Physiol. 2009;22:63–73. doi: 10.1159/000178865. [DOI] [PubMed] [Google Scholar]
- 147.Hirt-Burri N, de Buys Roessingh AS, Scaletta C, et al. Human muscular fetal cells: a potential cell source for muscular therapies. Pediatr Surg Int. 2008;24:37–47. doi: 10.1007/s00383-007-2040-5. [DOI] [PubMed] [Google Scholar]
- 148.Pioletti DP, Montjovent MO, Zambelli PY, et al. Bone tissue engineering using foetal cell therapy. Swiss Med Wkly. 2006;136:557–60. doi: 10.4414/smw.2006.11411. [DOI] [PubMed] [Google Scholar]
- 149.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–94. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Connolly JF, Guse R, Tiedeman J, et al. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;266:259–70. [PubMed] [Google Scholar]
- 151.Kruyt M, De Bruijn J, Rouwkema J Van Bliterswijk C, et al. Analysis of the dynamics of bone formation, effect of cell seeding density, and potential of allogeneic cells in cell-based bone tissue engineering in goats. Tissue Eng A. 2008;14:1081–8. doi: 10.1089/ten.tea.2007.0111. [DOI] [PubMed] [Google Scholar]
- 152.Connolly J, Guse R, Lippiello L, et al. Development of an osteogenic bone-marrow preparation. J Bone Joint Surg Am. 1989;71:684–91. [PubMed] [Google Scholar]
- 153.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 154.Montjovent MO, Bocelli-Tyndall C, Scaletta C, et al. In vitro characterization of immune-related properties of human fetal bone cells for potential tissue engineering applications. Tissue Eng A. 2009;15:1523–32. doi: 10.1089/ten.tea.2008.0222. [DOI] [PubMed] [Google Scholar]
- 155.Quintin A, Hirt-Burri N, Scaletta C, et al. Consistency and safety of cell banks for research and clinical use: preliminary analysis of fetal skin banks. Cell Transplant. 2007;16:675–84. doi: 10.3727/000000007783465127. [DOI] [PubMed] [Google Scholar]
- 156.Mirmalek-Sani SH, Tare RS, Morgan SM, et al. Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cells. 2006;24:1042–53. doi: 10.1634/stemcells.2005-0368. [DOI] [PubMed] [Google Scholar]
- 157.Montjovent MO, Burri N, Mark S, et al. Fetal bone cells for tissue engineering. Bone. 2004;35:1323–33. doi: 10.1016/j.bone.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 158.Montjovent MO, Mathieu L, Hinz B, et al. Biocompatibility of bioresorbable poly(L-lactic acid) composite scaffolds obtained by supercritical gas foaming with human fetal bone cells. Tissue Eng. 2005;11:1640–9. doi: 10.1089/ten.2005.11.1640. [DOI] [PubMed] [Google Scholar]
- 159.SwissMedics. Swiss Federal Council Transplantation Law. TxL;SR 810.21, 2007.
- 160.EU. Community code relating to medicinal products for hum use Parliament E editor. Directive 2001/83/EC, 2001. [Google Scholar]
- 161.Parliament E, editor. EU, Implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. editor. Directive 2006/17/EC, 2006.
- 162.Parliament E, editor. EU, Implementing Directive 2004/23/EC of the European Parliament and of the Council as regards traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells. editor. Directive 2006/86/EC, 2006.
- 163.EU. Advanced therapy medicinal products amending Directive 2001/83/EC and Regulation (EC) No 726/2004, Parliament, editor. Regulation (EC) No 1394/2007, 2007.
- 164.FDA. Human cells, tissues, and cellular and tissue-based products. 21 CFR 1271, 2006.
- 165.EU. Setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components, Directive 2002/98/EC, 2002.
- 166.EU. Setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells Parliament E editor. Directive 2004/23/EC, 2004.
- 167.Heinonen M, Oila O, Nordstrom K. Current issues in the regulation of human tissue-engineering products in the European Union. Tissue Eng. 2005;11:1905–11. doi: 10.1089/ten.2005.11.1905. [DOI] [PubMed] [Google Scholar]
- 168.Trommelmans L, Selling J, Dierickx K. A critical assessment of the directive on tissue engineering of the European union. Tissue Eng. 2007;13:667–72. doi: 10.1089/ten.2006.0089. [DOI] [PubMed] [Google Scholar]
- 169.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 170.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 171.Mazzucco L, Balbo V, Cattana E, et al. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110–8. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 172.Dohan DM, Choukrou J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006a;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 173.Anitua E, Sánchez M, Orive G, et al. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28:4551–60. doi: 10.1016/j.biomaterials.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 174.Mazzucco L, Balbo V, Cattana E, et al. Platelet-rich plasma and platelet gel preparation using Plateltex. Vox Sang. 2008;94:202–8. doi: 10.1111/j.1423-0410.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 175.Anitua E, Aguirre JJ, Algorta J, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84:415–21. doi: 10.1002/jbm.b.30886. [DOI] [PubMed] [Google Scholar]
- 176.Moojen DJ, Everts PA, Schure RM, et al. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26:404–10. doi: 10.1002/jor.20519. [DOI] [PubMed] [Google Scholar]
- 177.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006b;101:e51–55. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 178.Vogel JP, Szalay K, Geiger F, et al. Platelet-rich plasma improves expansion of human mesenchymal stem cells and retains differentiation capacity and in vivo bone formation in calcium phosphate. Platelets. 2006;17:462–9. doi: 10.1080/09537100600758867. [DOI] [PubMed] [Google Scholar]
- 179.Lucarelli E, Beccheroni A, Donati D, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 180.Gruber R, Varga F, Fischer MB, et al. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002a;13:529–35. doi: 10.1034/j.1600-0501.2002.130513.x. [DOI] [PubMed] [Google Scholar]
- 181.Ogino Y, Ayukawa Y, Kukita T, et al. The contribution of platelet-derived growth factor, transforming growth factor-beta1, and insulin-like growth factor-I in platelet-rich plasma to the proliferation of osteoblast-like cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:724–9. doi: 10.1016/j.tripleo.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 182.Gruber R, Karreth F, Kandler B, et al. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004;15:29–35. doi: 10.1080/09537100310001643999. [DOI] [PubMed] [Google Scholar]
- 183.Gruber R, Kandler B, Fischer MB, et al. Osteogenic differentiation induced by bone morphogenetic proteins can be suppressed by platelet-released supernatant in vitro. Clin Oral Implants Res. 2006;17:188–93. doi: 10.1111/j.1600-0501.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- 184.He L, Lin Y, Hu X, et al. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–13. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 185.Butcher A, Milner R, Ellis K, et al. Interaction of platelet-rich concentrate with bone graft materials: an in vitro study. J Orthop Trauma. 2009;23:195–200. doi: 10.1097/BOT.0b013e31819b35db. [DOI] [PubMed] [Google Scholar]
- 186.Cenni E, Perut F, Ciapetti G, et al. In vitro evaluation of freeze-dried bone allografts combined with platelet rich plasma and human bone marrow stromal cells for tissue engineering. J Mater Sci Mater Med. 2009a;20:45–50. doi: 10.1007/s10856-008-3544-9. [DOI] [PubMed] [Google Scholar]
- 187.Kasten P, Vogel J, Geiger F, et al. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29:3983–92. doi: 10.1016/j.biomaterials.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 188.Nair MB, Varma HK, John A. Platelet-rich plasma and fibrin glue-coated bioactive ceramics enhance growth and differentiation of goat bone marrow-derived stem cells. Tissue Eng A. 2009a;15:1619–31. doi: 10.1089/ten.tea.2008.0229. [DOI] [PubMed] [Google Scholar]
- 189.Zhang Z, Chen J, Jin D. Platelet-derived growth factor (PDGF)-BB stimulates osteoclastic bone resorption directly: the role of receptor beta. Biochem Biophys Res Commun. 1998;251:190–4. doi: 10.1006/bbrc.1998.9412. [DOI] [PubMed] [Google Scholar]
- 190.Niida S, Kaku M, Amano H, et al. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med. 1999;190:293–8. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Weicht B, Maitz P, Kandler B, et al. Activated platelets positively regulate RANKL-mediated osteoclast differentiation. J Cell Biochem. 2007;102:1300–7. doi: 10.1002/jcb.21360. [DOI] [PubMed] [Google Scholar]
- 192.Gruber R, Karreth F, Fischer MB, et al. Platelet-released supernatants stimulate formation of osteoclast-like cells through a prostaglandin/RANKL-dependent mechanism. Bone. 2002b;30:726–32. doi: 10.1016/s8756-3282(02)00697-x. [DOI] [PubMed] [Google Scholar]
- 193.Ogino Y, Ayukawa Y, Kukita T, et al. Platelet-rich plasma suppresses osteoclastogenesis by promoting the secretion of osteoprotegerin. J Periodontal Res. 2009;44:217–24. doi: 10.1111/j.1600-0765.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 194.Cenni E, Avnet S, Fotia C, et al. Platelet-rich plasma impairs osteoclast generation from human precursors of peripheral blood. J Orthop Res. 2010;28:792–7. doi: 10.1002/jor.21073. [DOI] [PubMed] [Google Scholar]
- 195.Gerard D, Carlson ER, Gotcher JE, et al. Effects of platelet-rich plasma at the cellular level on healing of autologous bone-grafted mandibular defects in dogs. J Oral Maxillofac Surg. 2007;65:721–7. doi: 10.1016/j.joms.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 196.Pilkington MF, Sims SM, Dixon SJ. Transforming growth factor-β induces osteoclast ruffling and chemotaxis: potential role in osteoclast recruitment. J Bone Miner Res. 2001;16:1237–47. doi: 10.1359/jbmr.2001.16.7.1237. [DOI] [PubMed] [Google Scholar]
- 197.Takai H, Kanematsu M, Yano K, et al. Transforming growth factor-β stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem. 1998;273:27091–6. doi: 10.1074/jbc.273.42.27091. [DOI] [PubMed] [Google Scholar]
- 198.Towler DA. The osteogenic-angiogenic interface: novel insights into the biology of bone formation and fracture repair. Curr Osteoporos Rep. 2008;6:67–71. doi: 10.1007/s11914-008-0012-x. [DOI] [PubMed] [Google Scholar]
- 199.Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006;21:183–92. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 200.von Schroeder HP, Veillette CJ, Payandeh J, et al. Endothelin-1 promotes osteoprogenitor proliferation and differentiation in fetal rat calvarial cell cultures. Bone. 2003;33:673–84. doi: 10.1016/s8756-3282(03)00215-1. [DOI] [PubMed] [Google Scholar]
- 201.Bouletreau PJ, Warren SM, Spector JA, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109:2384–97. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 202.Bertrand-Duchesne MP, Grenier D, Gagnon G. Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferation in vitro. J Periodontal Res. 2009;45:87–93. doi: 10.1111/j.1600-0765.2009.01205.x. [DOI] [PubMed] [Google Scholar]
- 203.Kilian O, Flesch I, Wenisch S, et al. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res. 2004;9:337–44. [PubMed] [Google Scholar]
- 204.Kandler B, Fischer MB, Watzek G, et al. Platelet-released supernatant increases matrix metalloproteinase-2 production, migration, proliferation and tube formation of human umbilical vascular endothelial cells. J Periodontol. 2004;75:1255–61. doi: 10.1902/jop.2004.75.9.1255. [DOI] [PubMed] [Google Scholar]
- 205.Bir SC, Esaki J, Marui A, et al. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50:870–9. doi: 10.1016/j.jvs.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 206.Fiedler J, Etzel N, Brenner RE. To go or not to go: migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem. 2004;93:990–8. doi: 10.1002/jcb.20219. [DOI] [PubMed] [Google Scholar]
- 207.Cenni E, Ciapetti G, Granchi D, et al. Endothelial cells incubated with platelet-rich plasma express PDGF-B and ICAM-1 and induce bone marrow stromal cell migration. J Orthop Res. 2009b;27:1493–8. doi: 10.1002/jor.20896. [DOI] [PubMed] [Google Scholar]
- 208.Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants. 2004;19:59–65. [PubMed] [Google Scholar]
- 209.Jensen TB, Rahbek O, Overgaard S, et al. Platelet rich plasma and fresh frozen bone allograft as enhancement of implant fixation. An experimental study in dogs. J Orthop Res. 2004;22:653–8. doi: 10.1016/j.orthres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 210.Roldán JC, Jepsen S, Miller J, et al. Bone formation in the presence of platelet-rich plasma versus bone morphogenetic protein-7. Bone. 2004;34:80–90. doi: 10.1016/j.bone.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 211.Yamada Y, Ueda M, Hibi H, et al. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: from basic research to clinical case study. Cell Transplant. 2004a;4:343–55. doi: 10.3727/000000004783983909. [DOI] [PubMed] [Google Scholar]
- 212.Dallari D, Fini M, Stagni C, et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877–88. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 213.Nagata M, Messora M, Okamoto R, et al. Influence of the proportion of particulate autogenous bone graft/platelet-rich plasma on bone healing in critical-size defects: an immunohistochemical analysis in rat calvaria. Bone. 2009a;45:339–45. doi: 10.1016/j.bone.2009.04.246. [DOI] [PubMed] [Google Scholar]
- 214.Suba Z, Takacs D, Gyulai-Gaal S, et al. Facilitation of β-tricalcium phosphate-induced alveolar bone regeneration by platelet-rich plasma in beagle dogs: a histologic and histomorphometric study. Int J Oral Maxillofac Implants. 2004;19:832–8. [PubMed] [Google Scholar]
- 215.Nair MB, Varma HK, Menon KV, et al. Reconstruction of goat femur segmental defects using triphasic ceramic-coated hydroxyapatite in combination with autologous cells and platelet-rich plasma. Acta Biomater. 2009b;5:1742–55. doi: 10.1016/j.actbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 216.Geuze RE, Everts PA, Kruyt MC. Orthotopic location has limited benefit from allogeneic or autologous multipotent stromal cells seeded on ceramic scaffolds. Tissue Engineering A. 2009;15:3231–9. doi: 10.1089/ten.TEA.2009.0023. [DOI] [PubMed] [Google Scholar]
- 217.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032–44. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 218.Yamada Y, Ueda M, Naiki T, et al. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004b;10:955–64. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 219.Ito K, Yamada Y, Naiki T, et al. Simultaneous implant placement and bone regeneration around dental implants using tissue-engineered bone with fibrin glue, mesenchymal stem cells and platelet-rich plasma. Clin Oral Implants Res. 2006;5:579–86. doi: 10.1111/j.1600-0501.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 220.Fuerst G, Gruber R, Tangl S, et al. Enhanced bone-to-implant contact by platelet-released growth factors in mandibular cortical bone: a histomorphometric study in minipigs. Int J Oral Maxillofac Implants. 2003;18:685–90. [PubMed] [Google Scholar]
- 221.Nagata MJ, Melo LG, Messora MR, et al. Effect of platelet-rich plasma on bone healing of autogenous bone grafts in critical-size defects. J Clin Periodontol. 2009b;36:775–83. doi: 10.1111/j.1600-051X.2009.01450.x. [DOI] [PubMed] [Google Scholar]
- 222.Wiltfang J, Kloss FR, Kessler P, et al. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. Clin Oral Implants Res. 2004;15:187–93. doi: 10.1111/j.1600-0501.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- 223.Fennis JP, Stoelinga PJ, Jansen JA. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg. 2004;33:48–55. doi: 10.1054/ijom.2003.0452. [DOI] [PubMed] [Google Scholar]
- 224.Choi BH, Im CJ, Huh JY, et al. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33:56–9. doi: 10.1054/ijom.2003.0466. [DOI] [PubMed] [Google Scholar]
- 225.Thor A, Franke-Stenport V, Johansson CB, et al. Early bone formation in human bone grafts treated with platelet-rich plasma: preliminary histomorphometric results. Int J Oral Maxillofac Surg. 2007;36:1164–71. doi: 10.1016/j.ijom.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 226.Intini G. The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials. 2009;30:4956–66. doi: 10.1016/j.biomaterials.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 227.Alsousou J, Thompson M, Hulley P, et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 228.Galasso O, Mariconda M, Romano G, et al. Expandable intramedullary nailing and platelet rich plasma to treat long bone non-unions. J Orthop Traumatol. 2008;9:129–34. doi: 10.1007/s10195-008-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kitoh H, Kawasumi M, Kaneko H, et al. Differential effects of culture-expanded bone marrow cells on the regeneration of bone between the femoral and the tibial lengthenings. J Pediatr Orthop. 2009;29:643–9. doi: 10.1097/BPO.0b013e3181b2afb2. [DOI] [PubMed] [Google Scholar]
- 230.Gandhi A, Bibbo C, Pinzur M, et al. The role of platelet-rich plasma in foot and ankle surgery. Foot Ankle Clin. 2005;10:621–37. doi: 10.1016/j.fcl.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 231.Bielecki T, Gazdzik TS, Szczepanski T. Benefit of percutaneous injection of autologous platelet-leukocyte-rich gel in patients with delayed union and nounion. Eur Surg Res. 2008;40:289–96. doi: 10.1159/000114967. [DOI] [PubMed] [Google Scholar]
- 232.Mariconda M, Cozzolino F, Cozzolino A, et al. Platelet gel supplementation in long bone nonunions treated by external fixation. J Orthop Trauma. 2008;22:342–5. doi: 10.1097/BOT.0b013e318172cea5. [DOI] [PubMed] [Google Scholar]
- 233.Calori GM, Tagliabue L, Gala L, et al. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39:1391–402. doi: 10.1016/j.injury.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 234.Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone. 2007;40:522–8. doi: 10.1016/j.bone.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 235.Jenis LG, Banco RJ, Kwon B. A prospective study of autologous growth factors (AGF) in lumbar interbody. Spine J. 2006;6:14–20. doi: 10.1016/j.spinee.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 236.Carreon LY, Glassman SD, Anekstein Y, et al. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30:E243–6. doi: 10.1097/01.brs.0000160846.85397.44. [DOI] [PubMed] [Google Scholar]
- 237.Coetzee JC, Pomeroy GC, Watts JD, et al. The use of autologous concentrated growth factors to promote syndesmosis fusion in the Agility total ankle replacement. A preliminary study. Foot Ankle Int. 2005;26:840–6. doi: 10.1177/107110070502601009. [DOI] [PubMed] [Google Scholar]
- 238.Barrow CR, Pomeroy GC. Enhancement of syndesmotic fusion rates in total ankle arthroplasty with the use of autologous platelet concentrate. Foot Ankle Int. 2005;26:458–61. doi: 10.1177/107110070502600606. [DOI] [PubMed] [Google Scholar]
- 239.Bibbo C, Bono CM, Lin SS. Union rates using autologous platelet concentrate alone and with bone graft in high-risk foot and ankle surgery patients. J Surg Orthop Adv. 2005;14:17–22. [PubMed] [Google Scholar]
- 240.Berghoff W, Pietrzak W, Rhodes R. Platelet-rich plasma application during closure following total knee arthroplasty. Orthopedics. 2006;29:590–8. doi: 10.3928/01477447-20060701-11. [DOI] [PubMed] [Google Scholar]
- 241.Yamada Y, Ueda M, Naiki T, et al. Tissue-engineered injectable bone regeneration for osseointegrated dental implants. Clin Oral Implants Res. 2004c;5:589–597. doi: 10.1111/j.1600-0501.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 242.Ueda M, Yamada Y, Ozawa R, et al. Clinical case reports of injectable tissue-engineered bone for alveolar augmentation with simultaneous implant placement. Int J Periodont Restorative Dent. 2005;2:129–37. [PubMed] [Google Scholar]
- 243.Marx RE. Reconstruction of defects caused by bisphosphonate-induced osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:107–19. doi: 10.1016/j.joms.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 244.Plachokova AS, Nikolidakis D, Mulder J, et al. Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res. 2008;19:539–45. doi: 10.1111/j.1600-0501.2008.01525.x. [DOI] [PubMed] [Google Scholar]
- 245.Griffin XL, Smith CM, Costa ML. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40:158–62. doi: 10.1016/j.injury.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 246.Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–20. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 247.Smith SE, Roukis TS. Bone and wound healing augmentation with platelet-rich plasma. Clin Podiatr Med Surg. 2009;26:559–88. doi: 10.1016/j.cpm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 248.Li X, Quigg RJ, Zhou J, et al. Early signals for fracture healing. J Cell Biochem. 2005;95:189–205. doi: 10.1002/jcb.20373. [DOI] [PubMed] [Google Scholar]
- 249.Li X, Wang H, Touma E, et al. Genetic network and pathway analysis of differentially expressed proteins during critical cellular events in fracture repair. J Cell Biochem. 2007;100:527–43. doi: 10.1002/jcb.21017. [DOI] [PubMed] [Google Scholar]
- 250.Deschaseaux F, Sensébé L, Heymann D. Mechanisms of bone repair and regeneration. Trends Mol Med. 2009;15:417–29. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 251.Kulterer B, Friedl G, Jandrositz A, et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. 2007;8:70–85. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Granchi D, Ochoa G, Leonardi E, et al. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during ex vivo expansion. Tissue Eng Part C Methods. 2010;16:511–24. doi: 10.1089/ten.TEC.2009.0405. [DOI] [PubMed] [Google Scholar]
- 253.Gronthos S, Simmons PJ, Graves SE, et al. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28:174–81. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 254.Hadjiargyrou M, Lombardo F, Zhao S, et al. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002;277:30177–82. doi: 10.1074/jbc.M203171200. [DOI] [PubMed] [Google Scholar]
- 255.Huang W, Yang S, Shao J, et al. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068–92. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473:188–92. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Deng ZL, Sharff KA, Tang N, et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–21. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 258.Papachroni KK, Karatzas DN, Papavassiliou KA, et al. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–16. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 259.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 260.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 261.Wan M, Cao X. BMP signaling in skeletal development. Biochem Biophys Res Commun. 2005;328:651–7. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 262.Lai CF, Cheng SL. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J Biol Chem. 2002;277:15514–22. doi: 10.1074/jbc.M200794200. [DOI] [PubMed] [Google Scholar]
- 263.Su N, Du X, Chen L. FGF signaling: its role in bone development and human skeleton diseases. Front Biosci. 2008;13:2842–65. doi: 10.2741/2890. [DOI] [PubMed] [Google Scholar]
- 264.Jacob AL, Smith C, Partanen J, et al. Fibroblast growth factor receptor 1 signaling in the osteochondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev Biol. 2006;296:315–28. doi: 10.1016/j.ydbio.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Montero A, Okada Y, Tomita M, et al. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest. 2000;105:1085–93. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005;16:205–13. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–59. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nakae J, Yoshiaki Kido Y, Accili D. Distinct and overlapping functions of Insulin and IGF-I receptors. Endocrine Reviews. 2001;22:818–35. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- 269.Tokunaga A, Oya T, Ishii Y, et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23:1519–28. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 270.Kumar A, Salimath BP, Stark GB, et al. Platelet-derived growth factor receptor signaling is not involved in osteogenic differentiation of human mesenchymal stem cells. Tissue Eng A. 2010;16:983–93. doi: 10.1089/ten.TEA.2009.0230. [DOI] [PubMed] [Google Scholar]
- 271.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 272.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 273.Jackson A, Vayssiere B, Garcia T, et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36:585–98. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 274.Fujita K, Janz S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71–83. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 276.Chung UI, Schipani E, McMahon AP, et al. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107:295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Long F, Chung UI, Ohba S, et al. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131:1309–18. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 278.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90:19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- 279.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 280.Canalis E. Notch signaling in osteoblasts. Sci Signal. 2008;1:1–4. doi: 10.1126/stke.117pe17. [DOI] [PubMed] [Google Scholar]
- 281.Chau JF, Leong WF, Li B. Signaling pathways governing osteoblast proliferation, differentiation and function. Histol Histopathol. 2009;24:1593–606. doi: 10.14670/HH-24.1593. [DOI] [PubMed] [Google Scholar]
- 282.Engin F, Yao Z, Yang T, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126:441–3. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 284.Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 285.Irie N, Takada Y, Watanabe Y, et al. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 2009;284:14637–44. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 287.Lian JB, Javed A, Zaidi SK, et al. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 288.Koga T, Matsui Y, Asagiri M, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11:880–5. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 289.Hartmann C. Transcriptional networks controlling skeletal development. Curr Opin Genet Dev. 2009;19:437–43. doi: 10.1016/j.gde.2009.09.001. [DOI] [PubMed] [Google Scholar]


