Abstract
In addition to roles in stress response, heat shock factors (HSFs) play crucial roles in differentiation and development. Heat shock transcription factor 4 (HSF4) deficiency leads to defect in lens epithelial cell (LEC) differentiation and cataract formation. However, the mechanism remains obscure. Here, we identified Src kinase-associated phosphoprotein 2 (SKAP2) as a downstream target of HSF4b and it was highly expressed at the anterior tip of lens elongating fibre cells in vivo. The HSF4-deficient lenses showed reduced SKAP2 expression and defects in actin reorganization. The disassembly of stress fibres and formation of cortical actin fibres are critical for the initiation of LEC differentiation. SKAP2 localized at actin-rich ruffles in human LECs (SRA01/04 cells) and knockdown SKAP2 using RNA interference impaired the disassembly of cellular stress fibres in response to fibroblast growth factor (FGF)-b. Overexpression of SKAP2, but not the N-terminal deletion mutant of SKAP2, induced the actin remodelling. We further found that SKAP2 interacted with the SH2 domain of non-catalytic region of tyrosine kinase adaptor protein 2 (NCK2) via its N-terminus. The complex of SKAP2-NCK2-F-actin accumulated at the leading edge of the lamellipodium, where FGF receptors and focal adhesion were also recruited. These results revealed an essential role for HSF4-mediated SKAP2 expression in the regulation of actin reorganization during lens differentiation, likely through a mechanism that SKAP2 anchors the complex of NCK2/focal adhesion to FGF receptors at the lamellipodium in lens epithelial cells.
Keywords: lens cell differentiation, actin reorganization, SKAP2, NCK2, HSF4b
Introduction
The lens starts development during the embryonic stage and continues to grow after birth, with the new secondary fibres being added from the outer mono-layer epithelium. Lens epithelial cells (LECs) start to differentiate, elongate, express lens fibre cell specific genes, lose contact with the capsule and epithelium, and finally lose their organelles as they become mature lens fibres [1]. Various signalling proteins are involved in this process, including fibroblast growth factor (FGF)-b, insulin-like growth factor (IGF)-1, transforming growth factor-β, N-cadherin and integrin [2–4]. Actin reorganization, which is profoundly affected by those signals, is essential for the differentiation induction and survival of LECs [5], and precise LEC differentiation is important for the transparency of the lens.
Previously we have reported that a DNA-binding domain mutant allele of heat shock factor (HSF)4 is associated with autosomal dominant lamellar and Marner cataracts [6]. Then, it has been reported that HSF4 knockout mice have defects in LECs differentiation and form cataracts [7, 8]. HSF4 belongs to the transcriptional regulator family of heat shock proteins (HSPs), and it binds to the heat shock element (HSE) that is composed of consensus inverted repeats of nGAAn [9]. Apart from the HSP genes, HSFs may also regulate distinct, non-HSP genes. Candidate targets of HSF4b include Crygf, Fgf7, Hspb2 and Bfsp2 [7, 8, 10]. There are two splicing isoforms of HSF4: HSF4a and HSF4b. However, only HSF4b is highly expressed in mouse lens. Although HSF4 knockout mice have obvious defects in lens development, the function of HSF4 in lens development remains elusive. To understand the role of HSF4 in lens development, it is imperative to identify its downstream targets.
In searching for the downstream targets of HSF4, we compared two independent sets of microarray expression data from HSF4 knockout mice with different backgrounds (C57BL/6-129/S3 and C57BL/6-129/SvJ) and found that the Src kinase-associated phosphoprotein 2 (SKAP2) gene is down-regulated in both HSF4−/– mouse lenses [8, 10].
SKAP2 (also called SCAP2, RA70, SKAP-HOM and SKAP55R) is a homolog of SKAP55 (encoded by Skap1). Unlike SKAP55, which is expressed exclusively in the thymus and in T lymphocytes, SKAP2 is expressed ubiquitously [11, 12]. The protein structure of SKAP2 is similar to that of SKAP55, both containing a pleckstrin homology (PH) domain, which can bind to membrane lipids [13], and both having a carboxyl-terminal src homology3 (SH3) domain. However, SKAP2 has a unique N-terminal coiled-coil (CC) domain and tyrosine phosphorylation sites. The amino acid sequence of mouse SKAP2 is 90% identical to human SKAP2, suggesting functional conservation of this protein. Previous studies on haematopoietic and immune systems have shown that SKAP2 binds to FYB (the Fyn binding protein) via its SH3 domain and is a substrate of Fyn kinase, which suggests a role of SKAP2 in T-cell receptor signalling similar to that of SKAP55 [12, 14]. It has also been reported that adhesion of activated B cells to fibronectin and to ICAM-1 is strongly reduced in the SKAP2−/– mouse, implying that SKAP2 might also be involved in the B-cell adhesion processes by coupling the B-cell receptor with the activation of integrin [15]. However, the mechanism of how SKAP2 is involved in integrin adhesion remains unclear, and much less is known for the function of SKAP2 beyond the immune system. Here, our results illustrate an essential role for SKAP2, a downstream target of HSF4b, in actin reorganization, providing a potential explanation for the cataracts formation in HSF4 knockout mice.
Material and methods
Cell culture and LEC differentiation induction
Cells from the human lens epithelial cell line SRA01/04 (a gift from Zhejiang University, China) were cultured at 37°C in low-glucose DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 15% FBS (GIBCO, Invitrogen, Grand Island, NY, USA) and 1× penicillin/streptomycin antibiotics (PAA Labs, Pasching, Austria). For the in vitro differentiation assays, the cells were starved for 24 hrs in DMEM with 0.15% FBS before treatment with 20 ng/ml of human recombinant basic FGF-b (ProSpec-Tany TechnoGene, Rehovot, Israel) to induce differentiation [16].
Plasmid transfection and antibodies used
Full-length SKAP2 was cloned into pcDNA3.1-triHA-5′[the trihemagglutinin (HA) sequence was inserted into pcDNA3.1 (Invitrogen)], while full-length NCK2 or NCK1 from mouse lens were cloned into pcDNA3.1-myc-3′[the myc sequence was inserted into pcDNA3.1 (Invitrogen)]. The Y to F mutation at position 75 of SKAP2 was made using a site-directed mutation kit (Sai Bai Sheng, Shanghai, China). The N-terminal 106 amino acids deletion mutant of SKAP2 (SKAP2Δ106aa) plasmid was also inserted into pcDNA3.1-triHA5′. The myc-tagged SH2 domain of NCK2 comprises residues 284-380. For knockdown assays using SRA01/04 cells, two duplexes that target different regions of hSKAP2 (5’-GATCCGCAAAGGAAGATGAGTCA GGTTCAAGAGACCTGACTCATCTTCCTTTGTTTTTTGGAAA-3′ and 5’-GATCCGCT GATGACCAACAGTTCCATTCAAGAGATGGAACTGTTGGTCATCAGTTTTTTGGAAA-3′, referred to as shRNA #1 and shRNA #2, respectively) were cloned into Psilencer 3.0 (Applied Biosystems/Ambion, Austin, TX, USA). SRA01/04 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Mouse anti-HA, rabbit anti-myc, rabbit anti-FAK and the monoclonal mouse anti-Phospho-fibroblast growth factor receptor (FGFR) antibodies were from Cell Signaling Technology, Inc. (Beverly, MA, USA). The primary goat anti-SKAP2 antibody was from Abcam (Cambridge, UK), and the rabbit anti-SKAP2 antibody was from Proteintech Group, Inc. (Chicago, IL, USA). The rabbit anti-HA antibody and the monoclonal mouse NCK antibody were from BD Biosciences (Franklin Lakes, NJ, USA). Phalloidin Alexa Fluor555, Alexa Fluor568-conjugated rabbit anti-goat IgG and goat anti-rabbit Alexa Fluor488 IgG were from Invitrogen/Molecular Probes. The goat antimouse CY3, donkey antimouse CY5, donkey anti-rabbit CY2 and donkey anti-goat CY3 secondary antibodies were from Jackson ImmunoResearch Labs (West Grove, PA, USA). The anti-actin antibody and Phalloidin-fluorescein isothiocyanate (FITC) were from Sigma-Aldrich (St. Louis, MO, USA).
Preparation and treatment of primary lens culture
The primary lens cell cultures from neonatal HSF4+/+ or HSF4−/− mice were prepared as described previously [5, 17]. Briefly, lenses were isolated from three HSF4+/+ or HSF4−/– mice at postnatal day 3, respectively. Lenes were then trypsinized in 2× trypsin- ethylenediaminetetraacetic acid (EDTA)/PBS buffer (GIBCO, Invitrogen) at 37°C for 5 min. and agitated. The collected lens cells were plated on 48-well dish (Greiner Bio-one, Stuttgart, Germany) and cultivated in M199 media supplemented with 20% FBS and 1× penicillin/streptomycin. The primary lens had well-spread epithelial morphology after 1 week culture. For the in vitro differentiation assays, the lens cells were treated with 40 ng/ml of FGF-b for 36 hrs after serum starvation in M199 supplemented with 0.15% FBS for 24 hrs.
Quantitative PCR
RNA from mouse lens or the SRA01/04 cells was extracted using Trizol (Invitrogen) and reverse transcribed using the MLV Transcription Kit (Invitrogen). Quantitative PCR was performed with the SYBR Green PCR kit (Applied Biosystems, Streetsville, ON, Canada) and the sequence detection system (ABI 7900HT). The following primers were used: 5′-ACCAGTTTCCTC CCATTGCA-3′ and 5′-CCATTCAAACCCCAGAAAGC-3′.
Chromatin immunoprecipitation
Lenses were isolated from postnatal, day-9 mice and treated as described previously [7]. Briefly, after cross-linking, the lens cells were lysed in cell lysis buffer [5 mM N-2-hydroxyethylpiperazine-N-ethane-sulphonicacid (HEPES) including 85 mM KCl, 0.5% NP40, PMSF (phenylmethanesulfonylfluoride) and protease inhibitor cocktail] and centrifuged. The precipitated fractions were then lysed in nucleus lysis buffer (50 mM Tris-HCl including 10 mM EDTA, 1% SDS, PMSF and protease inhibitor cocktail) and sonicated. The cross-linked chromatin fragments were immunoprecipitated using the anti-HSF4b antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or Normal IgG, following the Upstate manufacturer’s instructions. We determined the enrichment of specific DNA sequences in the immunoprecipitated material using PCR with the following primers: 5′-TGGGACTCCGTTA CCTTC-3′ and 5′-TTGCGTAGACCTTGC TTT-3′.
Immunostaining
The cultured cells were fixed with 4% paraformaldehyde in PBS for 10 min., permeabilized with 0.2% Triton X-100 in PBS for 10 min. and then blocked in 1% bovine serum albumin in PBS for 1 hr, all at room temperature. The cells were then incubated overnight at 4°C with the primary antibody. After washing, the cells were incubated with the secondary antibody in the dark or counterstained for polymerized actin (F-actin) using phalloidin for 1 hr at room temperature. The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI), and cover slips were mounted on glass slides using the antifade mounting medium from Sigma. Images were taken using a fluorescence microscope (ECLIPSE 80i, Nikon, Tokyo, Japan) or a confocal microscope (TCS sp5, Leica, Wetzlar, Germany). Quantification of the cells with actin remodelling (as visualized using a microscope) was described in [18].
Mouse eyes were isolated and fixed in 4% paraformaldehyde in PBS and embedded in paraffin (Sigma) using paraffin-embedding apparatus from Leica (EG1140H), and 7 μm serial sections were cut using a paraffin sectioning apparatus (Leica RM2126, Leica, Shanghai, China). After removal of the paraffin and retrieval of the antigen, the slices were blocked and incubated overnight at 4°C with the primary anti-SKAP2 antibody. The slices were then incubated with a fluorescent secondary antibody or counterstained with phalloidin. The animal protocol has been approved by the Ethics Committee of HIS.
Co-immunoprecipitation (co-IP) and immunoblotting
The cells were lysed in ice-cold lysis buffer (KangCheng, Shanghai, China) containing a protease inhibitor cocktail (Sigma). Normalized cell lysates were mixed with the primary antibodies at 4°C overnight and 30 μl of M-280 sheep anti-rabbit IgG paramagnetic Dynabeads (Dynal Biotech, Hamburg, Germany) was added following the manufacturer’s instructions. After vigorous washing, the immunoprecipitated samples were boiled for 10 min. in Laemmli buffer, separated on 4–12% NUPAGE Bis-tris gels (Invitrogen) and transferred to nitrocellulose membranes. After incubation with the HRP-conjugated secondary antibody, electrochemiluminescence (ECL) detection reagents (GE Healthcare, Little Chalfont, UK) were added to the membranes. The membranes were then exposed to photographic film. In some experiments, the membranes were labelled with IRDye 800 (LI-COR Bioscience, Lincoln, NE, USA) and then visualized using the ODYSSEY infrared imaging system (LI-COR Bioscience).
Results
SKAP2 is a downstream target of HSF4b in lens cells
HSF4b is expressed mainly in the lens, and it plays an important role in lens development [8]. To identify its downstream target, we examined two independent sets of microarray expression data from HSF4 knockout mice with different backgrounds (C57BL/6-129/S3 and C57BL/6-129/SvJ). We found that SKAP2 was down-regulated in both date sets [8, 10]. To determine the magnitude of SKAP2 repression in the HSF4−/− mouse lens cells, we extracted the mRNA from mouse lenses and analysed it by quantitative PCR. Compared to the wild-type mouse, the level of mSKAP2 mRNA was reduced more than 10-fold in the newborn HSF4−/− mouse lens (Fig. 1A, left part), and approximately 2-fold in the lens cells of 8-week-old adult knockout mouse lens (Fig. 1A, right part). Then we confirmed this result by immunofluorescent staining on lens sections (Fig. 1B) or by Western blot on proteins of lens at different developmental stages (P1, P5, P10, and 4 weeks old, Fig. 1D). The SKAP2 protein was barely detectable in the lens of HSF4 knockout mice (Fig.1B, part c and 1D). Taken together, these results indicate that SKAP2 is truly down-regulated in the HSF4 knockout mice lens. Furthermore, the expression level of SKAP2 was higher in the lens of neonatal mice than adult mice (Fig. 1A and D), synchronous with the expression time of HSF4b in the lens [8].
Fig 1.
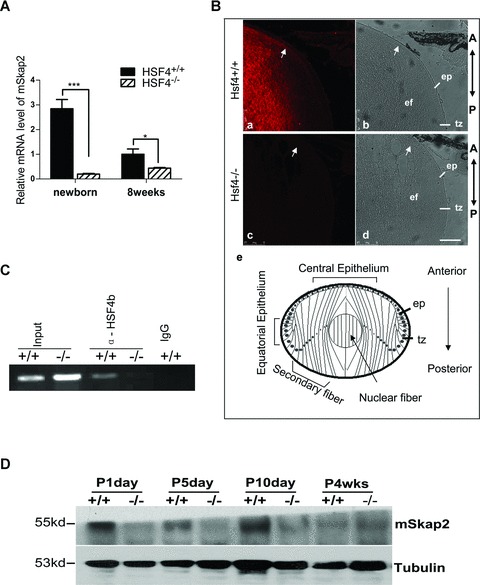
SKAP2 is down-regulated in HSF4−/− mouse lens. (A) mRNA was extracted from several lenses of newborn and adult (8 weeks) wild-type or HSF4−/− mice and assayed by quantitative RT-PCR. * denotes a significant difference compared to wild-type mice, P <0.05. (B) Fluorescent and light images of mid-sagittal sections from postnatal day 5 mice which were stained for endogenous mSKAP2. Parts a and b are from wild-type, parts c and d are from HSF4−/− mouse. The arrows point to the accumulating signal at the anterior tip of elongating secondary lens fibre cells, where they attach to the epithelium. Part e is a diagram of lens equatorial section. The bar represents 75 μm. ep: epithelium; tz: transition zone at equatorial epithelium; ef: elongating secondary fibre cells; A: anterior; P: posterior. (C) The nuclei of wild-type (+/+) and HSF4 knockout (−/−) mouse lens cells were extracted and immunoprecipitated with the goat anti-HSF4b antibody or with pre-immune IgG. Precipitated chromatin fragments were amplified using primers targeting the Skap2 promoter region around –500 bp. ‘Input’ indicates PCR amplification of the total DNA before precipitation. ‘IgG’ indicates pre-immune IgG. (D) SKAP2 protein was down-regulated in HSF4−/− mouse lens. SKAP2 was measured by the Western blotting from lens extracts (3 μg) of wild-type (+/+) and HSF4−/− (-/-) mice at postnatal days 1, 5 or 10; or at 4 weeks. Tubulin was re-blotted as the loading control.
We next investigated whether HSF4b regulates SKAP2 through binding to the promoter region of SKAP2. The nuclear proteins were extracted from both HSF4−/−and wild-type mouse lens cells and subjected to chromatin immunoprecipitation (ChIP) assays using the anti-HSF4b antibody or IgG control. Primers that targeted the promoter region of SKAP2 (from -100 to -500) were used in the PCR reaction of ChIP precipitates. HSF4b was co-precipitated with the promoter of SKAP2 from wild-type mouse lenses, but not from HSF4−/− mouse lens or from the negative control (Fig. 1C). This finding suggests that HSF4 can bind to the promoter region of Skap2.
The expression of SKAP2 correlates with the differentiation of LECs
To understand the function of SKAP2 in lens development, we checked the distribution of SKAP2 in vivo. The structure of lens mid-sagittal sections is diagrammed in Fig. 1B (part e). Immunofluorescence experiments showed that in the wild-type lens, the expression of SKAP2 was higher in the fibre cells than that in the epithelium (Fig. 1B, image a). SKAP2 particularly accumulated at the anterior tip of the elongating secondary fibre cells (Fig. 1B, white arrow in image a). The magnified confocal images in Fig. 2A also showed that endogenous SKAP2 clearly accumulated at the apical ends of the lens elongating fibre cells, where the fibre cells connect to the epithelium (white arrows in Fig. 2A). Thus, we proposed that the expression of SKAP2 was associated with the differentiation of LECs.
Fig 2.
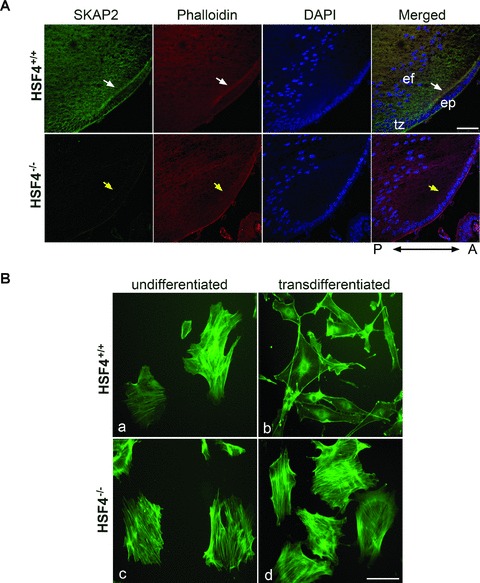
Defective reorganization of the actin cytoskeleton in HSF4−/− mouse lens cells. (A) SKAP2 was colocalized with F-actin at the anterior tip of elongating fibre cells in HSF4+/+ lens (white arrows), but not in HSF4−/− lens (yellow arrows). Mid-sagittal lens sections that were prepared from wild-type or HSF4−/– mice at postnatal day 4 were stained with phalloidin-Alexa Fluor 555. The images were taken under a confocal microscope. The bar represents 50 μm. ep: epithelium; tz: transition zone; ef: elongating fibre cell; A: anterior; P: posterior. (B) The HSF4−/− cells failed to reorganize their actin cytoskeleton in vitro. The primary undifferentiated lens cells (parts a, c) were cultured in medium with serum. To induce transdifferentiation in vitro, the primary lens cells were starved for 24 hrs and subsequently treated with 40 ng/ml of FGF-b for 36 hrs (parts b, d). The cells were fixed and stained with phalloidin-FITC. Bar 100 μm.
To investigate whether SKAP2 is associated with the differentiation of lens cells, we examined the levels of human SKAP2 mRNA in SRA01/04 cells, before and after FGF-b induced differentiation. LECs transdifferentiate into lens fibre-like mesenchymal cells when treated with FGF-b that is essential for the initiation of LEC differentiation [19–21]. This epithelial-to-mesenchymal transition (EMT) mimics the LEC differentiation in vivo[16, 17]. The levels of SKAP2 mRNA were significantly increased in response to FGF-b. The increase in SKAP2 mRNA after FGF-b treatment was correlated with increased expression levels of α-SMA (α-smooth muscle actin), which is a marker for EMT cells [22, 23] (Fig. S1). These results imply that SKAP2 probably plays an important role in the FGF-b induced lens cell differentiation.
Actin reorganization in primary HSF4−/− mouse lens cell cultures is inhibited during differentiation
As shown in Fig. 1B, SKAP2 concentrated at the anterior tip of elongating fibre cells, where cortical actin fibres were also assembled [24]. Indeed, SKAP2 colocalized with F-actin at the apical ends of the elongating fibre cells in wild-type mouse lens (Fig. 2A, white arrows). In contrast, not only was SKAP2 barely detectable in the HSF4−/− mouse lens, but also there were fewer cortical actin fibres in the anterior tip of the elongating fibre cells indicated by phalloidin staining (Fig. 2A, yellow arrows).
The primary lens culture system has been proved to be an ideal model to examine the mechanisms of differentiation [5, 17, 25]. Primary LECs cultured in medium with high serum have well-spread epithelial morphology [5, 17]. When the cells are starved with serum-free medium or treated with FGF-b, they begin to transdifferentiate and elongate; withdraw from the cell cycle, express differentiation-specific markers and assemble stable N-cadherin cell–cell junctions, similar to what happens in vivo[4, 17, 26]. At the beginning of lens differentiation, the disassembly of centre stress fibres and reorganization to cortical actin filaments provide an initiation signal for lens differentiation [5]. We used primary LECs culture to examine the effect of SKAP2 on actin reorganization in HSF4−/– lens cells during differentiation. The primary actin filament structures in the undifferentiated lens cells are actin stress fibres (Fig. 2B, parts a, c), as previously reported in studies of the LEC cultures [5]. After FGF-b treatment, LECs from wild-type mice started transdifferentiation, which was accompanied by the centre actin stress fibres disassembly and cell elongation (Fig. 2B, part b). In contrast, HSF4−/− LECs still contained strong actin stress fibres and had no morphological change after starvation and FGF-b treatment (Fig. 2B, part d). These results suggest that HSF4−/– LECs fail to reorganize their actin cytoskeleton during differentiation.
Next we tried to overexpress SKAP2 in the primary HSF4−/– cells for rescue experiment. Due to the low expression level in primary cells, we observed partial disassembly of centre stress fibre in the cells overexpressing SKAP2 after starvation and FGF-b treatment (Fig. S2, white arrowheads). In contrast, there are still plenty of centre stress fibres in the control HSF4−/−cells expressing GFP alone (Fig. S2, top parts) or in the untransfected cells nearby (Fig. S2, yellow arrows in lower parts). These results suggest that SKAP2 plays an important role in the remodelling of the actin cytoskeleton during the differentiation of lens cells.
SKAP2 associates with cortical actin fibres at the lamellipodium and is essential for the disassembly of centre stress fibre in SRA01/04 cells
Bourette et al. found that phosphorylated SKAP2 can be precipitated with α-actin in myeloid cells treated with M-CSF [27]. To confirm that SKAP2 localized in actin-rich membrane ruffles, we carried co-IP and immunostaining experiments in SRA01/04 LECs treated by FGF-b. Indeed, α-actin could be co-IPed with HA-SKAP2 in SRA01/04 cells (Fig. 3A). We also observed the subcellular colocalization of HA-SKAP2 and cortical actin fibres at the lamellipodia in the EMT cells (Fig. 3B), similar to what happens in vivo. As shown in Fig. 3B, lamellipodium are broad, flat, sheet-like structures at the leading edges and contain actin meshwork, which produce the major driving force of migration [28]. Meanwhile, the colocalization of endogenous SKAP2 and cortical actin fibres was showed in row 1 of Fig. 3D.
Fig 3.
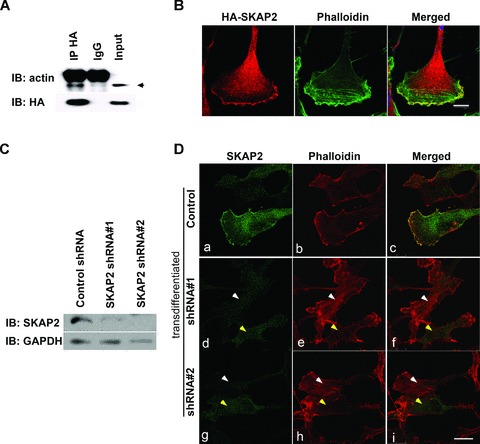
SKAP2 is essential for actin reorganization in lens epithelial cells. (A) SKAP2 was co-precipitated with α-actin in LECs. The SRA01/04 cells that had been transfected with HA-SKAP2 were lysed and immunoprecipitated with rabbit anti-HA antibody or with normal IgG. The immunoprecipitates were probed with rabbit anti-α-actin or mouse anti-HA antibodies. The arrowhead points to the band corresponding to α-actin, above which is the band corresponding to the IgG heavy chain. (B) HA-SKAP2 is colocalized with cortical actin fibres at the lamellipodia. The SRA01/04 cells that had been transfected with HA-SKAP2 were treated with FGF-b. Then cells were fixed and stained with anti-HA antibody (red) or counter-stained with phalloidin-FITC (green) and visualized using confocal microscopy. These results are representative of three independent experiments. Bar 10 μm. (C) The efficiency of SKAP2 depletion. SRA 01/04 cells were transiently transfected with either control or SKAP2 shRNA (#1 or #2) and then lysed and blotted with SKAP2 and GAPDH. (D) SKAP2 was necessary for actin remodelling during differentiation. After starvation and FGF-b treatment, the control (parts a–c) or shRNA (parts d–i) transfected SRA 01/04 cells were fixed and stained with rabbit anti-SKAP2 antibody and counterstained with phalloidin Alexa Fluor 555. Cells with high levels of SKAP2 have less F-actin (yellow arrows), while cells with less SKAP2 have much higher F-actin (white arrows). Bar 25 μm.
Rapid loss of actin stress fibres, the reorganization of actin from cellular stress fibres to cortical actin fibres, and the assembly of N-cadherin-based cell–cell junctions are characteristic markers for the initiation of LEC differentiation [1]. The FGF-b induced human LECs differentiation in vitro is also accompanied by the disassembly of actin stress fibres and the formation of cortical actin fibres (Fig. 3D, row 1). The function of the actin-binding protein SKAP2 in actin reorganization was then examined. In fact, overexpressing SKAP2 alone in the SRA01/04 cells could cause a clear disassembly of the centre stress fibres (Fig. 4A, top part), similar to that seen in differentiated LECs that had been induced by FGF-b (Fig. 3D, row 1).
Fig 4.
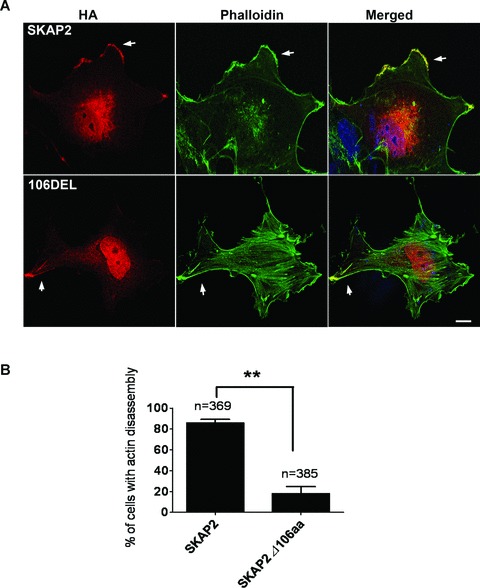
The N-terminus of SKAP2 is necessary for the disassembly of centre stress fibres. (A) The full length SKAP2 but not the N-terminal truncated form of SKAP2 induced the disassembly of actin stress fibres. After parallel transfection with full-length HA-SKAP2 or HA-SKAP2Δ106aa (106DEL), the cells were fixed and stained with anti-HA antibody (red), phalloidin-FITC (green) and DAPI. Bar 10 μm. (B) The percentage of transfected SRA01/04 cells that exhibit the disassembly of cellular F-actin was quantified. The bars represent the mean ± S.E.M. of two different experiments.
To confirm the effect of SKAP2 on actin reorganization, we knocked down SKAP2 expression in human LECs with shRNA and then examined the remodelling of actin during the initiation of LEC differentiation induced by FGF-b. The knockdown efficiency is shown in Fig. 3C. The SRA01/04 LECs were transfected with control shRNA, SKAP2 shRNA#1, SKAP2 shRNA#2, respectively. They were next treated by FGF-b to induce differentiation. In the control cells, the cellular actin stress fibres were disassembled as expected (Fig. 3D, parts a, b, c). The cells that had been transfected with SKAP2 shRNA had different knockdown efficiency. This turned out to be a very useful tool to analyse the effect of SKAP2 on the dynamics of actin. The cells with down-regulated SKAP2 (Fig. 3D, white arrows in parts f, i) exhibited clear cellular actin stress fibres. However, nearby cells, that had a higher level of SKAP2, disassembled their centre actin stress fibres in response to FGF-b (Fig. 3D, yellow arrows in parts f, i). These results suggest that SKAP2 is essential for actin reorganization during lens differentiation.
The N-terminal 106 amino acids of SKAP2 are necessary for actin remodelling
To understand the mechanism of SKAP2 in actin reorganization, we first compared the two SKAP homologues. SKAP2 has unique tyrosine phosphorylation sites in the N-terminal region. To investigate the function of the N-terminal 106 amino acids, especially in the SKAP2-dependent reorganization of actin, we then transfected SRA01/04 cells with plasmids encoding either HA-tagged full-length SKAP2 or HA-tagged SKAP2Δ106aa (the N-terminal 106 amino acids deletion mutant of SKAP2). The cells were subsequently fixed and stained with anti-HA antibody and phalloidin-FITC. Both the full-length SKAP2 and SKAP2Δ106aa localized at membrane ruffles (Fig. 4A, arrows). Centre actin stress fibres were absent in the cells overexpressing full-length SKAP2 (Fig. 4A, top part), whereas the cells overexpressing SKAP2Δ106aa exhibited obvious cellular stress fibres without actin reorganization (Fig. 4A, bottom part). The efficiency of cellular F-actin disassembly in SKAP2-expressing cells was much higher than that in the SKAP2Δ106aa cells (Fig. 4B). These results suggest that the N-terminal fragment of SKAP2 has an important role in actin remodelling.
Identification of the interaction between SKAP2 and NCK2 at membrane ruffles
SKAP2 PH domain can bind directly to membrane lipids [13]. However the precise ruffle-targeting signal for SKAP2 remains unclear. We next examined the downstream proteins of SKAP2 that play a role in the actin dynamics. We observed two conserved tyrosine-based motifs (Y75DDP and Y93DKD) within the N-terminus of SKAP2 (Fig. 5A). Previous analysis showed that the consensus motif phospho(p)-Y-D-E/D/K-V/P is a binding site for the NCK SH2 domain [29]. Thus, the Y75DDP motif is a potential binding site for the NCK adaptor proteins. There are two NCK proteins in mammals, NCK1 and NCK2, each of which contains three consecutive SH3 domains and a C-terminal SH2 domain. NCK2 is highly expressed in the mouse lens as we cloned full length NCK2 from lens sample. It has been shown that NCK2 functions in coupling phosphotyrosine (pTyr) signals to actin cytoskeletal reorganization, through a mechanism that the SH2 domian binds to phosphotyrosine (pTyr) signals, the second SH3 domain interacts with PAK (p21-activated kinase) that leads to the disassembly of stress fibre and the formation of lamellipodia upon Rac activation, and the third SH3 domain recruits focal adhesions [30–35] (Fig. 7). Therefore, it is probable that SKAP2 signals to actin via NCK2/PAK pathway.
Fig 5.
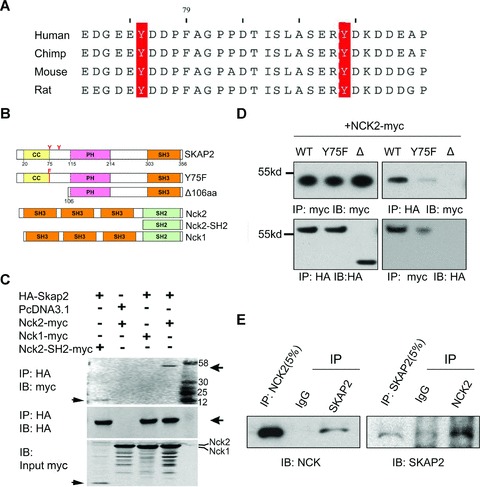
Identification of the interaction between SKAP2 and NCK2. (A) YDDP, the predicted NCK SH2-binding motif in SKAP2, is highly conserved among human beings, chimps, mice and rats. (B) Vector construction of full length SKAP2, NCK2 and NCK1; site mutant of SKAP2 Y75F; N-terminal 106aa deletion mutant of SKAP2; and the SH2 domain of NCK2 expression vectors. (C) SKAP2 interacted with the SH2 domain of NCK2 but not with NCK1. HA-SKAP2 was cotransfected into the SRA01/04 cells with NCK2-myc, NCK1-myc or the myc-tagged SH2 domain of NCK2 (NCK2-SH2-myc), respectively. The cell lysates were then immunoprecipitated with rabbit anti-HA antibody. The immunoprecipitates were probed with either mouse anti-myc or mouse anti-HA antibodies. Arrows indicate the specific bands. (D) The association of SKAP2 with NCK2 was dependent on the N-terminus containing the YDXP motif. Myc-tagged NCK2 was cotransfected with HA-tagged full-length SKAP2 (WT), mutant SKAP2-Y75F (Y75F) or HA-SKAP2Δ106aa (Δ) into the SRA01/04 cells. The cell lysates were precipitated with rabbit anti-HA or rabbit anti-myc antibodies and analysed using immunoblotting (IB) with mouse anti-HA or mouse anti-myc antibodies. (E) Endogenous SKAP2 interacted with NCK2. The SRA01/04 cell lysates were precipitated with rabbit SKAP2 or rabbit NCK2 antibodies. The precipitates were blotted with mouse anti-NCK or goat anti-SKAP2 antibodies.
Fig 7.
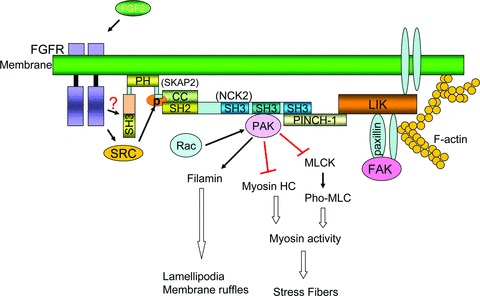
Model for membrane recruitment of SKAP2 and actin cytoskeleton. SKAP2 is directed to membrane via its PH domain [13]. The N-terminus of SKAP2 interacts with the SH2 domain of NCK2, while the second SH3 domain of NCK2 interacts with PAK and the third SH3 domain links the LIM4 domain (a zinc finger structure initially discovered in the proteins Lin11, Isl-1 & Mec-3) of PINCH, an integrin receptor partner [48]. SKAP2 could be phosphorylated by Src kinase family, which is activated by FGFR, or might be activated by FGFR directly [11]. PAK signal activated by RAC, as the downstream signal of NCK2 complex, is presented at the bottom [33, 53]. Direct activating signals are depicted as solid arrows. Inhibitory signals are presented by red bars. Double-lined arrows and bars represent the net result of multi-step signalling transduction.
We then examined the interaction between SKAP2 and NCK1 or NCK2 by co-IP experiments. As shown in Fig. 5C, HA-SKAP2 was co-precipitated with full-length NCK2 and the SH2 domain of NCK2 (Fig. 5B), but not with NCK1. This indicates different binding properties between NCK1 and NCK2. To investigate whether the Y75DDP motif in the N-terminal domain of SKAP2 mediates the association with NCK2; we introduced a tyrosine (Y) to phenylalanine (F) mutation into the full-length SKAP2 at position 75 (designated hereafter as Y75F) (Fig. 5B). Plasmids containing either full length HA-tagged SKAP2, mutant SKAP2 Y75F or SKAP2Δ106aa were each co-transfected into SRA01/04 cells with myc-tagged NCK2. The cells were then lysed and precipitated with the anti-HA antibody or the anti-myc antibody. The interaction of NCK2 was reduced with SKAP2 Y75F and was virtually undetectable with SKAP2Δ106aa (Fig. 5D). We also confirmed the interaction between endogenous SKAP2 and NCK2 by co-IP using antibodies against endogenous SKAP2 or NCK2 (Fig. 5E). Together, the N-terminal domain of SKAP2 interacts with the SH2 domain of NCK2, giving the potential explanation for the different effects between SKAP2 and SKAP2Δ106aa.
We further determined the subcellular localization of SKAP2 and NCK2. Interestingly, the endogenous SKAP2, NCK2 and the cortical actin fibres were clearly co-localized at the lamellipodial leading edge (indicated by the white arrows in Fig. 6A). Taken together, these results suggest that SKAP2 associates exclusively with the SH2 domain of NCK2 via its N-terminal domain at the leading edge.
Fig 6.
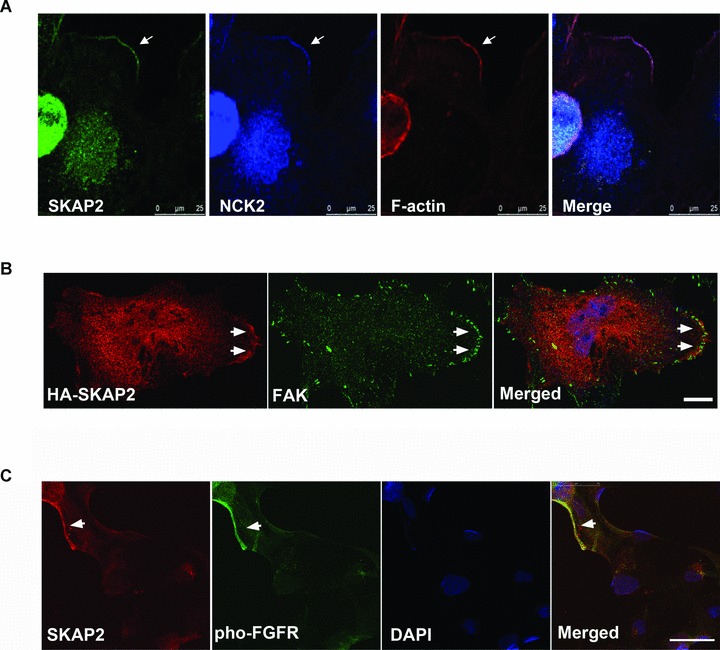
SKAP2 accumulates with NCK2, FAK and FGF receptor at the leading edges. (A) Endogenous SKAP2, NCK2 and the cortical actin fibres colocalized at the leading edge of the lamellipodia. The SRA01/04 cells were stained with rabbit anti-SKAP2 antibody, monoclonal mouse anti-NCK antibody and Phalloidin Alexa Flour 555. The arrow indicates the lamellipodia. Bar 25 μm. (B) The cells expressing HA-SKAP2 were stained with mouse anti-HA and rabbit anti-FAK antibodies. The accumulation of SKAP2 and FAK at the leading edge is indicated by arrows. Bar 10 μm. (C) Colocalization of SKAP2 and phospho-FGFR in the SRA01/04 cells. The cells were starved and treated with FGF-b. Endogenous SKAP2 and the phospho-FGF receptor (Tyr653/654) were visualized with rabbit anti-SKAP2 antibody and monoclonal mouse anti-Phospho-FGFR antibody. Bar 50 μm.
Previous studies have identified that the third SH3 domain of NCK2 interacts with PINCH (particularly interesting new Cys-His protein 1, also named as LIMS1, LIM and senescent cell antigen-like domains 1), a linker between integrin and focal adhesions [36]. NCK2 has also been proven to interact with focal adhesion kinase (FAK) directly at cell periphery and to be involved in the formation of dorsal ruffles [35, 37–39]. It has been known that focal adhesions are sites where integrin adhesion complexs link to the actin cytoskeleton. There are various components of focal adhesions, including scaffolding molecules, GTPases, kinases and phosphatases [40]. Therefore, it is possible that SKAP2 locates with NCK2 at focal adhesions. To address this point, we transfected HA-SKAP2 into SRA0/04 cells and then stained with anti-HA antibody or anti-FAK antibody. FAK was concentrated at cell periphery when the cells were overexpressed with SKAP2. SKAP2 accumulated with FAK particularly at the leading edge (Fig. 6B, arrowheads)
Integrin adhesion complexes usually recruit actin cytoskeleton regulatory proteins that initiate or terminate actin polymerization. This event also requires other membrane receptors activation [41]. FGF receptors can activate NCK signal, however there is no evidence showing the direct interaction between NCK2 and FGFR [42]. Thus, there might be a connection between SKAP2 and FGF receptor signalling. Indeed, SKAP2 and FGFR were also co-localized at membrane (Fig. 6C).
In summary, our data provides a potential explanation on how SKAP2 regulates actin reorganization in LECs. SKAP2 might function as the link between membrane receptors and the ‘NCK-PINCH-FAK’ focal adhesion complexes at the membrane protrusions (Fig. 7).
Discussion
Lens cells are an ideal cell type to study the mechanisms of differentiation. During the process of differentiation into lens fibre cells, LECs undergo dramatic morphological changes including cell elongation, membrane remodelling and cell polarization. These are concomitant with migration towards the interior of the lens. Most of these events are influenced in large part by the dynamic reorganization of the actin cytoskeleton [5, 43]. HSF4 knockout mouse, which display abnormal differentiation of LECs, is a good model for identifying novel target genes that are essential for the differentiation of LECs.
In our study, we identified a downstream target of HSF4b, SKAP2. ChIP assay revealed that HSF4 could bind to the promoter region of Skap2 (Fig. 1C). The perfect HSF binding site is composed of at least three inverted repeats of nGAAn (nTTCnnGAAnnTTCn) [9] and the atypical HSEs contain gap-type HSE (nTTCnnGAAn(5 bp)nGAAn), step-type HSE (nTTCn(5 bp)nTTCn(5 bp)nTTCn) and DR-type HSE (nnGAAnnnnnnnGAAn) [44]. Besides, Fujimoto et al. have reported that the motif with at least three inverted repeats of nGnnn is also HSF4 binding site [45]. Sequence analysis on mSkap2 promoter region (from –650 to 0) suggests that there are nine putative HSEs in this region, seven of which are motifs with at least three inverted repeats of nGnnn, one is DR-type HSE and the other is gap-type HSE (Fig. S3). Further experiments will be needed to identify which motif is essential for HSF4 binding.
Previous studies have shown that SKAP2 is involved in the differentiation of myeloid cells and in cell cycle arrest [27, 46]. Consistent with that finding, expression of SKAP2 in the lens also correlated with the differentiation of LECs, indicating that SKAP2 may be involved in some process of lens cell differentiation. The facts that SKAP2 was phosphorylated after stimulation by MCS-F and that it was co-immunoprecipitated with α-actin in myeloid cells in vitro[27], inspired us to investigate the role of SKAP2 in regulating actin cytoskeleton in LECs. Our study demonstrates that SKAP2 is apt to accumulate with cortical actin fibres at membrane ruffles and is essential for the disassembly of actin stress fibres in response to FGF-b. The disruption of actin stress fibres regulates LEC differentiation [5, 43]. The failure of actin disassembly unmasks the mechanism of the abnormal differentiation in HSF4−/− len cells. In agreement with our findings, Reinhold and his colleagues recently revealed that actin polymerization was enhanced when triggered by integrin in SKAP2-deficient dendritic cells [47]. Furthermore, we found that the N-terminal domain of SKAP2 is important for its function in actin reorganization as SKAP2 interacts with NCK2 via its N-terminal domain.
NCK2 is one of the proteins that control the rearrangement of the actin cytoskeleton by linking membrane receptors to actin regulatory proteins. The SH3 domain of NCK2 has been reported to bind to the proline-rich motifs of N-WASP, WASP-interacting protein (WIP), PAK serine/threonine kinase, which are all involved in control of cellular actin dynamics [36, 39, 48–50]. When cells are stimulated, the complex of NCK-PAK-PINCH-PKL (the paxillin kinase linker) is recruited to nascent focal complexes at the leading edge upon intergrin engagement and Rac activation, where lamellipodia forms and the regulatory signal of actin cytoskeletion are transduced [31]. As the downstream effector of NCK signal, the membrane-attached and activated PAK induces the disassembly of stress fibres through inhibiting myosin light chain kinase and myosin HC activity [33, 51–53], and promotes the formation of lamellipodia through filamin [32, 54] (Fig. 7). In the upstream signal, the SH2 domain of NCK binds to specific pTyr-containing sites on activated receptors directly, such as the ß-platelet-derived growth factor-receptor (ß-PDGFR) and EGFR [34, 55, 56]. NCK has also been reported to link to FGF receptors indirectly [42]. Our observations of the association of SKAP2 with the SH2 domain of NCK2 at the lamellipodia, and the essential role for SKAP2 in actin remodelling induced by FGF-b, suggest that SKAP2 may be the missing link between NCK2 and FGF receptor. Here, we also observed that SKAP2 and the activated FGF receptor colocalized in LECs (Fig. 6B). However, whether they interact with each other directly or through other proteins requires further examination. Importantly, there is a positive feedback that the expression of SKAP2 is significantly increased when LECs were induced by FGF-b. Our data raises the possibility that SKAP2 might act cooperatively with NCK2 at the leading edge to regulate the PAK activity. However, further investigations are required to fully understand the signal transduction in lens downstream of FGFR/SKAP2/NCK2.
SKAP2 can also be phosphorylated by SRC kinases, including Src, Fyn, Lyn and Hck [11]. Src and related kinases can phosphorylate tyrosine sites of many actin-associated proteins, such as FAK, paxillin, vinculin and so on, which will regulate the formation of various phosphotyrosine-based macromolecular signaling complexes like focal adhesion complexes, or directly affect the activity of the downstream substrates, and thereby influence the actin cytoskeleton and cell shape [57, 58]. In addition, kinases in the Src family are known to play a central role in the migration of epithelial cells. They accomplish this through their ability to recruit proteins to the sites of integrin attachment for regulating the focal adhesion turn-over and actin reorganization and to regulate the activity of the small GTPases Rac and Rho [57–59]. Therefore, it is probable that SKAP2, as the actin-binding protein phosphorylated by SRC kinases or directly by FGFR, recruits NCK2/F-actin to transmembrane integrin /focal adhesion in the lamella.
Taken together, these results reveal an essential role for SKAP2 in actin remodelling and uncover a novel ruffle-targeting complex. Our new findings are crucial to understand the role of HSF4 in LEC differentiation and the molecular mechanism of cataract.
Acknowledgments
We thank Dr. Dangsheng Li for helpful discussions. We also thank three anonymous referees for their constructive comments. This work was supported by the National Natural Science Foundation of China, Key Program (No. 30530450), the National High Technology Research and Development Program of China (2006AA02Z330, 2006AA02A301), the National Basic Research Program of China (Nos. 2007CB512202, 2004CB518603) and the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KSCX1-YW-R-74).
Supporting Information
SKAP2 is associated with lens epithelial cellsdifferentiation. Semi-quantitative RT-PCR of 1 μg ofpolyA+ mRNA from the SRA01/04 cells was performed withprimers for the genes that are specified. The cells were incubatedin the presence of FBS (+), serum-free DMEM- or 20 ng/ml FGF-b for different amounts of time.
Overexpression of SKAP2 in HSF4-/-lens cells partially causes the disassembly of centre stressfibres. The primary HSF4-/- lens culture cells weretransfected with SKAP2-GFP or pEGFP vectors. After 24 hrs, thecells were starved for 24 hrs and then treated with 40 ng/ml FGF-bfor 12 hrs. Finally, the cells were fixed and stained withphalloidin Alexa Fluor 555 to visualize F-actin. The magnifiedimages were from the region pointed by the white arrows. Bar 50μm.
The nucleotide sequences of putative HSEs in the promoter region of mouse Skap2. The sequences consisting of at least three inverted repeats of nGnnn include #2, #3, #4, #6, #7, #8, #9 sequences. Among these, #2 sequence has two perfect inverted repeats of nGAAn; #3 sequence has one perfect repeat of nGAAn. #1 sequence is DR-type HSE (nnGAAnnnnn nnGAAn) and #5 sequence is the gap-type HSE (nTTCnnGAAn(5 bp)nGAAn). The inverted repeats of nGnnn or nGAAn sequences are shown in red.
References
- 1.Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–90. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain CG, McAvoy JW. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF) Growth Factors. 1989;1:125–34. doi: 10.3109/08977198909029122. [DOI] [PubMed] [Google Scholar]
- 3.Walker JL, Zhang L, Zhou J, et al. Role for alpha 6 integrin during lens development: evidence for signaling through IGF-1R and ERK. Dev Dyn. 2002;223:273–84. doi: 10.1002/dvdy.10050. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira-Cornwell MC, Veneziale RW, Grunwald GB, et al. N-cadherin function is required for differentiation-dependent cytoskeletal reorganization in lens cells in vitro. Exp Cell Res. 2000;256:237–47. doi: 10.1006/excr.2000.4819. [DOI] [PubMed] [Google Scholar]
- 5.Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295:714–29. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 6.Bu L, Jin Y, Shi Y, et al. Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet. 2002;31:276–8. doi: 10.1038/ng921. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto M, Izu H, Seki K, et al. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004;23:4297–306. doi: 10.1038/sj.emboj.7600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min JN, Zhang Y, Moskophidis D, et al. Unique contribution of heat shock transcription factor 4 in ocular lens development and fiber cell differentiation. Genesis. 2004;40:205–17. doi: 10.1002/gene.20087. [DOI] [PubMed] [Google Scholar]
- 9.Xiao H, Lis JT. Germline transformation used to define key features of heat-shock response elements. Science. 1988;239:1139–42. doi: 10.1126/science.3125608. [DOI] [PubMed] [Google Scholar]
- 10.Shi X, Cui B, Wang Z, et al. Removal of Hsf4 leads to cataract development in mice through down-regulation of gamma S-crystallin and Bfsp expression. BMC Mol Biol. 2009;10:10. doi: 10.1186/1471-2199-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouroku Y, Soyama A, Fujita E, et al. RA70 is a src kinase-associated protein expressed ubiquitously. Biochem Biophys Res Commun. 1998;252:738–42. doi: 10.1006/bbrc.1998.9637. [DOI] [PubMed] [Google Scholar]
- 12.Marie-Cardine A, Verhagen AM, Eckerskorn C, et al. SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 1998;435:55–60. doi: 10.1016/s0014-5793(98)01040-0. [DOI] [PubMed] [Google Scholar]
- 13.Swanson KD, Tang Y, Ceccarelli DF, et al. The Skap-hom dimerization and PH domains comprise a 3’-phosphoinositide-gated molecular switch. Mol Cell. 2008;32:564–75. doi: 10.1016/j.molcel.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Kang H, Raab M, et al. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc Natl Acad Sci USA. 1998;95:8779–84. doi: 10.1073/pnas.95.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Togni M, Swanson KD, Reimann S, et al. Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol Cell Biol. 2005;25:8052–63. doi: 10.1128/MCB.25.18.8052-8063.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–84. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- 17.Menko AS, Klukas KA, Johnson RG. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol. 1984;103:129–41. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 18.Lim MA, Yang L, Zheng Y, et al. Roles of PDK-1 and PKN in regulating cell migration and cortical actin formation of PTEN-knockout cells. Oncogene. 2004;23:9348–58. doi: 10.1038/sj.onc.1208147. [DOI] [PubMed] [Google Scholar]
- 19.Symonds JG, Lovicu FJ, Chamberlain CG. Posterior capsule opacification-like changes in rat lens explants cultured with TGFbeta and FGF: effects of cell coverage and regional differences. Exp Eye Res. 2006;82:693–9. doi: 10.1016/j.exer.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Huang JX, Feldmeier M, Shui YB, et al. Evaluation of fibroblast growth factor signaling during lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2003;44:680–90. doi: 10.1167/iovs.01-1177. [DOI] [PubMed] [Google Scholar]
- 21.Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–40. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia CM, Kwon GP, Beebe DC. alpha-Smooth muscle actin is constitutively expressed in the lens epithelial cells of several species. Exp Eye Res. 2006;83:999–1001. doi: 10.1016/j.exer.2006.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagamoto T, Eguchi G, Beebe DC. Alpha-smooth muscle actin expression in cultured lens epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1122–9. [PubMed] [Google Scholar]
- 24.Lee A, Fischer RS, Fowler VM. Stabilization and remodeling of the membrane skeleton during lens fiber cell differentiation and maturation. Dev Dyn. 2000;217:257–70. doi: 10.1002/(SICI)1097-0177(200003)217:3<257::AID-DVDY4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekher G, Sailaja D. Differential activation of phosphatidylinositol 3-kinase signaling during proliferation and differentiation of lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:4400–11. doi: 10.1167/iovs.03-0136. [DOI] [PubMed] [Google Scholar]
- 26.Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- 27.Bourette RP, Therier J, Mouchiroud G. Macrophage colony-stimulating factor receptor induces tyrosine phosphorylation of SKAP55R adaptor and its association with actin. Cell Signal. 2005;17:941–9. doi: 10.1016/j.cellsig.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–44. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- 29.Frese S, Schubert WD, Findeis AC, et al. The phosphotyrosine peptide binding specificity of Nck1 and Nck2 Src homology 2 domains. J Biol Chem. 2006;281:18236–45. doi: 10.1074/jbc.M512917200. [DOI] [PubMed] [Google Scholar]
- 30.Bladt F, Aippersbach E, Gelkop S, et al. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol. 2003;23:4586–97. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13:1550–65. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–7. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 33.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Fan J, Woodley DT. Nck/Dock: an adapter between cell surface receptors and the actin cytoskeleton. Oncogene. 2001;20:6403–17. doi: 10.1038/sj.onc.1204782. [DOI] [PubMed] [Google Scholar]
- 35.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–13. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu Y, Li F, Wu C. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol Biol Cell. 1998;9:3367–82. doi: 10.1091/mbc.9.12.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goicoechea SM, Tu Y, Hua Y, et al. Nck-2 interacts with focal adhesion kinase and modulates cell motility. Int J Biochem Cell Biol. 2002;34:791–805. doi: 10.1016/s1357-2725(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 38.Ruusala A, Pawson T, Heldin CH, et al. Nck adapters are involved in the formation of dorsal ruffles, cell migration, and Rho signaling downstream of the platelet-derived growth factor beta receptor. J Biol Chem. 2008;283:30034–44. doi: 10.1074/jbc.M800913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velyvis A, Vaynberg J, Yang Y, et al. Structural and functional insights into PINCH LIM4 domain-mediated integrin signaling. Nat Struct Biol. 2003;10:558–64. doi: 10.1038/nsb938. [DOI] [PubMed] [Google Scholar]
- 40.Geiger B, Bershadsky A, Pankov R, et al. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 41.Westhoff MA, Serrels B, Fincham VJ, et al. SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol. 2004;24:8113–33. doi: 10.1128/MCB.24.18.8113-8133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu JF, Chevet E, Kebache S, et al. Functional Rac-1 and Nck signaling networks are required for FGF2-induced DNA synthesis in MCF-7 cells. Oncogene. 1999;18:6425–33. doi: 10.1038/sj.onc.1203027. [DOI] [PubMed] [Google Scholar]
- 43.Rao PV, Maddala R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Semin Cell Dev Biol. 2006;17:698–711. doi: 10.1016/j.semcdb.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai H, Takemori Y. Interaction between heat shock transcription factors (HSFs) and divergent binding sequences: binding specificities of yeast HSFs and human HSF1. J Biol Chem. 2007;282:13334–41. doi: 10.1074/jbc.M611801200. [DOI] [PubMed] [Google Scholar]
- 45.Fujimoto M, Oshima K, Shinkawa T, et al. Analysis of HSF4 binding regions reveals its necessity for gene regulation during development and heat shock response in mouse lenses. J Biol Chem. 2008;283:29961–70. doi: 10.1074/jbc.M804629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis DJ, Jane SM, Hilton DJ, et al. Adaptor protein SKAP55R is associated with myeloid differentiation and growth arrest. Exp Hematol. 2000;28:1250–9. doi: 10.1016/s0301-472x(00)00537-3. [DOI] [PubMed] [Google Scholar]
- 47.Reinhold A, Reimann S, Reinhold D, et al. Expression of SKAP-HOM in DCs is required for an optimal immune response in vivo. J Leukoc Biol. 2009;86:61–71. doi: 10.1189/jlb.0608344. [DOI] [PubMed] [Google Scholar]
- 48.Wu C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15:460–6. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Anton IM, Lu W, Mayer BJ, et al. The Wiskott-Aldrich syndrome protein-interacting protein (WIP) binds to the adaptor protein Nck. J Biol Chem. 1998;273:20992–5. doi: 10.1074/jbc.273.33.20992. [DOI] [PubMed] [Google Scholar]
- 50.Bagrodia S, Taylor SJ, Creasy CL, et al. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995;270:22731–7. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- 51.Sanders LC, Matsumura F, Bokoch GM, et al. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–5. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 52.van Leeuwen FN, van Delft S, Kain HE, et al. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1:242–8. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- 53.Manser E, Huang HY, Loo TH, et al. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–43. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vadlamudi RK, Li F, Adam L, et al. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–90. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 55.Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–9. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- 56.Chen M, She H, Kim A, et al. Nckbeta adapter regulates actin polymerization in NIH 3T3 fibroblasts in response to platelet-derived growth factor bb. Mol Cell Biol. 2000;20:7867–80. doi: 10.1128/mcb.20.21.7867-7880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 58.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–92. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 59.Frame MC, Fincham VJ, Carragher NO, et al. v-Src’s hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–45. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SKAP2 is associated with lens epithelial cellsdifferentiation. Semi-quantitative RT-PCR of 1 μg ofpolyA+ mRNA from the SRA01/04 cells was performed withprimers for the genes that are specified. The cells were incubatedin the presence of FBS (+), serum-free DMEM- or 20 ng/ml FGF-b for different amounts of time.
Overexpression of SKAP2 in HSF4-/-lens cells partially causes the disassembly of centre stressfibres. The primary HSF4-/- lens culture cells weretransfected with SKAP2-GFP or pEGFP vectors. After 24 hrs, thecells were starved for 24 hrs and then treated with 40 ng/ml FGF-bfor 12 hrs. Finally, the cells were fixed and stained withphalloidin Alexa Fluor 555 to visualize F-actin. The magnifiedimages were from the region pointed by the white arrows. Bar 50μm.
The nucleotide sequences of putative HSEs in the promoter region of mouse Skap2. The sequences consisting of at least three inverted repeats of nGnnn include #2, #3, #4, #6, #7, #8, #9 sequences. Among these, #2 sequence has two perfect inverted repeats of nGAAn; #3 sequence has one perfect repeat of nGAAn. #1 sequence is DR-type HSE (nnGAAnnnnn nnGAAn) and #5 sequence is the gap-type HSE (nTTCnnGAAn(5 bp)nGAAn). The inverted repeats of nGnnn or nGAAn sequences are shown in red.


