Abstract
The onset of human labour resembles inflammation with increased synthesis of prostaglandins and cytokines. There is evidence from rodent models for an important role for nuclear factor-κB (NF-κB) activity in myometrium which both up-regulates contraction-associated proteins and antagonizes the relaxatory effects of progesterone. Here we show that in the human, although there are no differences in expression of NF-κB p65, or IκB-α between upper- or lower-segment myometrium or before or after labour, there is nuclear localization of serine-256-phospho-p65 and serine-536-phospho-p65 in both upper- and lower-segment myometrium both before and after the onset of labour at term. This shows that NF-κB is active in both upper and lower segment prior to the onset of labour at term. To identify the range of genes regulated by NF-κB we overexpressed p65 in myocytes in culture. This led to NF-κB activation identical to that seen following interleukin (IL)-1β stimulation, including phosphorylation and nuclear translocation of p65 and p50. cDNA microarray analysis showed that NF-κB increased expression of 38 genes principally related to immunity and inflammation. IL-1β stimulation also resulted in an increase in the expression of the same genes. Transfection with siRNA against p65 abolished the response to IL-1β proving a central role for NF-κB. We conclude that NF-κB is active in myocytes in both the upper and lower segment of the uterus prior to the onset of labour at term and principally regulates a group of immune/inflammation associated genes, demonstrating that myocytes can act as immune as well as contractile cells.
Keywords: labour, myometrium, NF-κB, cytokines, chemokines
Introduction
Abnormalities of the duration of human pregnancy, resulting in preterm or postdate labour, are major causes of neonatal morbidity and mortality [1, 2]. A full understanding of the biochemical mechanisms underlying the onset of human parturition is required to predict and prevent early and late delivery. Considerable attention has been focused upon the endocrine and mechanical stimuli leading to the change in the myometrium from a quiescent to a contractile state. The current paradigm is that foetal cortisol production increases the synthesis of corticotrophin releasing hormone (CRH) and estrogens by the fetoplacental unit, leading to increased expression or activity of genes that increase myometrial electrical connectivity, excitability and contractility [3]. These events do not represent a sudden switch at term, but evolve throughout gestation and involve paracrine, endocrine and mechanical stimuli [4].
A substantial body of evidence supports the link between increased cytokine synthesis and both term and preterm labour [5, 6]. Early studies suggested that prior to the onset of uterine contractions, activated inflammatory cells infiltrate both the cervix and foetal membranes. More recent data demonstrate that the inflammatory infiltration also involves the myometrium [7, 8] and that the influx of activated inflammatory cells results in an increase in the synthesis and release of cytokines and chemokines [9–11], the former promoting the synthesis of prostaglandins and the latter further inflammatory infiltration [9–11].
The nuclear factor-κB (NF-κB) transcription factor family is classically associated with inflammation and is activated in response to infection and pro-inflammatory cytokines. As a cytokine-inducible transcription factor, it plays a key role in the expression of a variety of genes involved in inflammatory responses and cell survival [12]. NF-κB is composed of homo- or heterodimeric complexes of members of the Rel family of proteins. The best studied and most abundant of these complexes is the p65:p50 heterodimer. Activation of NF-κB within the uterus appears to be a key event in parturition. In the mouse, the onset of labour is associated with release of surfactant proteins from the foetal lung, indicating pulmonary maturity, which stimulates NF-κB activation in the myometrium and the onset of labour. Inhibition of NF-κB in the mouse delays the onset of labour [13]. In a mouse model of inflammation-associated preterm labour, the anti-inflammatory cyclopentenone prostaglandin 15-deoxy-delta prostaglandin J2 inhibits NF-κB and its downstream inflammatory mediators and delays lipopolysaccharide-induced parturition [14].
In the human, labour is associated with increased NF-κB activity in amnion which precedes labour [15]. Chapman et al.[16] examined the overall cellular expression of NF-κB proteins in human myometrium and found that the principal NF-κB dimers at term are p65:p50, and that there is a decrease in the overall expression of both p65 and p50 in the lower but not the upper segment in association with labour. In a subsequent study Condon et al.[17] examined NF-κB p65 nuclear localization in lower- and upper-segment myometrium of women before and after the initiation of labour and found that p65 was principally cytoplasmic in tissues collected before the onset of labour but was nuclear in upper- but not lower-segment myometrium after the onset of labour. These studies suggest regionalization of the activation of NF-κB within the human myometrium at the time of the onset of labour. However, most studies of labour-associated gene expression in myometrium have used lower-segment myometrial biopsies and have shown up-regulation of NF-κB regulated genes such as cyclooxygenase (COX)-2, interleukin (IL)-8 and chemokines in the lower segment of the uterus beginning before the onset labour [10, 18, 19]. This suggests that activation of NF-κB occurs in both upper and lower segment of the uterus at the time of the onset of labour.
Here we use matched upper- and lower-segment myometrial biopsies taken from women at elective caesarean section before the onset of labour and at emergency caesarean section during labour to determine the pattern of NF-κB activation. NF-κB can be activated in the uterus by cytokines such as IL-1β and tumour necrosis factor (TNF)-α[20]; however, this also leads to activation of a range of other signal transduction pathways including c-Jun N-terminal kinase (JNK), extracellular-signal-regulated kinase (ERK) and p38 MAP kinases and transcription factors such as activator protein 1 (AP-1), CCAAT-enhancer-binding protein (C/EBP) and Signal Transducers and Activator of Transcription (STATs) [21–24]. To identify the genes and signalling pathways directly regulated by NF-κB in myometrium, we established overexpression of NF-κB p65 as an appropriate model of NF-κB activation in myocytes and used this model to investigate the effects of NF-κB overexpression on human myocyte gene expression and define the major pathways involved. We validated the array and pathway data by using a combined overexpression and siRNA knockdown approach.
Materials and methods
Tissue collection
Ethics approval was obtained for upper- and lower-segment myometrial biopsies. Tissue was collected with informed consent from patients at Queen Charlotte’s and Chelsea Hospital, London. Lower-segment tissue was obtained from the upper edge of lower uterine incision, made at the location of the bladder fold at the time of Caesarean section. Simultaneously, upper-segment biopsies were taken using a cervical punch biopsy instrument from the top of the uterine fundus through the uterine incision. All biopsies were taken by the same surgeon. Elective caesarean section was performed in each case at or after 39 weeks in uncomplicated pregnancies. Indications were breech presentation and previous caesarean section. Samples were taken from caesarean section in labour only in patients with uncomplicated pregnancies, who had established in labour spontaneously. Indications for caesarean section were cephalopelvic disproportion and risk of foetal hypoxia.
Immunostaining
Five micrometre sections were cut from formalin-fixed and paraffin wax-embedded myometrial tissue. Sections were deparaffinized in histoclear and rehydrated using serial ethanol dilutions. Antigen retrieval was performed in 1 mM citrate buffer. Sections were then blocked in 5% normal goat serum followed by primary antibody (p65, Santa Cruz Biotechnology, Santa Cruz, CA, USA; p65 serine-276, Cell Signalling, Inc., Danvers, MA, USA; α-smooth-muscle actin, Sigma, St. Louis, MO, USA) for 12–18 hrs at 4°C. Slides were washed with PBS and incubated with a fluorescently conjugated secondary antibody; p65 and p65 serine-276 were visualized with red-fluorescent Alexa Fluor 594 dye (Invitrogen, Carlsbad, CA, USA), and α-smooth-muscle actin was visualized with green-fluorescent Alexa Fluor 488 dye (Invitrogen). Sections were then washed with PBS and mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA). Images were captured with the IPlab 3.7 programme using a Hamamatsu camera (Hamamatsu Photonics, Iwata, Japan). All images were taken with the same exposure time with the same dynamic range.
Cell culture
Lower uterine segment myometrial tissue from pre-labour caesarean section, was minced and digested for 45 min. in DMEM with 1 mg/ml collagenase types IA and IX (Sigma-Aldrich, St. Louis, MO, USA). Cells were centrifuged at 400 ×g for 10 min. and grown in DMEM with 10% foetal calf serum, L-glutamine, and penicillin-streptomycin (37°C and 5% CO2). Cells were serum starved for 16 hrs before IL-1β (R&D Systems, Minneapolis, MN, USA) was added to a final concentration of 1 ng/ml. To confirm that the cells established in our cultures are myocytes and not fibroblasts or epithelial cells, we undertook Western analysis for α-smooth-muscle actin, a smooth-muscle-specific marker that is not expressed in fibroblasts or epithelial cells [25]. α-smooth-muscle actin was expressed strongly in both upper- and lower-segment uterine myocytes.
Transient transfection
Transfection of adherent myocytes was performed with FuGENE 6 (Roche, Indianapolis, IN, USA). Final volume of FuGENE 6 necessary for transfection was calculated as FuGENE:DNA ratio (μl:μg) of 3:1. The FuGene/plasmid DNA/media mix was gently swirled and incubated at room temperature for 15 min. Transfection was performed when myocytes reached a confluence level of approximately 70–80%. Expression construct for NF-κB p65 in a basic PSG5 vector was transfected at 0.2 μg/well. The cells were transfected with either empty PSG5 or PSG5 plasmid containing p65 and grown for 48 hrs, after which protein and RNA were extracted via established protocols, as outlined below. The transfection efficiency in our system is approximately 40%, as assessed by using a green fluorescent protein (GFP) construct. Forty-eight hours was set as a time point for transfecting p65 as preliminary experiments showed a good response at this time point (see Fig. 4K).
Fig 4.
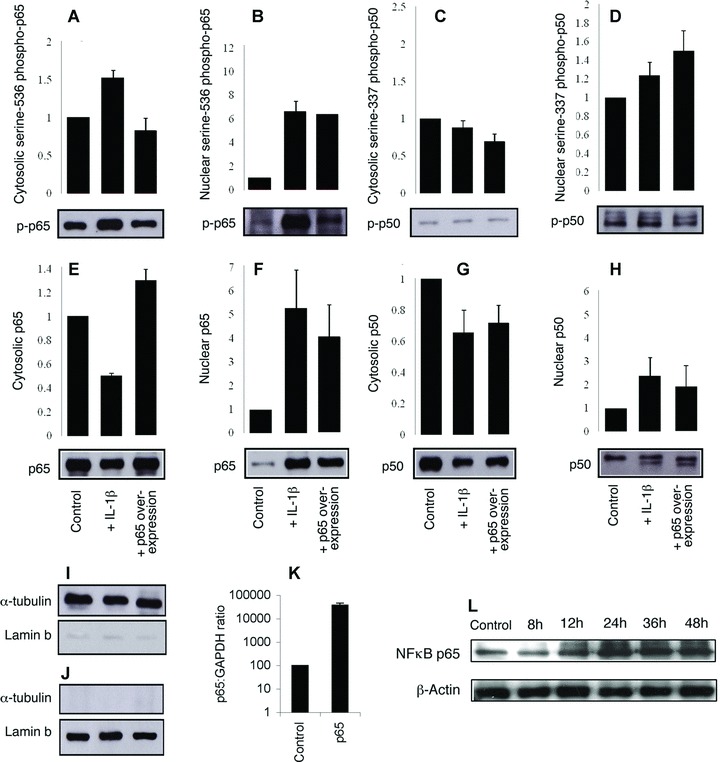
Overexpression of NF-κB p65 increases the nuclear localization and phosphorylation of both p65 and p50. Myometrial cells were either transfected with p65 or stimulated with IL-1β (1 ng/mL) for 30 min. Nonstimulated cells transfected with empty vector were used as control. Separate nuclear and cytoplasmic protein fractions were used for Western analysis for serine-536-phospho-p65 (A, B), serine-337-phospho-p50 (C, D), NF-κB p65 (E, F), NF-κB p50 (G, H). To confirm nuclear/cytoplamsic separation Western analysis was performed on the same proteins for α-tubulin (cytosol specific) and Lamin b (nuclear specific) (I, J). Results were normalized against the nuclear and cytoplasmic controls α-tubulin and lamin-b respectively and are expressed as mean ± S.E.M. (n= 3). RT-PCR data showed an increase in NF-κB p65 48 hrs following transfection of a p65 expression vector (K) and are expressed on a log scale as mean ± S.E.M. (n= 6). A time course study shows that p65 protein overexpression is maximal as early as 24 hrs but sustained for at least 48 hrs (L).
SiRNA transfection
ON-TARGETplus SMART pool human RelA SiRNA (Dharmacon, Lafayette, CO, USA) was used to knock down p65. SiGLO (Dharmacon) was used as a positive control, gaving a high transfection efficiency of approximately 90%, and ON-TARGETplus Non-Targeting Pool (Dharmacon) was used as a negative control. The SiRNAs were transfected using Dharmafect 2 (Dharmacon) transfection reagent at a final concentration of 20 μM according to manufacturer’s instructions.
Protein extraction
Cultured cells
Myocytes were scraped into buffer A [10 mM HEPES pH 7.4, 10 mM KCl, 0.1 mM ethylenediaminetetraacetic acid ethylene glycol tetraacetic acid (EDTA), 0.1 mM EGTA, 2 mM dithiothreitol (DTT), complete protease inhibitor tablets (Roche) and 2 M KOH to adjust pH to 7.4]. The resulting suspension was collected and incubated on ice for 20 min. To the incubated mixture, NP-40 (Nonidet P-40) was added to give a final concentration of 1% and carefully mixed. The cytosolic protein fraction was extracted by centrifuging at 13,000 ×g for 30 sec. The pellet was then resuspended in buffer B (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 2 mM DTT, 400 mM NaCl, 1% NP-40 and complete protease inhibitor tablets [Roche]) to lyse the nuclear membrane. Samples were incubated on a shaking platform for 15 min. Cell suspensions were centrifuged at 13,000 ×g for 5 min. and supernatant (nuclear protein fraction) was collected, snap-frozen and stored at –80°C for later use.
Tissue biopsies
Tissue was washed in PBS, dissected, flash frozen in liquid nitrogen and stored at –80°C. Tissue samples were homogenized in the presence of radioimmunoprecipitation assay (RIPA)/SDS buffer (RIPA buffer; [1% NP-40, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Tris-HCl (pH 8.0), 2 mM NaF]), with a Dounce homogenizer. The lysate was incubated on ice for 15 min., and the supernatant collected after a 5 min. centrifugation at 4°C, 12,000 ×g. Supernatant was aliquoted, snap frozen and stored at –80°C.
Protein quantification protocol
Protein concentration was determined for all protein lysates using DC protein assay reagents (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions.
Western blot analysis
Cultured cells
Twenty-five micrograms protein samples were mixed with Laemmli sample buffer (1:1) containing β-mercaptoethanol (5%), and boiled for 5 min. Protein was separated by SDS-PAGE gels and transferred onto nitrocellulose membrane (Amersham Biosciences, Arlington Heights, IL, USA). The membrane was blocked in buffer containing 5% milk powder, PBS and 0.1% Tween 20 for 30 min., and immunoblotted with primary antibody for 1 hr in 1% milk buffer followed by secondary antibody for 45 min. Horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) were used with ECL Plus (Amersham Biosciences) chemiluminescent reagents for signal detection.
Tissue extracts
A total of 20–70 μg proteins were run as per the cultured cell protocol. To detect multiple signals from a single membrane, the blot membrane was treated with a stripping buffer (2% SDS, 62.5 mM Tris-HCl [pH 6.7], and 100 mM 2-mercaptoethanol) for 30 min. at 50°C, washed in PBS and Tween 20 and then pre-blocked and reprobed (once only) with a different primary antibody. The levels of cellular Gβ (Santa Cruz Biotechnology) were used as a control for intersample variability.
Antibodies against p65, IκB-α, p50, Ser337p50, α–tubulin, laminB and Gβ were purchased from Santa Cruz Biotechnology. Smooth muscle actin and β-actin were purchased from Sigma. Ser536p65 and Ser276p65 were from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Electrophoresis motility shift assay (EMSA)
Equal quantities of sense and antisense oligonucleotides were incubated for 10 min. at 65°C in annealing buffer (10 mM Tris-HCl pH7.5, 100 mM NaCl, 1 mM EDTA), and allowed to cool at room temperature. Duplex oligonucleotides (3.5pmol) were 5′-end-labelled with [γ32P] ATP (0.37 MBq/μl; MP Biomedical, Irvine, CA, USA; Limited Liability Company) by incubating with T4 polynucleotide kinase at 37°C for 10 min. Radioactively labelled oligonucleotides were purified over a G-25 Sephadex column. Five micrograms of nuclear protein extracts of either p65 overexpressed or IL-1β treated cells were incubated on ice for 1 hr in binding buffer (4% glycerol, 1 mM MgCl2, 0.4 mM EDTA, 10 mM Tris-HCl pH 7.5, 50 mM NaC, 0.4DTT) with non-radiolabelled consensus NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) and non-consensus Oct-1 oligonucleotide (5′-TGT CGA ATG CAA ATC ACT AGA A-3′). Oct-1 was used as control for protein binding. Binding specificity was assessed by using a 100-fold molar addition of non-consensus and consensus unlabelled oligonucleotides. Supershift analysis was performed by incubating samples with 2 μl of p65 antibody (Santa Cruz Biotechnology) and p50 (Santa Cruz Biotechnology) antibodies for 1 hr on ice. The mix was further incubated with [γ32P] ATP-end labelled NF-κB probe on ice for 45 min. The DNA/protein complexes were resolved by electrophoresis in a 4% polyacrylamide gel and dried under vacuum at 80°C. EMSA blots were exposed overnight at –80°C.
RNA extraction
Total RNAs were isolated using RNeasy mini Kit (Qiagen, Hilden, Germany). RNA integrity and purity were assessed using a ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Quantitative real-time PCR
One microgram of total RNA was reverse transcribed with oligo dT random primers using MuLV reverse transcriptase (Applied Biosystems Ltd., Foster City, CA, USA). Paired oligonucleotide primers for amplification of genes of interest were designed using Primer 3 software against the sequences downloaded from GenBank. The primer sets used (Table 1) produced amplicons of the expected size and where possible flanked intron/exon junctions. Quantitative PCR was performed with Power SYBR Green PCR master mix (Applied Biosystems Ltd.) and amplicon yield was monitored during cycling in a RotorGene Sequence Detector (Corbett Research Ltd., Sydney, Australia) that continually measures fluorescence caused by the binding of the dye to double-stranded DNA. Pre-PCR cycle was 10 min. at 95°C followed by up to 45 cycles of 95°C for 20 sec., 58–60°C for 20 sec. and 72°C for 20 sec. followed by an extension at 72°C for 15 sec. The final procedure involves a melt over the temperature range of 72–99°C rising by 1 degree steps with a wait for 15 sec. on the first step followed by a wait of 5 sec. for each subsequent step. The cycle at which the fluorescence reached a preset threshold (cycle threshold) was used for quantitative analyses. The cycle threshold in each assay was set at a level where the exponential increase in amplicon abundance was approximately parallel between all samples. mRNA data were expressed relative to the amount of the constitutively expressed housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GADPH).
Table 1.
Primer pairs used
| mRNA | Forward primer sequence | Reverse primer sequence | Genebank accession no. |
|---|---|---|---|
| GAPDH | 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ | 5′-TCCTTGGAGGCCATGTAGGCCAT-3′ | BC014085 |
| IL-8 | 5′-GCCTTCCTGATTTCTGCAGC -3′ | 5′- CGCAGTGTGGTCCACTCTCA -3′ | NM000584 |
| CCL5 | 5′-CCATATTCCTCGGACACCAC-3′ | 5′- TGTACTCCCGAACCCATTTC-3′ | NM002985 |
| CXCL1 | 5′-GAAAGCTTGCCTCAATCCTG-3′ | 5′-GCCTCTGCAGCTGTGTCTCT-3′ | NM001511 |
| IL-6 | 5′-CCTTCCAAAGATGGCTGAAA-3′ | 5′-AGCTCTGGCTTGTTCCTCAC-3′ | NM000600 |
| CCL2 | 5′-TCTGTGCCTGCTGCTCATAG | 5′-AGATCTCCTTGGCCACAATG-3′ | X14768 |
| IL-1β | 5′-TACCTGAGCTCGCCAGTGAA-3′ | 5′-TCGGAGATTCGTAGCTGGATG-3′ | NM000576 |
| CCL11 | 5′- TCCCCAGAAAGCTGTGATCTTC-3′ | 5′- ATCCTGCACCCACTTCTTCTTG-3′ | NM002986 |
| CCL20 | 5′-TTTATTGTGGGCTTCACACG | 5′-TGGGCTATGTCCAATTCCAT-3′ | NM004591 |
| SOD2 | 5′-TTACAACTCAGGTCGCTCTT-3′ | 5′-GCTGTCAGCTTCTCCTTAAA-3′ | R34841 |
| MGC5618 | 5′-CCAAGAAGGGTTTTTGTGACTGA-3′ | 5′-TCCAACACTAATCATGCTGAGGTT-3′ | BF575213 |
Statistical analysis
For Western blotting, data densitometry was performed to quantify protein. Either all samples were separated on the same Western blot (Fig. 1) or expression was normalized to the control value (Fig. 4). Data are expressed as mean ± S.E.M. and statistical analysis was performed by ANOVA with the level of significance defined as P < 0.05. For RT-PCR, data were expressed as mean ± S.E.M. and statistical analysis was performed by Wilcoxon matched pairs analysis with the level of significance defined as P < 0.05.
Fig 1.
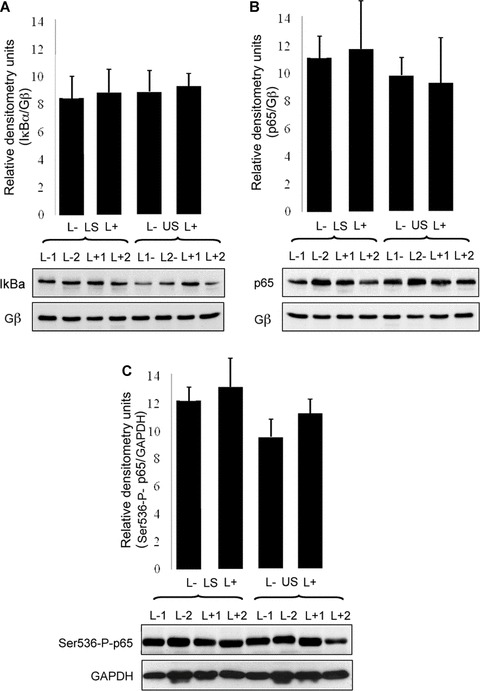
No labour-associated or regional differences in NF-κB proteins in myometrium at term. Whole-cell tissue protein was extracted from matched upper and lower-segment myometrial biopsies collected before (L−) or after (L+) labour onset (example Western blots n=2 for each tissue type, bar graphs total n=4 for each tissue type). Western analysis was performed for IkBa (A; P= 0.9938 by ANOVA), p65 (B; P= 0.4177 by ANOVA), and Ser536-P-p65 (C; P= 0.4414 by ANOVA). Blots were scanned for densitometric analysis and values normalized against densitometry values for Gβ or GAPDH.
Affymetrix HgU133 plus 2.0 array processing
Term myocytes from caesarean section prior to labor were transfected with pSG5/p65 and grown for 48 hrs, with total RNA extracted as previously described. Samples were profiled by Almac Diagnostics (Craigavon, UK) using the Affymetrix HgU133 Plus 2.0 GeneChip® (Affymetrix, Inc., Santa Clara, CA, USA). Briefly, cDNA was generated from 2 μg of total RNA using the GeneChip® Expression 3′-Amplification One-Cycle cDNA Synthesis kit, in conjunction with the GeneChip® Eukaryotic PolyA RNA Control Kit (Affymetrix, Inc.). The cDNA was cleaned up using the GeneChip® Sample Cleanup Module and subsequently processed to generate biotin-labelled cRNA using the GeneChip® Expression 3′-Amplification IVT Labeling Kit (Affymetrix, Inc.). Twenty-five micrograms of labelled cRNA was fragmented using 5× fragmentation buffer and Rnase-free water at 94°C for 35 min. Fifteen micrograms of the fragmented, biotin-labelled cRNA was made up in a hybridization cocktail and hybridized to the HgU133 Plus 2.0 array at 45°C for 16 hrs. Following hybridization the arrays were washed and stained using the Affymetrix Fluidics Station 450 and scanned using the Affymetrix GeneChip® Scanner 3000. All steps of the process were quality controlled by measuring yield (μg), concentration (μg /l) and 260:280 ratios via spectrophotometry using the Nanodrop ND-1000 and sample integrity using the Agilent 2100 bioanalyser (Agilent Agilent Technologies, Inc., Santa Clara, CA, USA).
Data quality control and analysis
All data quality control and analysis was performed by Almac Diagnostics. Following scanning of the arrays, grids were aligned using the Affymetrix GeneChip® Operating Software (GCOS; Affymetrix, Inc.) and intensity values captured for each transcript. Initial GeneChip® QC quality control was performed according to Affymetrix recommendations to ensure samples were suitable for analysis. In addition data QC was performed by assessing sample and replicate profiles using hierarchical clustering and principal components analysis to identify any potential outliers.
Generation of differentially expressed gene lists was performed with GeneSpring v 7.0 (Agilent Technologies, Inc.). In brief, the data were normalized by flooring the values to 0.01, followed by a per-chip normalization to the median value and gene expression ratios generated by per-gene normalization to the control group (PSG5).
Data were filtered using a sequential filtering approach. A combination of flag, signal-to-noise, fold-change and confidence filtering was applied to the entire array profile. Data that were ‘present’ or ‘marginal’ in all samples, had intensity values above the signal-to-noise floor value, were greater than 2-fold differentially expressed (comparison dependent) and had a fold-change P-value of <0.05 with Benjamini–Hochberg false discovery rate (FDR) applied were retained. This filtering resulted in a less-stringent gene list (Flag, Signal-to-noise and fold-change filters) and a stringent gene list (Flag, Signal-to-noise, fold-change and confidence filters).
Functional analysis
Functional analysis was performed on the less-stringent gene list generated based on NF-κB overexpression, using the Metacore™ Integrated Software Suite (GeneGo Bioinformatics Software, Inc., St. Joseph, MI, USA). The gene list contained 457 transcripts, of which 405 transcript IDs were recognized by Metacore™. The analysis suite was used to perform pathway analysis. The recognized transcript IDs were compared against all available pathways and gene networks were created using the ‘transcription regulation’ algorithm.
Results
No differences in expression of p65, IκBα or serine-536-phospho-p65, between either the upper and lower uterine segments of the myometrium or before or after the onset of labour
No significant differences were detected in total p65 protein expression between tissues derived before (n= 11) or after (n= 11) labour onset in lower segment (LS) myometrium, as assessed by Western immunoblot analysis (data not shown). No significant labour-associated or regional differences were detected in IκBα expression (Fig. 1A). Similarly, no differences in p65 expression were found when comparing matched upper segment (US) and LS myometrium either prior to or following labour onset (Fig. 1B). Gβ levels were used to control for equal loading since expression of this protein in myometrium has been shown not to vary with labour status or region of the uterus sampled [16]. To study serine-536- phosphorylation of p65 we used an antibody for serine-536-phospho-p65 which is suitable for Western analysis but is unsuitable for immunohistochemistry. We also found no differences in overall cellular serine-536-phospho-p65 between US and LS myometrium or before or after labour (Fig. 1C).
No differences in nuclear localization of p65 or serine-276-phospho-p65 between either the upper and lower uterine segments of the myometrium or before or after the onset of labour
To study nuclear localization and phosphorylation of p65 we used antibodies for total NF-κB p65 and serine-276-phospho-p65 which are suitable for immunohistochemical studies Matched US and LS myometrial biopsies taken from women undergoing caesarean section were subjected to immunohistochemical studies using antibodies to total NF-κB p65, serine-276-phospho-p65 and smooth-muscle actin. Nuclei were identified by 4′,6-diamidino-2-phenylindole (DAPI) staining. NF-κB p65 was found to be present in all myocytes (co-incident with smooth-muscle actin staining) (Fig. 2). Total NF-κB p65 was present both in the nucleus and in the cytoplasm; however, serine-276-phospho-p65 was seen only in the nucleus in the majority, but not all of the cells. There were no differences in nuclear localization of p65 or serine-276-phospho-p65 between US or LS myometrium or before or after labour (Fig. 3).
Fig 2.
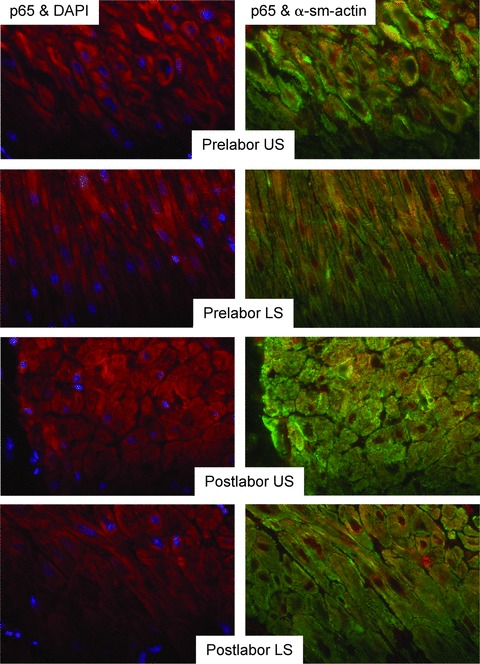
No labour-associated or regional differences in expression or nuclear localization of p65 in myometrium at term. Paraffin-embedded tissue taken from matched upper and lower-segment myometrial biopsies collected prior to (L−) or following (L+) labour onset was subject to immunofluorescent analysis, using p65 with red-fluorescent Alexa Fluor 594 dye and a-smooth muscle actin (α-sm-actin) was visualized with green-fluorescent Alexa Fluor 488 dye. Cell nuclei were visualized with DAPI (blue). (L−, n=5, L+, n=6). Objective lens used was x40.
Fig 3.
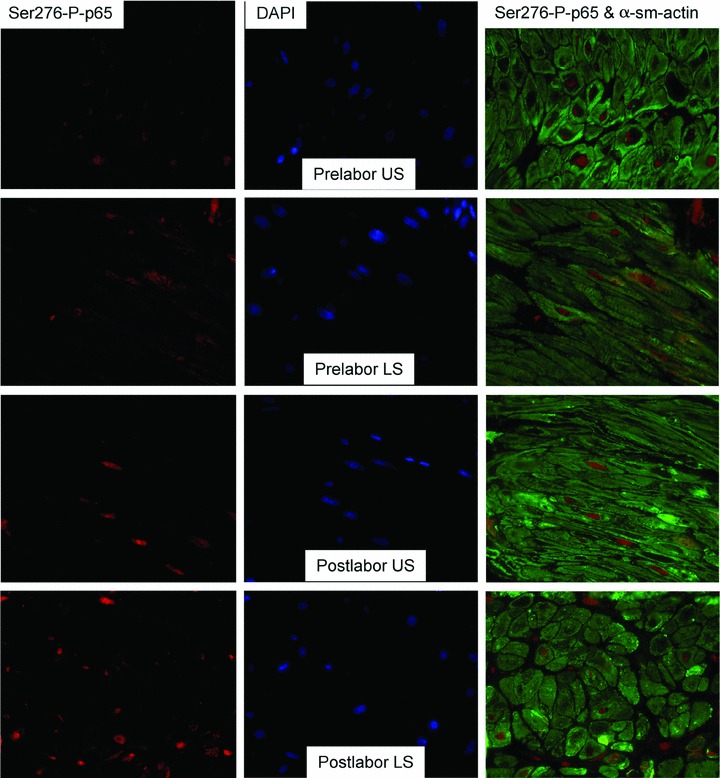
No labour-associated or regional differences in serine 276 phosphorylation of p65 in myometrium at term. Immunofluorescent analysis of serine 276-phosphorlyated p65 (Ser276-P-p65) expression in paraffin-embedded matched upper (US) and lower (LS) segment myometrial biopsies taken at term either prior (L−) or following (L+) labour onset. Ser276-P-p65 was visualized with red-fluorescent Alexa Fluor 594 dye and a-smooth muscle actin (α-sm-actin) was visualized with green-fluorescent Alexa Fluor 488 dye. Cell nuclei were visualized with DAPI (blue). (L−, n=3, L+, n=6). Objective lens used was x63.
Overexpression of p65 results in phosphorylation, nuclear localization and DNA binding of both p65 and p50
We have previously shown that in myometrium the principal NF-κB dimer that translocates to the nucleus, binds DNA and drives gene expression is the p65:p50 heterodimer [20]. To specifically drive NF-κB gene expression, we explored overexpression of p65 and p50. We found that p65 could be reliably overexpressed, leading to increased phosphorylation, nuclear localization and DNA binding of both p65 and p50. Western immunoblotting analysis was performed on cytosolic and nuclear extracts from term myocytes transfected with p65 and/or stimulated with IL-1β. As expected, in non-transfected cells, IL-1β stimulation resulted in a reduction in cytosolic p65 and a concomitant increase in nuclear p65. Likewise, IL-1β increased the levels of nuclear Ser536-p-p65. IL-1β stimulation also increased nuclear p50 and Ser337-p-p50 levels. In transfected cells overexpressing p65, there was a 380-fold increase in p65 mRNA expression measured by RT-PCR and a 1.2-fold increase in cytosolic total p65 protein levels but a 4-fold increase in p65 protein levels in the nuclear fractions. p65 overexpression also caused a 1.8-fold increase in p50 and a 1.4-fold increase in Ser337-p-p50 in the nucleus (Fig. 4).
These data show that overexpression of p65 leads to the phosphorylation and nuclear translocation of both p65 and p50 similar to that stimulated by IL-1β. EMSA analysis with supershift using p65 and p50 antibodies showed that overexpression of p65 leads to an increase in the nucleus of both p65 homodimers and p65/p50 heterodimers (Fig. 5).
Fig 5.
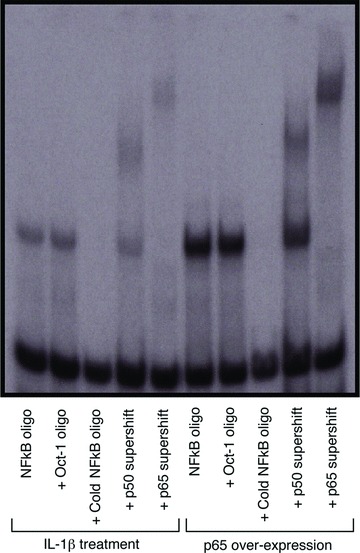
Overexpression of NF-κB p65 increases the DNA binding of both p65 and p50 to levels that are comparable to IL-1β stimulation. A 100-fold excess of unlabelled κB cold (NF-κB oligo) or irrelevant (oct-1) oligonucleotide were added before the addition of 32P-labelled probe as DNA binding competitor to verify specificity of binding. NF-κB Nuclear proteins were supershifted with both p50 (lanes 4, 9) and p65 antibodies (lanes 5, 10).
NF-κB regulates a group of immune/inflammatory genes
An Affymetrix Human Gene Chip array (HG_U95Av2) was used to characterize the change in gene expression profile of uterine myocytes (n= 3 in each group) overexpressing NF-κB p65 (Array Express; E-MEXP-2160). The fold-change difference for each gene was quantified by generating a gene expression ratio between p65-overexpressing and control cells. Of the genes present on the microarray profiling platform, nine were expressed only in p65-overexpressing cells (Table 2). These nine genes included p65 (RelA) itself, IL-1β and the chemokines CC chemokine ligand (CCL)-20, CCL-11 and chemokine (C-X-C motif) ligand (CXCL)-2. A further 33 genes showed a significant 2-fold or greater change in their expression level in p65-overexpressing cells compared to controls (Table 3). Of those 33 genes, 28 were up-regulated and 5 were down-regulated. Of the up-regulated genes, IL-8 was the most dramatically up-regulated, with an increase of 17.6-fold. Other up-regulated genes included the chemokines CCL-2, CCL-5, CXCL1 and the cytokines IL-6 and PTX3.
Table 2.
Genes which are only present upon NF-κB activation
| Gene symbol | Gene name | |
|---|---|---|
| 1 | CCL20 | Chemokine (C-C motif) ligand 20 |
| 2 | BIRC3 | Baculoviral IAP repeat-containing 3 |
| 3 | TSLP | Thymic stromal lymphopoietin |
| 4 | RELA | Nuclear factor of κ light polypeptide gene enhancer in B-cells 3 |
| 5 | IL1B | Interleukin 1, β |
| 6 | ICAM1 | Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor |
| 7 | CCL11 | Chemokine (C-C motif) ligand 11 |
| 8 | CXCL2 | Chemokine (C-X-C motif) ligand 2 |
| 9 | SELE | Selectin E (endothelial adhesion molecule 1) |
Table 3.
Genes regulated by NF-κB activation
| Gene symbol | Gene name | Fold change | |
|---|---|---|---|
| 1 | IL-8 | Interleukin 8 | 17.6 |
| 2 | TNFAIP3 | Tumour necrosis factor, α-induced protein 3 | 4.3 |
| 3 | CCL2 | Chemokine (C-C motif) ligand 2 | 3.7 |
| 4 | CCL5 RANTES | Chemokine (C-C motif) ligand 5 | 3.5 |
| 5 | PTX3 | Pentraxin-related gene, rapidly induced by IL-1β | 3.5 |
| 6 | SOD2 | Superoxide dismutase 2, mitochondrial | 3.5 |
| 7 | CXCL1 | Chemokine (C-X-C motif) ligand 1 | 3.2 |
| 8 | MGC5618 | Hypothetical protein MGC5618 | 3.2 |
| 9 | NFKBIA | NF-k light polypeptide gene enhancer in B-cells inhibitor, α | 2.9 |
| 10 | GCH1 | GTP cyclohydrolase 1 (dopa-responsive dystonia) | 2.9 |
| 11 | TNFAIP2 | Tumour necrosis factor, α-induced protein 2 | 2.8 |
| 12 | IRAK2 | Interleukin-1 receptor-associated kinase 2 | 2.7 |
| 13 | IL-6 | Interleukin 6 (interferon, β2) | 2.7 |
| 14 | SERPINB2 | Serpin peptidase inhibitor, clade B (ovalbumin), member 2 | 2.7 |
| 15 | CLCF1 | Cardiotrophin-like cytokine factor 1 | 2.7 |
| 16 | IFI44L | Interferon-induced protein 44-like | 2.6 |
| 17 | LOC129607 | Hypothetical protein LOC129607 | 2.6 |
| 18 | RSAD2 | Radical S-adenosyl methionine domain containing 2 | 2.5 |
| 19 | CD40 | CD40 molecule, TNF receptor superfamily member 5 | 2.3 |
| 20 | CD83 | CD83 molecule | 2.3 |
| 21 | TNFSF13B | Tumour necrosis factor (ligand) superfamily, member 13b | 2.3 |
| 22 | OAS1 | 2′,5′-oligoadenylate synthetase 1, 40/46kDa | 2.2 |
| 23 | BDKRB1 | Bradykinin receptor B1 | 2.2 |
| 24 | TNFAIP8 | Tumour necrosis factor, α-induced protein 8 | 2.2 |
| 25 | IFIH1 | Interferon induced with helicase C domain 1 | 2.2 |
| 26 | MX1 | Myxovirus (influenza virus) resistance 1 | 2.1 |
| 27 | C9orf64 | Chromosome 9 open reading frame 64 | 2.0 |
| 28 | MX2 | Myxovirus (influenza virus) resistance 2 (mouse) | 2.0 |
| 29 | OPN3 | Opsin 3 (encephalopsin, panopsin) | –2.0 |
| 30 | POLR2A | Polymerase (RNA) II (DNA directed) polypeptide A, 220kDa | –2.0 |
| 31 | BAT4 | HLA-B associated transcript 4 | –2.0 |
| 32 | EEF1D | Eukaryotic translation elongation factor 1 delta | –2.0 |
| 33 | SUOX | Sulphite oxidase | –2.2 |
Pathway analysis of the genes differentially expressed due to overexpression of NF-κB p65
In pathway analysis, genes and their expression pattern are visually characterized based on their location within a cellular pathway. The original 2-fold threshold for the gene list proved to be too stringent for the pathway analysis. Therefore, the selection criterion for the gene list used for pathway analysis was relaxed; a reduced fold-change threshold of 1.5 was used. This identified 175 genes whose expression was increased by activation of NF-κB. Of over 500 possible canonical pathways on the interrogated database, 60 were significantly associated with the genes regulated by activation NF-κB in uterine myocytes. Of these 60, 31 pathways are involved in cytokine and chemokine regulation, or other immune responses, and 7 in apoptotic pathways (Table S1). Of the 10 pathways with the most significant associations, 8 are involved in immunological pathways and 2 with apoptosis.
Validation of gene expression profiles for genes found to be differentially expressed by microarray analysis
We used a dual overexpression/knockdown approach in cultures from a different group of patients to those used in the microarray studies to validate the microarray results. To confirm the cDNA microarray data, we used quantitative real-time PCR (qPCR) on a selection of genes that were found to be central in our pathway analysis. These were the cytokines IL-6 and IL-1β, the chemokines 1, 5 and 8 (IL-8) of the CXCL family, and the chemokines 2, 5, 11 and 20 of the CCL family. We also examined Mammalian Gene Collection (MGC)5618 and superoxide dismutase (SOD)2 as genes with less relevance to the pathway analysis.
Treatment of uterine myocytes with IL-1β similarly resulted in increased expression of each of the selected gene (Fig. 6A). mRNA isolated from uterine myocytes in which p65 was overexpressed also showed consistent increases in expression of all of our selected genes (Fig. 6B). In each case, the largest increases were seen for CXCL8 (IL-8) and CCL20. SiRNA to p65 caused a reduction in p65 expression to levels almost undetectable on Western analysis (Fig. 7). SiRNA to p65 reduced the effect of IL-1β on the expression of all the chemokines by greater than 80% (Fig. 6C). The expression of IL-6 and IL-1β, were also reduced by about 50%. Statistical analysis was performed by Wilcoxon matched pairs analysis with the level of significance defined as P < 0.05 as shown in Figure 6.
Fig 6.
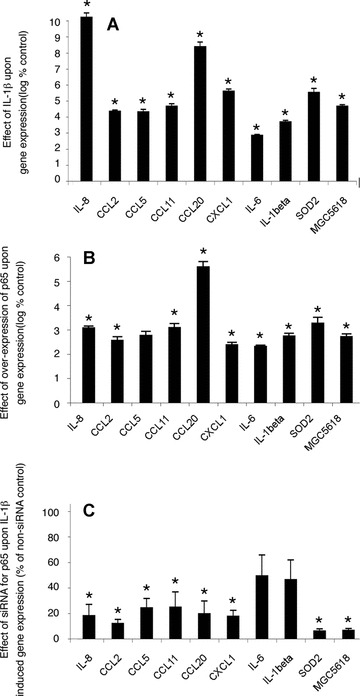
Effect of IL-1β, overexpression of p65 and knockdown of p65 on mRNA expression of selected genes. Myometrial cells were incubated with IL-1 (1 ng/ml) for 24 hrs after which total RNA was extracted and mRNA expression of selected genes was measured by qPCR. Note the logarithmic scale. (A) Mean ± S.E.M. n= 6, *P < 0.05 Wilcoxon matched pairs analysis. Myometrial cells were transfected with the expression construct for p65. The empty expression vector pSG5 was used as control. After 24 hrs, total RNA was extracted and mRNA expression of selected genes was measured by qPCR. Note the logarithmic scale. (B) Mean ± S.E.M. n= 7, *P < 0.05 Wilcoxon matched pairs analysis. Myometrial cells were transfected with either siRNA for p65 or a non-targeting siRNA control. After 72 hrs cells were incubated with IL-1 (1 ng/ml) for a further 24 hrs, after which total RNA was extracted and mRNA expression of selected genes was measured by qPCR. Note the linear scale. (C) Mean ± S.E.M. n= 6, *P < 0.05 Wilcoxon matched pairs analysis.
Fig 7.
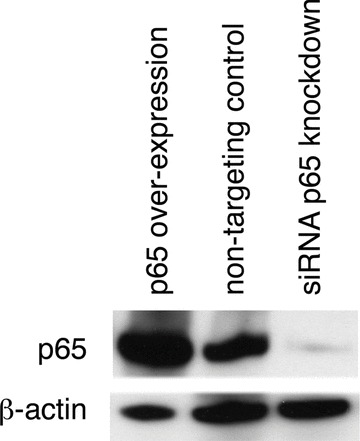
siRNA in myometrial cells effectively knocks down NF-κB p65 protein expression. Myometrial cells were transfected with either NF-κB p65 expression vector or siRNA against NF-κB p65. Non targeting siRNA was used as control. Western analysis of myometrial whole cell lysate was performed for NF-κB p65 after 72 hours incubation.
Discussion
This study has shown that that NF-κB is active in myocytes in both the upper and lower segment of the uterus prior to the onset of labour at term and regulates principally a group of immune/ inflammation associated genes, demonstrating that myocytes can act as immune as well as contractile cells. We found no differences in NF-κB activation between myometrium collected before or after the onset of labour. In our immunohistochemistry studies the majority of myocytes showed phospho-p65 nuclear localization, indicating NF-κB activation. It is unlikely that NF-κB is active in myometrium throughout pregnancy, more likely it becomes active close to the time of labour, and our failure to see differences is a reflection of our clinical practice to undertake elective caesarean section after 39 weeks, when the process leading to the onset of labour has already begun. In support of this Condon et al.[17], have previously shown that there is no activation of NF-κB in myometrium during pregnancy remote from the onset of labour (i.e. at 35 weeks). More recently Vora et al.[26] have also examined NF-κB p65 nuclear staining by immunohistochemistry in myometrium collected from women both preterm, and at term before and after the onset of labour. In their studies samples were taken at term from 37 weeks onwards. They found that even following term labour only a proportion of myocytes show NF-κB activation but that the highest degree of NF-κB activation was seen only in samples taken following the onset of labour. This, taken together with data which shows that inhibition of NF-κB in the mouse prevents normal term labour [13] suggests that NF-κB is activated in myometrium close to the onset of labour at term.
Regionalization within the uterus is important for its function. The early biochemical events in the process of parturition lead to cervical changes days or weeks before the onset of contractions. There is evidence that release of prostaglandins from the foetal membranes occurs first in the lower pole of the uterus, possibly through regional control of prostaglandin dehydrogenase expression [27].
Condon et al. have reported that activation of NF-κB in the myometrium occurs only in the upper-segment myometrium and only after the onset of labour [17]; however, they compared preterm pre-labour myometrium with post-labour myometrium and so could not exclude activation of NF-κB prior to the onset of labour. Animal data from both Condon et al. and our group show that inhibition of NF-κB in the mouse delays the onset of labour [13, 14]. This suggests that activation of NF-κB occurs either before or early in the process of labour. We have not been able to replicate the human myometrium data of Condon et al. since, when using appropriate controls, we have been unable to completely separate nuclear from cytoplasmic protein from myometrial tissue samples. We therefore used immunohistochemistry to show that NF-κB p65 and serine-276-phospho-p65 is present in the nucleus of myocytes and Western analysis using whole tissue protein to show serine-536 phosphorylation of NF-κB p65. These three factors demonstrate that NF-κB is active, and we found activation in both upper and lower segments and in tissues obtained at term, both before and after the onset of labour. We therefore conclude that there is no regionalization within the uterus in respect of activation of NF-κB and that activation of NF-κB precedes the onset of labour at term.
There is an influx of inflammatory cells into the uterus in association with labour, which themselves express NF-κB; however, our immunohistochemical studies show that the overwhelming majority of cells which express p65 within both upper- and lower-segment myometrium are myocytes. Condon et al. showed a dramatic increase in nuclear localization of NF-κB [17]. Here we show no differences in overall expression but there is activation of NF-κB prior to labour. This is consistent with it being activation of NF-κB in the pre-existing cells of the myometrium rather than an influx of NF-κB expressing leucocytes that constitute the majority of NF-κB activity in myometrium.
Activation of NF-κB within the uterus appears therefore to be a central and essential event leading to labour and delivery (15, 17). NF-κB can be activated by inflammatory cytokines (IL-1β or TNF-α) or via the TLR system [12, 28, 29]. These stimuli, however, activate other pathways in addition to NF-κB. Our secondary aim was to identify which genes are specifically up-regulated by NF-κB in human uterine myocytes. To dissect out the specific effect of NF-κB activation, we explored the use of overexpression of NF-κB subunits as a model for NF-κB activation. We have previously found that the principal NF-κB dimers that translocate to the nucleus and drive gene expression are p65 homodimers and p65:p50 heterodimers. In preliminary experiments we determined that transfection of an expression vector for p65 alone was sufficient to lead to nuclear translocation of both p65 and p50. Following overexpression of p65, nuclear NF-κB comprises principally p65 homodimers and p65:p50 heterodimers (Fig. 5). In resting cells NF-κB constantly shuttles between nucleus and cytoplasm but the balance is towards cytoplasmic retention [29]. Activation of Iκ kinase by cytokines or TLR-agonist stimulus leads to decreased inhibitory IκB binding to NF-κB dimers in the cytoplasm and therefore to a shift in the balance towards the nucleus [12]. We presume that overexpressed p65 is able to partner existing p50, overwhelming available IκB binding, and therefore leading to an increase in p65:p50 heterodimers in the nucleus. We found a transfection efficiency of 40%. Although other techniques such as cell selection by flow cytometry or stable transfection might have been used to increase apparent transfection efficiency, we considered that p65 overexpression is a valid model of NF-κB activation because immunhistochemistry had shown that not all myocytes in the intact tissue show nuclear localization of phosphorylated p65 (see Fig. 3) and the pattern of NF-κB phosphorylation and nuclear translocation following overexpression of p65 was similar to that seen following IL-1β stimulation. This approach means that we will detect changes in the expression of genes which are highly sensitive to NF-κB activation but may not detect changes in those genes that are less sensitive. The cDNA array data are based upon only three individual patients in each group which is the minimum number to allow analysis; however, the changes in gene expression were all statistically significant. This may be another reason for the relatively small number of NF-κB responsive genes that were detected; however, it does not diminish our principal conclusion that the most highly regulated genes are chemokines and cytokines.
We validated the cDNA array data using RT-PCR for mRNA for five of the ‘on-off’ genes and seven of the increased expression genes. These genes were mainly chemokines or cytokines. MGC5618 and SOD2 were selected as random genes that exhibited a greater than 3-fold change in expression but are not directly involved in immunity. Expression of each of these genes was increased by both p65 overexpression and IL-1β stimulation. Knockdown of p65 led to an 80% reduction in the expression of the chemokines and a 50% reduction in cytokine expression, suggesting that the chemokines are probably more dependent upon NF-κB activity for their expression.
Therefore, it appears that the principal gene family regulated by NF-κB in human uterine myocytes are the chemokines. The validation studies showed that CCL-20 and CXCL-8, usually known as IL-8, appear to be the most highly expressed following NF-κB activation. Expression of IL-8 in myometrium has previously been associated with labour [9, 10] and is known to be regulated by NF-κB [30]. IL-8 induces chemotaxis of inflammatory cells and is particularly responsible for recruitment of neutrophils, the signature cell of the acute inflammatory response and the principal component of the labour-associated inflammatory infiltrate into myometrium [31]. CCL-20 synthesis within the uterus has also been associated with both term and preterm labour [32]. CCL-20 is the specific chemokine ligand of the CC chemokine receptor 6 [33]. It is regulated by NF-κB in other cell types [34–38] and plays roles in the recruitment of immature dendritic cells to sites of antigen entry, mediates effector/memory T-cell interactions with endothelial cells and stimulates migration of memory B lymphocytes [39, 40]. These, together with the other chemokines that we identified to be up-regulated by activation of NF-κB in uterine myocytes, are likely to be responsible for the inflammatory infiltrate seen in myometrium with labour [8].
Results from previous studies using cDNA microarrays to examine myometrial gene expression before and after the onset of labour are not wholly consistent with each other [41, 42]. Bollapragada et al. recently compared myometrium and cervix from term pre-labour and labour samples [43]. They found that the major genes up-regulated in the uterus and cervix at the time of labour belong to inflammatory pathways and overlap considerably with the genes that we have identified to be up-regulated by NF-κB. In both studies, the gene whose expression increased the most was CXCL8/IL-8.
The changes that occur within the uterus leading to the onset of labour result from the integration of endocrine and mechanical stimuli throughout pregnancy. A current concept in the human is that CRH production from the placenta rises at term, leading to an increase in oestrogen synthesis and a reduction in the rate of progesterone production. Stretch of the uterus also modulates expression of proteins such as connexins and oxytocin receptor [44, 45], which increase myometrial responsiveness. In the mouse, a final signal to labour is increased foetal surfactant release, which stimulates NF-κB activation. It is possible that a similar mechanism functions in the human since surfactant protein A is a ligand for toll-like receptor 4, which is expressed on amnion and chorion decidua [46]. Current evidence from animal models suggests that inhibition of NF-κB will inhibit labour [13, 14]; however, our data suggest that activation of NF-κB does not drive the expression of any proteins that are directly involved in uterine contractions. Activation of NF-κB probably mediates the final commitment to labour and delivery through two mechanisms. Firstly, NF-κB has a negative interaction with progesterone receptor, leading to a final withdrawal of the quiescent effects of progesterone on the myometrium. Secondly, NF-κB leads to inflammatory cell migration into the myometrium, with a consequent marked increase in local cytokine concentrations. These cytokines would then stimulate synthesis of prostaglandins [47, 48], induce calcium entry into myometrial smooth-muscle cells [49], increasing their contractility, and stimulate phosphodiesterase activity, leading to a decrease in cAMP (adenosine monophosphate) concentrations [50].
Many researchers have shown that individual chemokines are up-regulated within the uterus with labour [11, 32, 51–54] and have suggested that these (e.g. CCL-2) may be targeted in prevention of preterm labour. However, there is considerable redundancy and promiscuity in the chemokine superfamily. Chemokines are redundant in their effects on target cells, any given leucocyte population will express receptors for several chemokines and most chemokines will interact with a range of chemokine receptors [55]. The present work demonstrates a central and essential role for NF-κB in cytokine-driven chemokine expression in myometrium. This suggests that targeting of myometrial NF-κB itself, rather than individual chemokines, would circumvent redundancy and promiscuity and is likely to be a more effective approach to prevention of preterm labour [14, 20, 28].
Acknowledgments
This study was funded by Action Medical Research Ref SP4241. PRB is supported by the NIHR Biomedical Research Centre funding scheme.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Pathways found to have significant associationswith the genes regulated by activation of NF-κB
References
- 1.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Bjog. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty L, Norwitz ER. Prolonged pregnancy: when should we intervene. Curr Opin Obstet Gynecol. 2008;20:519–27. doi: 10.1097/gco.0b013e328314b6f8. [DOI] [PubMed] [Google Scholar]
- 3.Smith R. Parturition. N Engl J Med. 2007;356:271–83. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 4.Challis JR, Sloboda DM, Alfaidy N, et al. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002;124:1–17. doi: 10.1530/rep.0.1240001. [DOI] [PubMed] [Google Scholar]
- 5.Keelan JA, Blumenstein M, Helliwell RJ, et al. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24:S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 6.Berry SM, Romero R, Gomez R, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173:1315–20. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 7.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–5. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Goncalves LF, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott CL, Slater DM, Dennes W, et al. Interleukin 8 expression in human myometrium: changes in relation to labor onset and with gestational age. Am J Reprod Immunol. 2000;43:272–7. doi: 10.1111/j.8755-8920.2000.430505.x. [DOI] [PubMed] [Google Scholar]
- 10.Tattersall M, Engineer N, Khanjani S, et al. Pro-labour myometrial gene expression: are preterm labour and term labour the same. Reproduction. 2008;135:569–79. doi: 10.1530/REP-07-0461. [DOI] [PubMed] [Google Scholar]
- 11.Shynlova O, Tsui P, Dorogin A, et al. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–9. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–86. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 13.Condon JC, Jeyasuria P, Faust JM, et al. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA. 2004;101:4978–83. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirianov G, Waddington SN, Lindstrom TM, et al. The cyclopentenone 15-deoxy-delta12,14-prostaglandin J2 delays LPS-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2008;150:699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- 15.Allport VC, Pieber D, Slater DM, et al. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581–6. doi: 10.1093/molehr/7.6.581. [DOI] [PubMed] [Google Scholar]
- 16.Chapman NR, Europe-Finner GN, Robson SC. Expression and deoxyribonucleic acid-binding activity of the nuclear factor kappaB family in the human myometrium during pregnancy and labor. J Clin Endocrinol Metab. 2004;89:5683–93. doi: 10.1210/jc.2004-0873. [DOI] [PubMed] [Google Scholar]
- 17.Condon JC, Hardy DB, Kovaric K, et al. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–75. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 18.Elliott CL, Loudon JA, Brown N, et al. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol. 2001;46:260–7. doi: 10.1034/j.1600-0897.2001.d01-11.x. [DOI] [PubMed] [Google Scholar]
- 19.Slater DM, Dennes WJ, Campa JS, et al. Expression of cyclo-oxygenase types-1 and -2 in human myometrium throughout pregnancy. Mol Hum Reprod. 1999;5:880–4. doi: 10.1093/molehr/5.9.880. [DOI] [PubMed] [Google Scholar]
- 20.Lindstrom TM, Bennett PR. 15-Deoxy-{delta}12,14-prostaglandin j2 inhibits interleukin-1{beta}-induced nuclear factor-{kappa}b in human amnion and myometrial cells: mechanisms and implications. J Clin Endocrinol Metab. 2005;90:3534–43. doi: 10.1210/jc.2005-0055. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JA, Lye SJ. Differential expression of activator protein-1 transcription factors in pregnant rat myometrium. Biol Reprod. 2002;67:240–6. doi: 10.1095/biolreprod67.1.240. [DOI] [PubMed] [Google Scholar]
- 22.Sooranna SR, Engineer N, Liang Z, et al. Stretch and interleukin 1 beta: pro-labour factors with similar mitogen-activated protein kinase effects but differential patterns of transcription factor activation and gene expression. J Cell Physiol. 2007;212:195–206. doi: 10.1002/jcp.21019. [DOI] [PubMed] [Google Scholar]
- 23.Markovic D, Vatish M, Gu M, et al. The onset of labor alters corticotropin-releasing hormone type 1 receptor variant expression in human myometrium: putative role of interleukin-1beta. Endocrinology. 2007;148:3205–13. doi: 10.1210/en.2007-0095. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht U, Yang X, Asselta R, et al. Activation of NF-kappaB by IL-1beta blocks IL-6-induced sustained STAT3 activation and STAT3-dependent gene expression of the human gamma-fibrinogen gene. Cell Signal. 2007;19:1866–78. doi: 10.1016/j.cellsig.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Condon J, Yin S, Mayhew B, et al. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67:506–14. doi: 10.1095/biolreprod67.2.506. [DOI] [PubMed] [Google Scholar]
- 26.Vora S, Abbas A, Kim CJ, et al. Nuclear factor-kappa B localization and function within intrauterine tissues from term and preterm labor and cultured fetal membranes. Reprod Biol Endocrinol. 2010;8:8–20. doi: 10.1186/1477-7827-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel FA, Clifton VL, Chwalisz K, et al. Steroid regulation of prostaglandin dehydrogenase activity and expression in human term placenta and chorio-decidua in relation to labor. J Clin Endocrinol Metab. 1999;84:291–9. doi: 10.1210/jcem.84.1.5399. [DOI] [PubMed] [Google Scholar]
- 28.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–81. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 29.Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111–30. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 30.Elliott CL, Allport VC, Loudon JA, et al. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol Hum Reprod. 2001;7:787–90. doi: 10.1093/molehr/7.8.787. [DOI] [PubMed] [Google Scholar]
- 31.Norman JE, Bollapragada S, Yuan M, et al. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7:S7–11. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamill N, Romero R, Gotsch F, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217–27. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba M, Imai T, Nishimura M, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272:14893–8. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- 34.Imaizumi Y, Sugita S, Yamamoto K, et al. Human T cell leukemia virus type-I Tax activates human macrophage inflammatory protein-3 alpha/CCL20 gene transcription via the NF-kappa B pathway. Int Immunol. 2002;14:147–55. doi: 10.1093/intimm/14.2.147. [DOI] [PubMed] [Google Scholar]
- 35.Sugita S, Kohno T, Yamamoto K, et al. Induction of macrophage-inflammatory protein-3alpha gene expression by TNF-dependent NF-kappaB activation. J Immunol. 2002;168:5621–8. doi: 10.4049/jimmunol.168.11.5621. [DOI] [PubMed] [Google Scholar]
- 36.Rumbo M, Sierro F, Debard N, et al. Lymphotoxin beta receptor signaling induces the chemokine CCL20 in intestinal epithelium. Gastroenterology. 2004;127:213–23. doi: 10.1053/j.gastro.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Kao CY, Huang F, Chen Y, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–85. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 38.Inoue T, Kimura T, Azuma C, et al. Structural organization of the human oxytocin receptor gene. J Biol Chem. 1994;269:32451–6. [PubMed] [Google Scholar]
- 39.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–26. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 40.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 41.Bukowski R, Hankins GD, Saade GR, et al. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med. 2006;3:e169. doi: 10.1371/journal.pmed.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bethin KE, Nagai Y, Sladek R, et al. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol. 2003;17:1454–69. doi: 10.1210/me.2003-0007. [DOI] [PubMed] [Google Scholar]
- 43.Bollopragada S, Youssef R, Jordan F, et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(104):e1–11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Terzidou V, Sooranna SR, Kim LU, et al. Mechanical stretch up-regulates the human oxytocin receptor in primary human uterine myocytes. J Clin Endocrinol Metab. 2005;90:237–46. doi: 10.1210/jc.2004-0277. [DOI] [PubMed] [Google Scholar]
- 45.Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398–407. doi: 10.1210/endo.138.12.5624. [DOI] [PubMed] [Google Scholar]
- 46.Holmlund U, Wahamaa H, Bachmayer N, et al. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology. 2007;122:430–7. doi: 10.1111/j.1365-2567.2007.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43:152–9. doi: 10.1111/j.8755-8920.2000.430304.x. [DOI] [PubMed] [Google Scholar]
- 48.Liang Z, Sooranna SR, Engineer N, et al. Prostaglandin F2-alpha receptor regulation in human uterine myocytes. Mol Hum Reprod. 2008;14:215–23. doi: 10.1093/molehr/gan008. [DOI] [PubMed] [Google Scholar]
- 49.Tribe RM, Moriarty P, Dalrymple A, et al. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol Reprod. 2003;68:1842–9. doi: 10.1095/biolreprod.102.011403. [DOI] [PubMed] [Google Scholar]
- 50.Oger S, Mehats C, Dallot E, et al. Interleukin-1beta induces phosphodiesterase 4B2 expression in human myometrial cells through a prostaglandin E2- and cyclic adenosine 3′,5′-monophosphate-dependent pathway. J Clin Endocrinol Metab. 2002;87:5524–31. doi: 10.1210/jc.2002-020575. [DOI] [PubMed] [Google Scholar]
- 51.Andrews WW, Hauth JC, Goldenberg RL, et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 52.Romero R, Avila C, Santhanam U, et al. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mittal P, Romero R, Kusanovic JP, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60:246–57. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–47. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colobran R, Pujol-Borrell R, Armengol MP, et al. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin Exp Immunol. 2007;148:208–17. doi: 10.1111/j.1365-2249.2007.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pathways found to have significant associationswith the genes regulated by activation of NF-κB


