Abstract
The occurrence of neutrophils at the pannus-cartilage border is an important phenomenon for understanding the pathogenesis of rheumatoid arthritis (RA). Matrix metalloproteinases (MMPs) are predominant enzymes responsible for the cartilage degradation. The present article studied the expression of CD147 on neutrophils and its potential role in neutrophil chemotaxis, MMPs production and the invasiveness of fibroblast-like synoviocytes (FLS). The results of flow cytometry revealed that the mean fluorescence intensity of CD147 expression on neutrophils of peripheral blood from RA patients was higher than that in healthy individual. The potential role of CD147 in cyclophilin A (CyPA)-mediated cell migration was studied using chemotaxis assay and it was found that the addition of anti-CD147 antibody significantly decreased the chemotactic index of the neutrophils. Significantly elevated release and activation of MMPs were seen in the co-culture of neutrophil and FLS compared with cultures of the cells alone. An increased number of cells invading through the filters in the invasion assays were also observed in the co-cultured cells. The addition of anti-CD147 antibody had some inhibitory effect, not only on MMP production but also on cell invasion in the co-culture model. Our study demonstrates that the increased expression of CD147 on neutrophils in RA may be responsible for CyPA-mediated neutrophil migration into the joints, elevated MMPs secretion and cell invasion of synoviocytes, all of which may contribute to the cartilage invasion and bone destruction of RA. Better knowledge of these findings will hopefully provide a new insight into the pathogenesis of RA.
Keywords: rheumatoid arthritis, neutrophils, CD147
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by pronounced synovial hyperplasia and bone destruction [1]. Articular cartilage to which synovium tissue can directly attach is progressively degraded even at the disease’s early stage. Accumulating evidence suggests that the fibroblast-like synovial cell (FLS, also called synovial fibroblasts) is not only space filling but also cartilage destruction causing by producing a great amount of matrix metalloproteinases (MMPs) [2, 3].
Although various cell types are present in the rheumatoid joint, neutrophils are the cells most abundant in number within RA synovial fluids and their activation products may serve as biomarkers of the disease activity [4]. As the disease worsens, the number of neutrophils in synovial fluid is strongly correlated with the severity of disease [5]. One suggested reason may be that the activated neutrophils and the synovial fibroblasts are both important sources of MMPs, which have been found involved in the process of cartilage and sub-chondral bone degradation [6].
CD147, a 57-KD transmembrane glycosylated immunoglobulin, also called extracellular matrix metalloproteinase inducer (EMM PRIN), has been proven to stimulate the stromal cells to produce several MMPs [7]. In our previous study, we found that CD147, rich on the surface of RA synovium cells, is involved in promoting the secretion and activation of MMPs, which, in turn increases the invasive potential of FLS [8, 9]. However, the exact mechanism underlying the up-regulated expression of MMPs during the cell–cell interaction remains unclear yet. This study reported here, using an all-trans retinoic acid (ATRA)-induced cell differentiation model of HL-60 (a human neutrophil lineage), was designed to explore the possible functions of CD147 in the pathogenesis of RA by investigating the expression of CD147 on FLS, HL-60 cells, and neutrophils from peripheral blood and synovial fluid in RA.
Patients and methods
Patients
Samples of peripheral blood and synovial fluid were obtained from 10 patients with active RA and joint synovium specimens were obtained from 6 RA patients and 5 patients with osteoarthritis (OA) undergoing joint replacement surgery or synovectomy at Xijing Hospital. All the RA patients met the 1987 revised diagnostic criteria of the American College of Rheumatology [10]. The patients with active RA from whom samples were obtained had received no treatment or were treated only with nonsteroidal anti-inflammatory drugs. Their mean erythrocyte sedimentation rate was 58 ± 16 mm/hr and the C-reactive protein was 35 ± 13 mg/l. Synovium tissues used as the control were obtained from five patients who met the clinical and radiographic criteria for OA. The normal control samples of peripheral blood were taken from 10 healthy human donor volunteers, with no significant age or sex differences compared with those of the patients. Ethics approval was granted for this study and all the individuals provided their informed consent.
Cells isolation and culture
FLS were isolated by enzymatic dispersion of synovial tissues. Briefly, with connective tissues and fat removed, tissues were digested with 2% collagenase II (Sigma, St. Louis, MO, USA) in serum-free DMEM for 2 hrs. The cell suspension was passed through a nylon mesh, and then collected by centrifugation and re-suspended in DMEM supplemented with 10% foetal bovine serum (FBS) (Gibco, Grand Island, NY, USA). The harvested cells were cultured with DMEM supplemented with 10% FBS containing 1% penicillin/streptomycin and 2% L-glutamine. When the cells had grown to confluence they were detached with 0.25% trypsin, split in a 1:3 ratio and were re-cultured in DMEM under the same conditions. To eliminate the non-adherent cells, the plated cells were washed thoroughly with PBS. Isolated synoviocytes were cultured in DMEM supplemented with 10% FBS. The cells used for experiments were at the third to fifth passage, because these cells were more purified than the first and second passages of FLS and had better biological functions than the cells above the fifth passage. The FLS were identified by morphologic study and flow cytometric analysis (phenotype: <1% CD14, <1% CD68 and >98% CD90) as a homogeneous population [11].
HL-60 cells, a human neutrophil lineage, were cultured in RPMI 1640 medium containing 10% FBS, 1% penicillin/streptomycin and 2% L-glutamine. For the induction of cell differentiation, cells were induced in RPMI 1640 serum medium with ATRA 1 μmol/l for 5 days. HL-60 cells were assayed for differentiation using the nitroblue tetrazolium reduction test, morphological analysis and cell surface differentiation markers (CD11b, CD66b) expression. Cell viability was determined by the trypan blue exclusion assay [12]. Neutrophils from heparinized human peripheral blood or synovial fluid were isolated by the gradient centrifugation method [13]. Neutrophils were co-cultured with FLS at a cell number ratio of 1:1 for 24 hrs.
Flow cytometry analysis
Cells were treated with fluorescein isothiocyanate-conjugated anti-CD147 monoclonal antibody IgG1 (1:500, BD Pharmingen, San Diego, CA, USA) in the experimental group and fluorescein isothiocyanate-conjugated Mouse IgG1 (1:500, BD Pharmingen) in the isotype control for 20 min. in dark conditions. Cells were analysed by a FACS Calibur flow cytometry and the positive cell count and the mean fluorescence intensity (MFI) of CD147 on the surface of the cells were both determined. The data were processed using Cell Quest software (BD Biosciences, San Jose, CA, USA).
Quantitative real-time PCR assay
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The concentration and purity of RNA were determined by absorbance at 260/280 nm and cDNA was synthesized using PrimeScript RT reagent Kit (TaKaRa, Shiga, Japan). Primers for CD147, MMP-1, -2, -3 and -9 (Table 1) were developed, validated and used for PCR analysis. To control variation in mRNA concentration, all results were normalized to the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GADPH). Quantitative real-time PCR was performed with the Mx3000P and Mx3005P QPCR Systems (Agilent Technologies, Santa Clara, CA, USA) and the SYBR Green (an asymmetrical cyanine dye used as a nucleic acid stain) Ex Taq (TaKaRa). PCR amplification specificity was ensured by examining the melting curve for non-specific peaks. MxPro QPCR System Software was used to analyse relative quantification.
Table 1.
Primer sequences used in the quantitative real-time PCR experiments
| Gene ID | Primer sequences (5′-3′) | Base pairs (bp) |
|---|---|---|
| CD147 (NM_001728.2) | Forward: GACTGGGCCTGGTACAAGATCAC | 128 |
| Reverse: GCCTCCATGTTCAGGTTCTCAA | ||
| MMP-1 (NM_001145938.1) | Forward: CTGGCCACAACTGCCAAATG | 103 |
| Reverse: CTGTCCCTGAACAGCCCAGTACTTA | ||
| MMP-2 (NM_001127891.1) | Forward: ATGACATCAAGGGCATTCAGGAG | 135 |
| Reverse: TCTGAGCGATGCCATCAAATACA | ||
| MMP-3 (NM_002422.3) | Forward: GGGTGAGGACACCAGCATGA | 178 |
| Reverse: CAGAGTGTCGGAGTCCAGCTT | ||
| MMP-9 (NM_004994.2) | Forward: CCCTTCTACGGCCACTACTGT | 75 |
| Reverse: GCGATGGCGTCGAAGATGTT | ||
| GAPDH (NM_002046.3) | Forward: GCACCGTCAAGGCTGAGAAC | 138 |
| Reverse: TGGTGAAGACGCCAGTGGA | ||
Chemotaxis assay
The chemotaxis of cyclophilin A (CyPA) was assessed in a 48-well modified Boyden chamber with the two compartments separated by a polyvinylpyrrolidone-free polycarbonate filter (pore size 5 μm, Neuro Probe, Gaithersburg, MD, USA). The neutrophils in RPMI-1640 supplemented with 2% bovine serum albumin were added to the compartment above the filter, chemoattractants and negative controls diluted in the same medium were put below the filters. The chambers were incubated at 37°C and 5% CO2 for 2 hrs before the filters were recovered, fixed and stained with Crystal Violet reagent (Sigma). The number of cells appearing on the lower face of the filter was recorded in four high-power fields for each well, and each experimental condition was assayed in triplicate wells. N-Formyl-Met-Leu-Phe (100 nmol/l) was used as a positive control. CyPA (100 ng/ml), cyclosporine A (CsA, 10 mmol/l) and CyPA Ab (20 μg/ml) were added separately to investigate their chemotactic effects. Anti-CD147 antibody (HAb18, 80 μg/ml), which was produced and characterized by our laboratory based on CD147/HAb18G, was added to the upper cells to investigate their blockage effects [14]. The irrelevant anti-Japanese encephalitis virus mAb (anti-JEV mAb, 80 μg/ml) provided by the Department of Microbiology, the Fourth Military Medical University (Xi’an, China) was used as a negative control antibody (nc-Ab).
Gelatine zymography
Serum-free conditioned media samples were mixed with SDS sample buffer and loaded onto a 10% polyacrylamide gel containing 0.1% gelatine. After electrophoresis, gels were washed in 2.5% Triton X-100 for 30 min. and incubated for 16 hrs in reaction buffer. The gels were subsequently stained with 0.5% Coomassie blue (R-250) and destained to visualize the zones of digestion as light areas against the darkly stained background. Gels were then scanned and analysed using GeneSnap from SynGene Tools. HAb18 and nc-Ab were added in advance. The same method was used to detect the secretion and activation of MMPs of FLS co-cultured with human neutrophils from peripheral blood of healthy individuals (H PB Neu), peripheral blood of RA patients (RA PB Neu) or synovial fluid of RA patients (RA SF Neu). MMP inhibitors, ethylenediaminetetraacetic acid (EDTA) (5 mmol/l, Sigma) and 1, 10-phenanthroline (20 mmol/l, Sigma) were added separately to verify that the bands of proteolytic activity were MMPs [15].
Enzyme-linked immunosorbent assay (ELISA) of MMP-1 and MMP-3
Serum-free conditioned media samples were collected and centrifuged at 10,000 ×g for 5 min. to remove particulates. Quantities of MMP-1 and MMP-3 were determined using Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s protocol. Optical density was determined with Microplate reader (Model 3550, Bio-Rad, Hercules, CA, USA). A standard curve of each cytokine was established using known concentrations of cytokine by plotting optical density versus log of the concentration.
Invasion assay
Cell invasion assay was performed with Boyden Chamber (Costar, Cambridge, NY, USA) equipped with a polycarbonate filter (pore size 8 μm) coated with diluted matrigel (1:3 dilution in RPMI-1640; Becton-Dickinson, Franklin Lakes, NJ, USA). The cells were added into the chamber and cultured for 24 hrs. HAb18 and nc-Ab were added in advance. The cells remaining in the upper compartment were completely removed with gentle swabbing. The filter was fixed and stained with crystal violet reagent. The cells invading the lower surface of the filter in five microscopic fields were counted in each filter. Triplicate samples were conducted and the data were expressed as the average cell number of 15 fields.
Statistical analysis
The results presented were representative of a minimum of three experiments and were expressed as the mean ± S.D. Statistical analysis was conducted using Student’s two-tailed t-test for paired or unpaired data where appropriate, and multiple comparisons with a single control were performed with ANOVA with Dunnett-t modification. Graphpad software (Cricket Software, Philadelphia, PA, USA) was used for the above analyses and P < 0.05 was considered significant.
Results
Expression of CD147 on FLS and neutrophils
Table 2 shows that the percentage of positive staining cells of CD147 on RA FLS was higher (P < 0.05) than that on OA FLS and no marked difference was observed in MFI, the quantity of CD147 expression on the surface of RA FLS and OA FLS. The MFI of CD147 on ATRA-induced differentiated HL-60 cells (d HL-60) was higher (P < 0.05) than that on the undifferentiated HL-60 cells (u HL-60). The MFI of CD147 on RA SF Neu was higher (P < 0.05) than that on the RA PB Neu, and the MFI of CD147 on RA PB Neu was higher (P < 0.05) than that on H PB Neu.
Table 2.
Expressions of CD147 on FLS and neutrophils
| Percentage of positive staining cells (%) | MFI | Number | |
|---|---|---|---|
| OA FLS | 87.97 ± 0.38 | 120.73 ± 15.34 | 5 |
| RA FLS | 98.25 ± 0.18* | 191.63 ± 13.84 | 6 |
| u HL-60 | 99.10 ± 0.25 | 134.36 ± 15.83 | 6 |
| d HL-60 | 98.79 ± 0.78 | 270.04 ± 32.15† | 6 |
| H PB Neu | 98.23 ± 0.16 | 89.01 ± 14.38 | 10 |
| RA PB Neu | 99.82 ± 0.54 | 179.42 ± 10.84‡ | 10 |
| RA SF Neu | 99.05 ± 0.63 | 182.07 ± 10.67# | 10 |
Values presented as the mean ± standard error (n= 4–6 independent samples per group). Expressions of CD147 on FLS and Neus were measured by flow cytometry. Two parameters were used: the percentage of positive staining cells and the MFI. *P < 0.05 versus OA FLS, †P < 0.05 versus u HL-60, ‡P < 0.05 versus H PB Neu and #P < 0.05 versus RA PB Neu.
OA: osteoarthritis; RA: rheumatoid arthritis; u HL-60: undifferentiated HL-60; d HL-60: differentiated HL-60; H PB Neu: healthy peripheral blood neutrophils; RA PB Neu: neutrophils from peripheral blood of RA; RA SF Neu: neutrophils from synovial fluid of RA.
Expression of CD147, MMP-1, -2, -3 and -9 mRNA in FLS and HL-60
The expressions of CD147, MMP-1, -2, -3 and -9 mRNA were higher (P < 0.05) in RA FLS than those in OA FLS. The expressions of CD147, MMP-1, -2, -3 and -9 mRNA in RA FLS increased (P < 0.05) after co-culture with HL-60 cells (Fig. 1A). The expressions of CD147, MMP-1, -2, -3 and -9 mRNA in HL-60 cells were enhanced (P < 0.05) after HL-60 cells were differentiated by ATRA stimulation (Fig. 1B).
Fig 1.
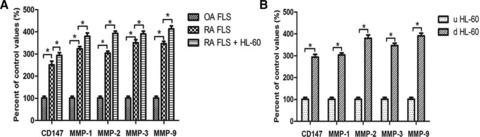
Expression of CD147, MMP-1, -2, -3 and -9 mRNA in FLS and HL-60 cells. (A) Expression of CD147, MMP-1, -2, -3 and -9 mRNA in RA FLS were higher than OA FLS; after co-culture with HL-60 cells (a human neutrophil lineage) they were higher than RA FLS alone. (B) Expression of CD147, MMP-1, -2, -3 and -9 mRNA of d HL-60 cells were higher than u HL-60 cells. In all these instances, mRNA expression was normalized to GAPDH values. *P < 0.05.
Chemoattraction of CyPA for neutrophils and its blockage by anti-CD147 antibody
Based on the reports that CD147 is a high-affinity receptor for CyPA and is responsible for a cyclophilin signalling cascade that culminates in extracellular signal-regulated kinase (ERK) activation and chemotaxis; we examined the chemoattraction of CyPA for neutrophils and the blockage effect of HAb18 on this. The CyPA chemotactic index for d HL-60 cells was higher than that for u HL-60 cells, indicating that CyPA had some significant chemotactic effect on HL-60 cells (P < 0.05). The CyPA chemotactic indexes decreased significantly in HAb18 groups compared with those in nc-Ab groups (P < 0.001) (Fig. 2). The blockage of CyPA chemoattraction for neutrophils by HAb18 was more obvious than that by CsA or CyPA Ab (P < 0.05).
Fig 2.
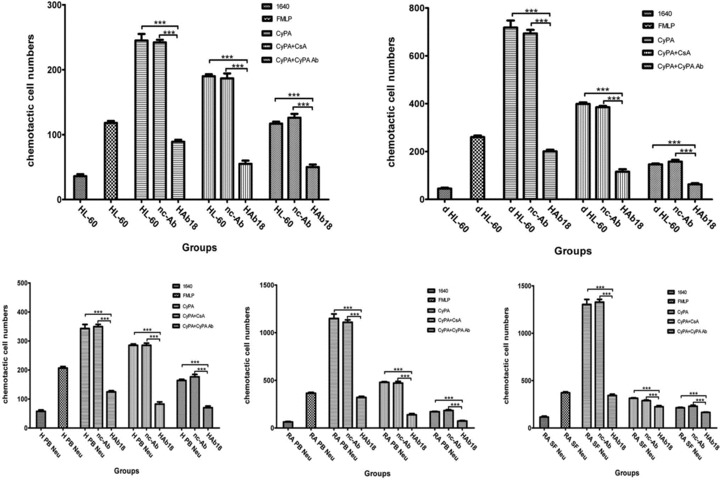
Effects of HAb18 on the chemoattraction of CyPA for neutrophils. FMLP: N-Formyl-Met-Leu-Phe; CyPA: Cyclophilin A; CsA: Cyclosporine A; nc-Ab: negative control antibody. ***P < 0.001.
MMPs secretion and activation in co-culture of RA FLS and HL-60
Gelatine zymography and ELISA showed that the secretion and activation of MMP-1, -2, -3 and -9 of RA FLS were higher (P < 0.05) than those of OA FLS. The increases were observed in the release of MMPs in d HL-60 compared with that in u HL-60 (P < 0.05). The secretion and activation of MMPs increased in the co-culture of RA FLS and d HL-60 compared with those in the co-culture of RA FLS and u HL-60 (P < 0.01). The values of MMPs activity of RA FLS co-cultured with u HL-60 or d HL-60 were higher (P < 0.05) than the values of co-cultured cells treated by HAb18, but no inhibitory effects were found in nc-Ab (Fig. 3).
Fig 3.
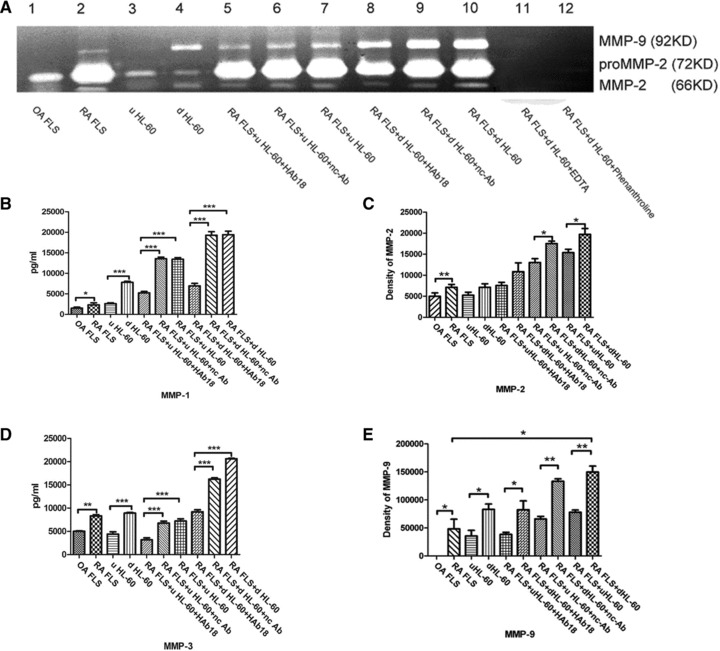
Secretion and activation of MMPs in FLS, HL-60, and co-cultured cells with or without treatment by HAb18 or nc-Ab, and EDTA or phenanthroline. (A) Shown are the results of gelatine zymography. Top band, MMP-9 gelatinase; lower two bands, MMP-2 gelatinase. (B) The quantification of MMP-1 levels by ELISA as described in the ‘Patients and methods’ section. (C) The value of MMP-2 activity by gelatine zymography. (D) The quantification of MMP-3 levels by ELISA as described in the ‘Patients and methods’ section. (E) The value of MMP-9 activity by gelatine zymography. The values of MMPs activity of RA FLS were higher than that of OA FLS. The values of MMPs activity of d HL-60 were higher than that of u HL-60 cells. The values of MMPs activity of RA FLS co-cultured with HL-60 were higher than that of co-cultured cells treated by HAb18, whereas no inhibitory effects were found in nc-Ab. *P < 0.05, **P < 0.01, ***P < 0.001.
MMPs secretion and activation in co-culture of RA FLS and human neutrophils
To further confirm the effect of cell–cell interactions in rheumatic joints, neutrophils from peripheral blood or synovial fluid of RA patients and peripheral blood of healthy individuals were co-cultured with RA FLS. The environments of co-cultured RA FLS and neutrophils from RA patients are more like the real environment in vivo. Gelatine zymography and ELISA showed that MMP-1, -2, -3 and -9 secretion and activation increased (P < 0.05) in both the co-culture group of RA FLS with RA PB Neus and the co-culture group of RA FLS with H PB Neus, compared with culture group of RA FLS alone. Significant increases were observed in the release of MMPs in the co-culture of RA FLS and RA PB Neus compared with that of H PB Neus (P < 0.05). The values of MMPs activity of RA FLS co-cultured with RA PB Neus or H PB Neus were higher (P < 0.05) than the values of co-cultured cells treated by HAb18, whereas no inhibitory effects were found in nc-Ab (Fig. 4). Figure 5 shows that the values of MMPs activity of RA SF Neus were higher (P < 0.05) than the values of RA PB Neus. MMPs secretion and activation in the co-culture of RA FLS with RA SF Neus were all higher than those in the culture of RA FLS alone or in the co-culture with RA PB Neus (P < 0.05). HAb18 was found to have inhibitory effects on MMPs release and activation of co-cultured RA FLS and neutrophils. However, no inhibitory effects were found in nc-Ab.
Fig 4.
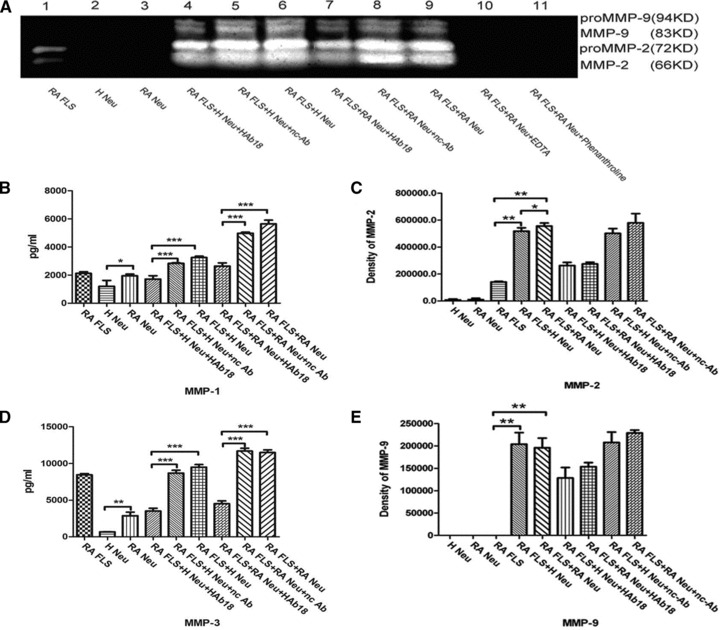
Secretion and activation of MMPs in RA FLS, neutrophils from peripheral blood of a healthy individual (H Neu) or RA patients (RA Neu), and co-cultured cells with or without treatment by HAb18 or nc-Ab, and EDTA or phenanthroline. (A) Shown are the results of gelatine zymography. Top two bands, MMP-9 gelatinase; lower two bands, MMP-2 gelatinase. (B) The quantification of MMP-1 levels by ELISA as described in the ‘Patients and methods’ section. (C) The value of MMP-2 activity by gelatine zymography. (D) The quantification of MMP-3 levels by ELISA as described in the ‘Patients and methods’ section. (E) The value of MMP-9 activity by gelatine zymography. The values of MMPs activity of RA PB Neus were higher than that of H PB Neus; The release of MMPs were all increased in the co-culture group of RA FLS and RA PB Neus when compared with that of RA FLS alone or the co-culture group with H PB Neus. The values of MMPs activity were decreased after treated by HAb18, whereas no inhibitory effects were found in nc-Ab. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig 5.
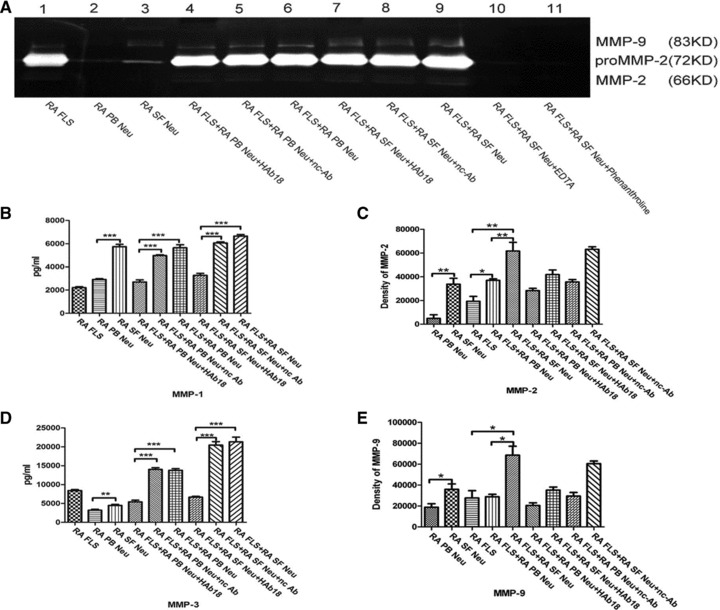
Secretion and activation of MMPs in RA FLS, neutrophils from peripheral blood of RA patients (RA PB Neu) or RA synovial fluid (RA SF Neu), and co-cultured cells with or without treatment by HAb18 or nc-Ab, and EDTA or phenanthroline. (A) Shown are the results of gelatine zymography. Top band, MMP-9 gelatinase; lower two bands, MMP-2 gelatinase. (B) The quantification of MMP-1 levels by ELISA as described in the ‘Patients and methods’ section. (C) The value of MMP-2 activity by gelatine zymography. (D) The quantification of MMP-3 levels by ELISA as described in the ‘Patients and methods’ section. (E) The value of MMP-9 activity by gelatine zymography. The values of MMPs activity of RA SF Neus were higher than that of RA PB Neus; The release of MMPs were all increased in the co-culture group of RA FLS and RA SF Neus when compared with that of the RA FLS alone or the co-culture group of RA FLS and RA PB Neus. The values of MMPs activity were decreased after treated by HAb18, whereas no inhibitory effects were found in nc-Ab. *P < 0.05, **P < 0.01, ***P < 0.001.
Invasive ability in co-culture of RA FLS and neutrophils
In order to testify whether the elevated expression of MMPs can enhance the invasive potential of FLS, cell invasion assay was conducted. A higher number of RA FLS (228.67 ± 6.62 cells/filter) were found to have invaded through transwell chambers compared with that of OA FLS (164.27 ± 4.28 cells/filter) (P < 0.05, Fig. 6A) and a higher number of RA FLS co-cultured with d HL-60 (765.31 ± 15.04 cells/filter) were found to have invaded compared with that of RA FLS co-cultured with u HL-60 (586.03 ± 22.08 cells/filter) (P < 0.05, Fig. 6A), both of which were higher than the number in the culture of RA FLS alone. RA FLS co-cultured with RA PB Neus were found to have a higher number invading through the transwell chambers (510.21 ± 10.03 cells/filter) compared with that of RA FLS co-cultured with H PB Neus (366.29 ± 13.80 cells/filter) (P < 0.05, Fig. 6B). RA FLS co-cultured with RA SF Neus were found to have a higher number invading through the transwell chambers (672.86 ± 19.91 cells/filter) compared with that of RA FLS co-cultured with RA PB Neus (P < 0.05, Fig. 6C). The invasion block assay showed that the number of cells decreased after the treatment with HAb18 in RA FLS co-cultured with HL-60 cells. The inhibitory rates of the invasive potential in RA FLS co-cultured with u HL-60 cells and in RA FLS co-cultured with d HL-60 were 32.84% and 38.23%, respectively (P < 0.05). The inhibitory rates of the invasive potential in RA FLS co-cultured with H PB Neus and in RA FLS co-cultured with RA PB Neus or with RA SF Neus were 37.24%, 41.06% and 45.37%, respectively (P < 0.05), whereas no inhibitory effects were found in nc-Ab.
Fig 6.
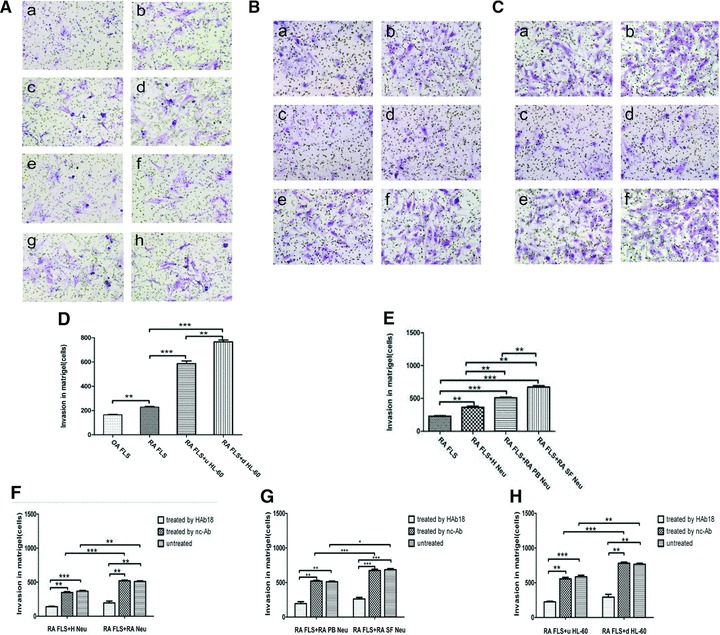
The invasive potential of FLS or in co-culture with neutrophils (original magnification ×200). (A) The invasive potential of FLS or in co-culture with HL-60 with or without the treatment of HAb18 or nc-Ab. (a) OA FLS; (b) RA FLS; (c) RA + HL-60; (d) RA+d HL-60; (e) RA + HL-60 + HAb18; (f) RA+d HL-60 + HAb18; (g) RA + HL-60 + nc-Ab and (h) RA+d HL-60 + nc-Ab. (B) The invasive potential in co-culture of RA FLS and H PB Neu or RA PB Neu with or without the treatment of HAb18 or nc-Ab. (a) RA + H Neu; (b) RA + RA Neu; (c) RA + H Neu + HAb18; (d) RA + RA Neu + HAb18; (e) RA + H Neu + nc-Ab and (f) RA + RA Neu + nc-Ab. (C) The invasive potential in co-culture of RA FLS and RA PB Neu or RA SF Neu with or without the treatment of HAb18 or nc-Ab. (a) RA + RA PB Neu; (b) RA + RA SF Neu; (c) RA + RA PB Neu + HAb18; (d) RA + RA SF Neu + HAb18; (e) RA + RA PB Neu + nc-Ab and (f) RA + RA SF Neu + nc-Ab. (D) The invasive potential of RA FLS was higher than OA FLS; the invasive potential of RA FLS co-cultured with d HL-60 was higher than that with u HL-60, and both of them were higher than that of RA FLS alone. (E) The invasive potential of RA FLS co-cultured with RA SF Neus, RA PB Ners, H PB Neus and RA FLS alone were increased one by one. (F) The invasive potential of RA FLS co-cultured with RA PB Neus was higher than that of co-cultured with H PB Neus, and the invasive potential of co-cultured cells inhibited by HAb18. (G) The invasive potential of RA FLS co-cultured with RA SF Neus was higher than that of co-cultured with RA PB Neus, and the invasive potential of co-cultured cells inhibited by HAb18. (H) The invasive potential of co-cultured cells inhibited by HAb18, whereas no inhibitory effects were found in nc-Ab. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we found that expression of CD147 on differentiated HL-60 cells were higher than that on u HL-60 cells. Gelatine zymography, ELISA and invasion assay all showed that the values of MMPs were significantly enhanced when HL-60 cells were stimulated into a maturation stage. The higher expression levels of CD147 observed in the differentiated process may explain the increased MMPs production. MMPs are known as a family of Zn2+-containing enzymes that cleave most of the components of extracellular matrix and are involved in cartilage invasion and bone destruction. Therefore, imbalanced activity between MMPs and tissue inhibitors of MMPs caused by enhanced expression of CD147 may eventually lead to RA joint destruction.
In this study, we had RA FLS co-cultured with HL-60 cells to explore the effect of cell–cell interaction on the production of MMPs and the invasiveness of RA synoviocytes. Gelatine zymography and ELISA both showed that the co-culture of HL-60 and RA FLS resulted in higher levels of MMPs than the culture of HL-60 or FLS alone. Cell invasion assay confirmed these results, which partly prove that CD147-up-regulated neutrophils may act as an amplifier of the pathogenetic cascade by interacting with FLS. The overexpression of CD147 on FLS and neutrophils, especially on neutrophils, suggests that CD147 may be important in both autocrine and paracrine stimulation of MMPs. On the basis of the findings that homophilic CD147-binding may occur in the context of both heterotypic and homotypic cell–cell interactions, which may play a key role in MMPs production and tumour cell invasion [16], we presume that the increased expression of CD147 on neutrophils in RA could possibly induce MMPs production through interaction with the surrounding FLS.
In this study, we also had neutrophils from peripheral blood or synovial fluid of RA co-cultured with RA FLS to further confirm the effect of neutrophil-FLS interactions in rheumatic joints as the environments of co-cultured FLS and neutrophils from RA are more like the real environments in vivo compared with those of co-cultured RA FLS and HL-60. The results from the co-culture of neutrophils from peripheral blood or synovial fluid of RA co-cultured with RA FLS were consistent with the results from the co-culture of RA FLS with HL-60, indicating that the overexpression of CD147 in vivo induces elevated levels of MMPs, which in turn increase the invasive potential of FLS.
We found that the addition of anti-CD147 monoclonal antibody has some inhibitory effects not only on MMPs production but also on the invasive potential of the co-cultured FLS, suggesting that the inhibition of CD147 can decrease the MMPs production and that CD147 may induce MMPs production. Though the mechanism by which CD147 enhances MMPs secretion and the invasion ability of FLS is largely unclear, Pushkarsky and colleagues have reported that CD147 is a high-affinity receptor for CyPA and is responsible for a cyclophilin signalling cascade in HIV-1 infection [17]. Some study from our lab also indicates that cyclophilin-CD147 interaction might contribute to the bone destruction in RA [18].
Beyond the proof that CD147-cyclophilin interactions might contribute to the pathogenesis of RA by promoting the recruitment of leucocytes into joint tissues [19], the results of this study have manifested that CyPA does have a chemotactic effect on neutrophils. Moreover, we have also found that this chemotaxis can be significantly suppressed by anti-CD147 antibody. These findings suggest that CyPA does interact with CD147 and it is highly possible that CyPA may act as a ligand of CD147 to induce the accumulation of inflammatory cells which highly express CD147. In RA synovial tissues, various cells, including macrophages and endothelial cells, are the sources of CyPA. When CyPA is released into the peripheral blood or the synovial fluid, abundant CyPA may be required to mediate the effect of CD147 on the inflammatory response by direct binding of CyPA to CD147. Our previous in vivo studies have also shown that anti-CD147 monoclonal antibody reduces cartilage erosion and synovitis by the inhibition of MMPs and reduction of inflammatory cytokines in severe combined immunodeficiency (SCID)-HuRAg mice [20], so the inhibition of CD147 may be a promising target for novel therapeutic strategies in RA. Better knowledge of these findings such as using a knockdown approach will hopefully provide a new insight into the pathogenesis of RA.
Acknowledgments
The authors thank G. C. Ge for his critical reading of the manuscript. This work was supported by grants from the Key Program of the National Natural Science Foundation of China (no. 30530720) and the National Basic Research Program (no. 2009CB521705).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Konttinen YT, Ainola M, Valleala H, et al. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999;58:691–7. doi: 10.1136/ard.58.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolboom TC, Pieterman E, van der Laan WH, et al. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 2002;61:975–80. doi: 10.1136/ard.61.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn R, Hellmark T, Leeb-Lundberg LM, et al. Neutrophil-derived proteinase 3 induces kallikrein-independent release of a novel vasoactive kinin. J Immunol. 2009;182:7906–15. doi: 10.4049/jimmunol.0803624. [DOI] [PubMed] [Google Scholar]
- 5.Chignard M, Selak MA, Smith JB. Direct evidence for the existence of a neutrophil-derived platelet activator (neutrophilin) Proc Natl Acad Sci USA. 1986;83:8609–13. doi: 10.1073/pnas.83.22.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore AR, Iwamura H, Larbre JP, et al. Cartilage degradation by polymorphonuclear leucocytes: in vitro assessment of the pathogenic mechanisms. Ann Rheum Dis. 1993;52:27–31. doi: 10.1136/ard.52.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konttinen YT, Li TF, Mandelin J, et al. Increased expression of extracellular matrix metalloproteinase inducer in rheumatoid synovium. Arthritis Rheum. 2000;43:275–80. doi: 10.1002/1529-0131(200002)43:2<275::AID-ANR6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Zhu P, Ding J, Zhou J, et al. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023–33. doi: 10.1186/ar1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu P, Lu N, Shi ZG, et al. CD147 overexpression on synoviocytes in rheumatoid arthritis enhances matrix metalloproteinase production and invasiveness of synoviocytes. Arthritis Res Ther. 2006;8:R44–55. doi: 10.1186/ar1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Lories RJ, Derese I, Ceuppens JL, et al. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 2003;48:2807–18. doi: 10.1002/art.11389. [DOI] [PubMed] [Google Scholar]
- 12.Atzeni F, Schena M, Ongari AM, et al. Induction of CD69 activation molecule on human neutrophils by GM-CSF, IFN-gamma, and IFN-alpha. Cell Immunol. 2002;220:20–9. doi: 10.1016/s0008-8749(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 13.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest. 1968;97:77–89. [PubMed] [Google Scholar]
- 14.Jiang JL, Zhou Q, Yu MK, et al. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem. 2001;276:46870–7. doi: 10.1074/jbc.M108291200. [DOI] [PubMed] [Google Scholar]
- 15.Stix B, Kahne T, Sletten K, et al. Proteolysis of AA amyloid fibril proteins by matrix metalloproteinases-1, -2, and -3. Am J Pathol. 2001;159:561–70. doi: 10.1016/S0002-9440(10)61727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/ extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–81. [PubMed] [Google Scholar]
- 17.Pushkarsky T, Zybarth G, Dubrovsky L, et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci USA. 2001;98:6360–5. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Lu N, Zhou J, et al. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatol. 2008;47:1299–310. doi: 10.1093/rheumatology/ken225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damsker JM, Okwumabua I, Pushkarsky T, et al. Targeting the chemotactic function of CD147 reduces collagen-induced arthritis. Immunology. 2009;126:55–62. doi: 10.1111/j.1365-2567.2008.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia J, Wang C, Shi Z, et al. Inhibitory effect of CD147/HAb18 monoclonal antibody on cartilage erosion and synovitis in the SCID mouse model for rheumatoid arthritis. Rheumatol. 2009;48:721–6. doi: 10.1093/rheumatology/kep099. [DOI] [PubMed] [Google Scholar]


