Abstract
The major stress-inducible heat shock protein 70 (Hsp70) is frequently present on the cell surface of human tumours, but not on normal cells. Herein, the binding characteristics of the cmHsp70.1 mouse monoclonal antibody (mAb) were evaluated in vitro and in a syngeneic tumour mouse model. More than 50% of the CT26 mouse colon carcinoma cells express Hsp70 on their cell surface at 4°C. After a temperature shift to 37°C, the cmHsp70.1-fluorescein isothiocyanate mAb translocates into early endosomes and lysosomes. Intraoperative and near-infrared fluorescence imaging revealed an enrichment of Cy5.5-conjugated mAb cmHsp70.1, but not an identically labelled IgG1 isotype-matched control, in i.p. and s.c. located CT26 tumours, as soon as 30 min. after i.v. injection into the tail vein. Due to the rapid turnover rate of membrane-bound Hsp70, the fluorescence-labelled cmHsp70.1 mAb became endocytosed and accumulated in the tumour, reaching a maximum after 24 hrs and remained detectable at least up to 96 hrs after a single i.v. injection. The tumour-selective internalization of mAb cmHsp70.1 at the physiological temperature of 37°C might enable a targeted uptake of toxins or radionuclides into Hsp70 membrane-positive tumours. The anti-tumoral activity of the cmHsp70.1 mAb is further supported by its capacity to mediate antibody-dependent cytotoxicity.
Keywords: membrane-bound Hsp70, Hsp70 antibody, tumour mouse model, flat panel volume CT, near-infrared fluorescence imaging
Introduction
Our search for innovative tumour-specific target structures for tumour therapy revealed heat shock protein 70 (Hsp70–1, HspA1A #3303) [1], the major stress-inducible member of the 70 kD heat shock proteins, as one such target. Both, Hsp70 and gp96, an endoplasmic reticulum resident member of the 90 kD heat shock protein group (gp96, HspC3 #7184), have been found on the plasma membrane of a variety of different human tumours [2–4]. During these studies we generated and characterized a mouse monoclonal antibody (mAb), termed cmHsp70.1, which specifically detects the cell surface localized Hsp70 on viable tumour cells with intact plasma membrane. The amino acid sequence of the Hsp70 molecule, which is exposed to the extracellular milieu of these tumours has been identified as being part of the 14-mer peptide TKDNNLLGRFELSG (TKD) [5, 6].
Screening of tumour biopsies and the corresponding normal tissues has indicated that primary diagnosed carcinoma samples, but none of the tested normal tissues, frequently exhibit an Hsp70 membrane-positive phenotype [7–9]. Moreover, an Hsp70 membrane-positive tumour phenotype has been associated with a significantly decreased overall survival in patients with lung cancer and lower rectal carcinomas suggesting that Hsp70 membrane-positivity might serve as a negative prognostic marker [10]. It has also been shown that the density of membrane Hsp70 on tumour cells can be further enhanced following therapeutic intervention such as radiotherapy or chemotherapy [11].
The anchorage of Hsp70 protein in the plasma membrane of non-stressed tumours is enabled by the glycosphingolipid globoyltriaoslyceramide (Gb3) [12, 13], which is frequently overexpressed in colorectal and gastric tumours and rarely found in the plasma membrane of normal cells. Following stress, elevated levels of Hsp70 are co-located with phosphatidylserine on the cell surface of tumour cells [14–16]. Moreover, an Hsp70 membrane-positive phenotype is associated with a higher resistance towards radiochemotherapy and membrane Hsp70 expression might therefore predict an unfavourable therapeutic outcome in lung and lower rectal tumours [17]. Taken together, these findings indicate the importance of determining the Hsp70 membrane status of tumours.
Within the last few years, non-invasive devices for the imaging of tumours in small animals have been developed [18]. Intraoperative and near-infrared fluorescence (NIRF) analyses are innovative approaches for tracking fluorophor-labelled probes, such as antibodies, in mice. Herein, we used a syngeneic tumour mouse model to study the distribution and binding characteristics of the cmHsp70.1 mAb in vivo. Screening of several mouse tumour cell lines revealed an Hsp70 membrane-positive phenotype on CT26 (mouse colon carcinoma cell line) colon carcinoma, ADJ (mouse plasmocytoma cell line) plasmocytoma, B16/F10 melanoma and MOS162 (mouse osteosarcoma cell line) mouse osteosarcoma cells at 4°C. Following a temperature shift to 37°C, the cmHsp70.1 mAb was rapidly taken up into early endosomes and lysosomes of CT26 tumour cells in vitro. Following i.v. injection of fluorescence-conjugated cmHsp70.1 mAb into the tail vein of CT26 tumour-bearing mice, the cmHsp70.1 mAb selectively and rapidly accumulates in endo-lysosomal compartments in vivo. In addition to its tumour imaging capacity, the cmHsp70.1 mAb can mediate cellular cytotoxicity (antibody-dependent cytotoxicity [ADCC]).
Materials and methods
Mouse tumour cell lines
The tumorigenic CT26 mouse colon adenocarcinoma (CT26.WT; ADCC CRL-2638) [19], 1048 mouse pancreatic carcinoma (kindly provided by Dieter Saur, Department of Medicine II, Technische Universität München, A20 B cell lymphoma [20], ADJ plasmocytoma cell lines, all derived from BALB/c mouse strains, the B16/F10 malignant mouse melanoma cells (C57/Bl6 mouse strain), and the MOS162 osteosarcoma cell line (C3H mouse strain; kindly provided by Dr. Michael Rosemann, HMGU Munich, Germany) were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% v/v heat-inactivated foetal calf serum, 2 mM L-glutamine, 1 mM sodium-pyruvate and antibiotics (100 IU/ml penicillin, 100 μg/ml streptomycin), at 37°C in 5% CO2. Cell lines were maintained in the exponential growth phase by regular cell passages twice a week and sub-cultivation at a ratio of 1:5 (seeding of 1 × 106 cells in 5 ml fresh culture medium). Doubling time of the cell lines was approximately 20 hrs. Single cell suspensions were derived by short-term (less than 1 min.) 0.25% (w/v) Trypsin 0.53 mM ethylenediaminetetraacetic acid treatment. All cell culture reagents were purchased from Life Technologies (Rockville, CA, USA).
Flow cytometry
The Hsp70 membrane phenotype in mouse tumour cell lines was determined by flow cytometry using the fluorescein isothiocyanate (FITC)-conjugated cmHsp70.1 mAb (IgG1; multimmune, Munich, Germany). For the CT26 mouse colon carcinoma cells, the Hsp70 status was routinely determined before their injection into the mice, and on single cell suspensions of random tumour samples after explanation on day 14. Briefly, after incubation of viable cells (0.2 × 106 cells) with the appropriate antibody for 30 min. at 4°C and following two washing steps, 7-AAD–, viable cells were analysed on a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany). An IgG1 isotype-matched control antibody was used to determine non-specific staining of the cell lines. The proportion of positively stained cells was defined as the difference of the number of cells stained with the relevant antibody minus the number of cells stained with the appropriate isotype-matched control immunoglobulin.
Fluorescence microscopy and kinetics of uptake of mAb cmHsp70.1-FITC
Microscopic immunofluorescence studies were performed with CT26 tumour cells which were cultured in 8-well chamber slides (Nunc, Rochester, NY, USA) at a cell density of 20,000 cells/well. After two washing steps in phosphate-buffered saline (PBS), viable cells were incubated for 30 min. with cmHsp70.1-FITC mAb either at 4°C or at 37°C. After fixation and permeabilization the cells were incubated with antibodies directed against Rab4, Rab5a, Rab7, Rab9, Rab11 (all obtained from Santa Cruz Biotechnology, Heidelberg, Germany), lysosomal associated membrane proteins (LAMP)1, LAMP2 (kindly provided by Prof. Stefan Höning, University of Cologne, Germany) for 1 hr and with the appropriate Cy3-conjugated secondary antibodies (anti-rabbit-Cy3 and anti-goat-Cy3, Jackson ImmunoResearch, West Grove, PA, USA) for 30 min. Cells were then washed twice in PBS and mounted in Vectashield containing 4,6-Diamidine-2-phenylindole (DAPI) solution (Vector Laboratories, Burlingame, CA, USA). The slides were analysed on a Zeiss Axioscop 2 plus scanning microscope (Zeiss, Jena, Germany) equipped with a ×100 oil-immersion objective and standard filters. Photographs of representative cells are shown; the localization and co-localization of Hsp70 and early (Rab4, Rab5a), late (Rab7, Rab11), trans golgi network, recycling endosomal (Rab11) and lysosomal (LAMP1, LAMP2) markers were visualized in green (FITC), red (Cy3) and yellow (merge) spectra.
The uptake of cmHsp70.1-FITC mAb and the identically labelled IgG1-FITC control antibody into tumour cells was measured by flow cytometry. For this, the tumour cells were incubated with the mAb for 2, 5, 10, 15, 30 and 60 min. either at 4°C or at 37°C. After two washing steps, viable cells were gated and analysed, as described above.
Biacore analysis
Kinetic measurements were performed with a Biacore X instrument (Biacore AB, Uppsala, Sweden) at 25°C with a CM5 chip (GE) and 25 mM HEPES, 150 mM KCl, 5 mM MgCl2 pH 7.6 as running buffer and at a flow rate of 10 μl/min.
For this assay, 70 μg/ml cmHsp70.1 mAb diluted in 20 mM acetate (pH 4.8) was covalently coupled to a CM5 chip surface with primary amine groups using a standard amine coupling method, which yielded in about 1700 RU. The second channel subjected to the same activation and deactivation treatments, but without the antibody was used as control. Solutions of human Hsp70 (0.78 – 50 nM) were prepared in running buffer and tested for binding.
To determine the binding constants, association (Kon) and dissociation (Koff) phase data from each concentration were globally fitted to a simple 1:1 interaction model (A + B = AB) using the BIAevalution software 4.1.
Animals
Female and male BALB/c mice were obtained from an animal breeding colony (Harlan Winkelmann, Borchen, Germany) and maintained in pathogen-free, individually ventilated cages (Tecniplast, Hohenpeissenberg, Germany). Animals were fed with sterilized, laboratory rodent diet (Meika, Großaitingen, Germany) and were used for experiments between 6 and 12 weeks of age. All animal experiments were approved by the ‘Regierung von Oberbayern’ (55.2–1-54–2531-30–07; 55.2–1-54–2531-52–07) and were performed in accordance with institutional guidelines.
Intraperitoneal (i.p.) and subcutaneous (s.c.) injection of tumour cells
CT26 tumour cells were thawed from a common frozen stock and cultured in vitro for 2 to 3 days before use. BALB/c mice were injected into the peritoneum (i.p.) or s.c. with 100 μl of the CT26 stock solution containing 2.5 × 104 cells, using a 1000 μl plastic syringe with a 22-gauge needle. Injection was visually controlled using a 7× Stereomicroscope (Zeiss, Göttingen, Germany) and tumour weights of single tumours were determined on days 4, 6, 8, 10, 12, 14, 19 and 21 after injection. From day 23 onwards, mice died from progressive tumour growth.
Injection of the antibodies
For intraoperative and NIRF imaging 100 μg cmHsp70.1 mAb or IgG1 isotype-matched control antibody [clone electron microscopy (EM)21, directed against O6-ethly-2-deoxyguanosine] conjugated to Cy5.5-NHS (Squarix GmbH, Marl, Germany) at dye to molar ratios of 0.74 and 1.02, respectively, were injected i.v. into tumour-bearing mice on day 14. As an alternative, both antibodies were labelled with FITC at identical fluorescence intensities, as determined on the multilabel Reader Victor X4 (Perkin Elmer, Rodgau-Jügesheim, Germany).
Intraoperative fluorescence imaging
For intraoperative imaging, mice were killed 30 min., 2, 4 and 8 hrs after i.v. injection of either cmHsp70.1 mAb or control IgG1 (100 μg per injection) labelled with Cy5.5. The fluorescence imaging measurements used a back illuminated EM-charge-coupled device (CCD) camera (iXon DV887, Andor, Belfast, Northern Ireland). Light from the tissue was collected using a variable zoom objective lens (NT58–240, Edmund Optics, Barrington, NJ, USA). Light collected by the objective was filtered using a 710/10 nm band pass filter. A 670 nm CW diode laser (B&W Tek, Newark, DE, USA) with maximum power 300 mW was used for the excitation. The laser light beam was guided through a multimode fibre (200 μm core/ 0.22 NA) to a collimator and a diffuser (F260SMA-b, ED1-S20, Thorlabs, Newton, NJ, USA) for beam expansion and uniform illumination. A 24-bit colour CCD camera (PCO AG, Donaupark, Kelheim, Germany) coupled with the same objective lens was used to obtain colour images of the measured tissue. A 250 W halogen lamp (KL-2500 LCD, Edmund Optics) was used for white light illumination.
Immunofluorescence studies of tissue sections
On day 14, CT26 tumour-bearing mice were injected i.v. either with Cy5.5- or FITC-labelled cmHsp70.1 mAb or with identically labelled IgG1 control immunoglobulin (100 μg) into the tail vein. Mice were killed 3, 8, 24 and 72 hrs after injection of the antibodies and tumours and organs such as liver, lung, kidney, heart and spleen of the mice were collected, cut in four equal pieces and cryo-conserved. Consecutive sections of the tumours and organs (5–10 μm) were prepared using a Leica Cryostat (Leica CM1950, Leica Microsystems GmbH, Wetzlar, Germany) from the ventral margin of each piece for a distance of 250 μm. After fixation in formalin (10%) and counterstaining with Hoechst 33342, to visualize the nuclei, the sections were mounted with an antifade solution (Vectashield mounting media H-1000, Vector Laboratories). Sections were analysed on an upright epifluorescence microscope (Zeiss Axio Imager.Z1, Carl Zeiss MicroImaging GmbH, Jena, Germany) equipped with a C-Apochromat 40×/1.2 W Korr UV-VIS-IR objective and an AxioCam MRm camera. The visualization of the distribution of the fluorescence signals was performed with the AxioVision software (AxioVS40 V 4.8.1.9, Zeiss). Nuclei were visualized in blue (DAPI) and Hsp70 was visualized either in green (FITC) or in red (Cy5.5).
Flat-panel volume CT (VCT)
A flat-panel VCT (Fig. 4A), a non-clinical CT prototype equipped with two flat-panel detectors (GE Global Research, Niskayuna, NY, USA) [21] was used for the CT analyses. Briefly, anaesthetized mice were placed on a multimodality bed throughout the imaging session and injected i.v. with 150 μl of iodine-containing contrast agent Isovist 300 approximately 30 sec. before starting of the scan. All data sets were acquired with a step-and-shoot technique, using 1000 views per 1 full rotation, 8 sec. of rotation time per step, 360 used detector rows, 80 kVp, and 100 mA. For high-resolution image reconstructions a modified Feldkamp algorithm implemented on a simultaneous computer with eight nodes was used.
Fig 4.
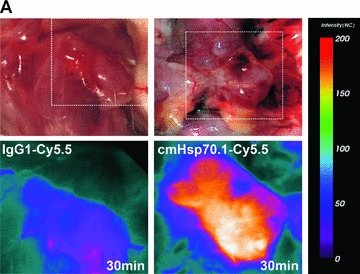
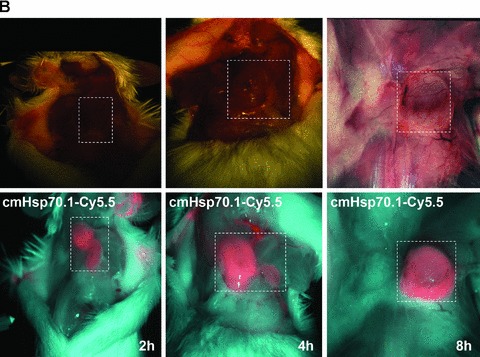
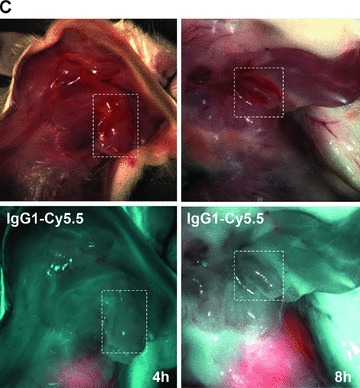
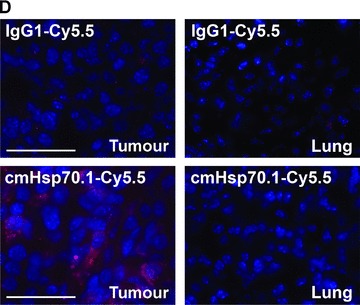
(A) Optical imaging. Intraoperative detection of cmHsp70.1-Cy5.5 mAb in tumour-bearing BALB/c mice. 100 μg of cmHsp70.1-Cy5.5 mAb as well as the IgG1-Cy5.5 control were injected i.v. into the tail vein of CT26 tumour-bearing mice on day 14. Representative views of the Cy5.5 fluorescence and autopsy images of the dorsal part of the mice were taken 30 min. after i.v. injection of the antibodies. Upper panel, autopsy image of the dorsal located mouse tumour in true colours. Lower panel, false colour images of the Cy5.5 staining within the tumour. The massive fluorescence signal corresponds to the anatomic position of the CT26 tumour which is stained with cmHsp70.1-Cy5.5 mAb. Almost no staining with an equivalently labelled IgG1 isotype-matched control is detectable. (B) Kinetics of the intraoperative detection of cmHsp70.1-Cy5.5 mAb in CT26 tumour-bearing mice on day 14. Representative views of the Cy5.5 fluorescence images of the dorsal part of the mice were taken 2, 4 and 8 hrs after i.v. injection of the antibody. Upper panel, autopsy images of the dorsal located mouse tumour in true colours. Lower panel, false colour images of the Cy5.5 staining within the tumour indicated in red. (C) Kinetics of the intraoperative detection of IgG1-Cy5.5 control immunoglobulin in CT26 tumour-bearing mice on day 14. Representative views of the Cy5.5 fluorescence images of the ventral part of the mice were taken 4 and 8 hrs after i.v. injection of the antibody. Upper panel, autopsy images of the ventral located mouse tumour in true colours. Lower panel, false colour images of the Cy5.5 staining indicated in red. No staining was detectable within the tumour following the administration of the IgG1-Cy5.5 isotype-matched control. (D) Immunofluorescence studies of tumour and normal tissue (lung) sections (10 μm) derived from the same animals, 8 hrs after i.v. injection of IgG1-Cy5.5 (upper panel) or cmHsp70.1-Cy5.5 mAb (lower panel). The nuclei are visualized in blue (DAPI) and the localization of Hsp70 is visualized in red (Cy5.5). The scale bar represents 50 μm.
Near-infrared fluorescence imaging
The Optix system (Advanced Research Technologies, Montreal, Canada) is a 2D imaging system that works in a reflection scheme. The output of the system consists of maps of intensity and lifetime of the fluorescence distribution, in relation to a previously acquired camera image of the animal. Lifetime analysis describes the mean residence time of the fluorophor in an excited state and provides a characteristic parameter for the fluorescent probe. The mean transit time of an emitted photon following an excitation pulse can be used to calculate the depth and concentration of the fluorescence intensity by time-resolved measurements [18]. The fluorescence intensity was determined in anaesthetized, viable mice that developed s.c. tumours before (0 hr), as well as 24, 48, 72 and 96 hrs after i.v. injection of the Cy5.5-labelled antibodies into the tail vein.
Antibody dependent cellular cytotoxicity assay
ADCC was measured using a standard 4-hr 51Cr-release assay. Briefly, viable CT26 mouse colon carcinoma cells were labelled with 0.1 μCi of Na51CrO4 at 37°C for 1 hr. After two washes with RPMI 1640 medium, 51Cr-labelled target cells (1 × 104) were transferred into triplicate wells of a 96-well plate and the cmHsp70.1 (IgG1) mAb was added (0.7, 1, 1.4 μg/ml). Freshly isolated mouse (BALB/c) spleen cells were added at various effector to target cell ratios (E:T). After a 4-hr co-incubation period supernatants (100 μl) were harvested and the levels of radioactive 51Cr was counted using a gamma counter (Coulter-Counter, Billerica, MA, USA). Percentage of ADCC was calculated using the formula: %specific lysis = (experimental release – spontaneous release) / (maximum release – spontaneous release) × 100. The spontaneous release in each target cell ranged between 10% and 15%.
Statistics
Comparative analysis of the in vitro data was undertaken using the t-test for the analysis of two paired and unpaired samples. A significance level of α= 0.05 was used.
Results
Binding of mAb cmHsp70.1 mAb to cell surface-bound Hsp70 on mouse tumour cell lines in vitro
Screening of different mouse tumour cell lines with the IgG1 anti-human Hsp70-specific mAb cmHsp70.1, which detects the cell surface localized form of Hsp70 on human tumours, revealed that this antibody also recognizes membrane Hsp70 on mouse tumour cell lines [22]. An Hsp70 membrane-positive phenotype was determined in mouse colon carcinoma (CT26), plasmocytoma (ADJ), malignant melanoma (B16/F10) and MOS162 osteosarcoma cells (C3H) derived from different mouse strains (Table 1). In contrast, the mouse pancreatic carcinoma (1048) and the A20 B cell lymphoma cell line are considered as being membrane Hsp70– (Table 1). A representative flow cytometric image of the mouse CT26 colon carcinoma cell line is illustrated in Figure 1A. Cytosolic Hsp70 staining was excluded, as the entire staining procedure was performed at 4°C, and only 7-AAD–, viable tumour cells with intact plasma cell membranes were analysed. Figure 1B illustrates the different binding patterns of the cmHsp70.1-FITC mAb to CT26 tumour cells at 4°C (left panel) and at 37°C (right panel). At 4°C, the binding of cmHsp70.1-FITC mAb to CT26 tumour cells reveals a typical ring-shaped cell surface staining pattern (Fig. 1B, left panel). The dotted staining pattern reflects the localization of membrane-bound Hsp70 in lipid rafts [23, 24]. Measurements, using fluorescence-conjugated marker beads revealed that approximately 10,000 Hsp70 molecules are present on the plasma membrane of the mouse tumour cell line CT26 at 4°C (data not shown).
Table 1.
Proportion of Hsp70 membrane positive cells in different malignant mouse tumour cell lines. Hsp70 membrane positivity on mouse tumour cell lines was determined by flow cytometry using the cmHsp70.1-FITC mAb at 4°C. In line with previous reports [6–8] a sample was considered as Hsp70 membrane positive when more than 15% of the cells were positively stained with the cmHsp70.1-FITC mAb. The data represent the mean of at least six independent experiments ± S.E.
| Mouse tumour cells (mouse strain) | Origin | Hsp70 membrane-positive cells (%) |
|---|---|---|
| CT26 (BALB/c) | Colon | 44 ± 5.2 |
| 1048 (BALB/c) | Pancreas | 12 ± 10.3 |
| A20 (BALB/c) | B cell | 5 ± 4.3 |
| ADJ (BALB/c) | Plasmocytoma | 45 ± 5.4 |
| B16/F10 (C57/Bl6) | Melanoma | 97 ± 6.2 |
| MOS162 (C3H) | Osteosarcoma | 70 ± 3.6 |
Fig 1.
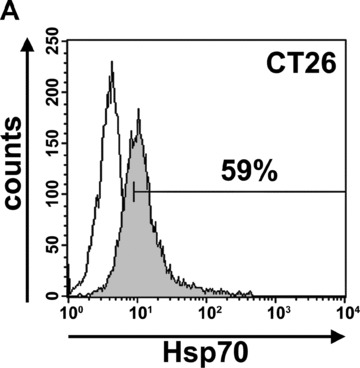
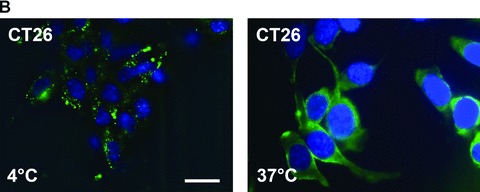
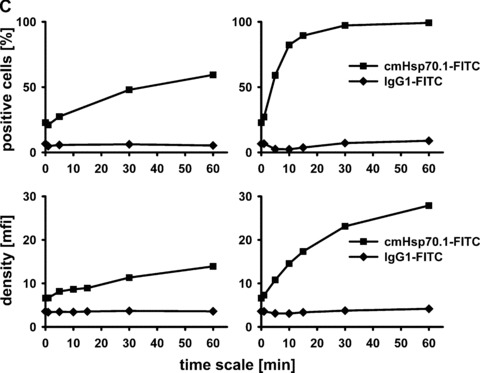
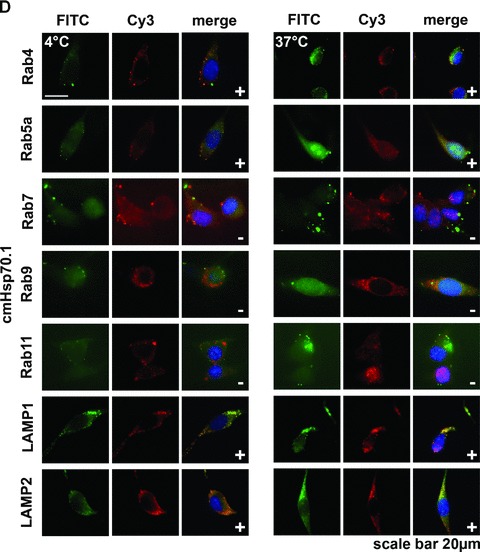
(A) Flow cytometric analysis. Representative view of the Hsp70 cell surface expression on CT26 mouse colon carcinoma cells after flow cytometric analysis using the cmHsp70.1-FITC conjugated mAb. Results are expressed as log green fluorescence intensity versus relative cell numbers. The IgG1 isotype-matched control is indicated in white and membrane Hsp70+ population is shown in grey. Only viable (propidium-iodide negative) cells were gated and analysed. (B) Representative immunofluorescence images of CT26 tumour cells stained with cmHsp70.1-FITC mAb at 4°C (cell surface staining; left), or following a temperature shift to 37°C for 15 min. (cytosolic staining; right). The scale bar represents 20 μm. (C) Representative views of the kinetics of cmHsp70.1-FITC mAb uptake into CT26 tumour cells. Tumour cells were washed and analysed by flow cytometry following incubation with cmHsp70.1-FITC mAb or IgG1-FITC for 2, 5, 10, 15, 30 and 60 min. cells at 4°C (left panel) and 37°C (right panel). The upper graphs indicate the percentage of positively stained cells the lower graphs indicate the antigen densities at the indicated time-points, expressed as the mfi. The increase in the proportion of membrane Hsp70+ cells and in the mfi was significant (P < 0.05) at 37°C but not at 4°C. (D) Representative immunofluorescence images of CT26 tumour cells either stained with cmHsp70.1-FITC (green, first row) or with Cy3-secondary antibody labelled (red, second row) Rab4 (early endosome), Rab5a (early endosome), Rab7 (late endosome), Rab9 (late endosome), Rab11 (trans golgi network, recycling endosome), LAMP1 (CD107, lysosome), LAMP2 (lysosome) antibodies at 4°C (left three rows) and after an incubation of 30 min. at 37°C (right three rows). A co-localization of the FITC (green) and Cy3 (red) fluorescence, as indicated in a yellow spectrum (third row), is marked with ‘+’ in the merged fluorescence staining pattern. Isotype-matched control antibodies did not show any staining (data not shown). Similar results were obtained in three independent experiments. The scale bar represents 20 μm.
A shift to 37°C resulted in a strong intracellular staining pattern, which likely results from the translocation of the FITC-conjugated antibody cmHsp70.1-FITC into the cytosol (Fig. 1B, right panel). Kinetic studies show a significant increase in the proportion of Hsp70 membrane-positive cells at 37°C between 2 and 15 min. after incubation with cmHsp70.1-FITC mAb, but not with an isotype-matched control antibody (Fig. 1C, upper right panel). In contrast, at 4°C the proportion of Hsp70 membrane-positive tumour cells remained nearly unchanged up to 60 min. (Fig. 1C, upper left panel). Furthermore, the mean fluorescence intensity (mfi) of Hsp70 per cell significantly increased after incubation with antibody at 37°C from 2 to 60 min. (Fig. 1C, lower right panel), but remained stably low at 4°C (Fig. 1C, lower left panel). These data indicate that the cmHsp70.1-FITC staining accumulates in Hsp70 membrane-positive tumour cells at the physiological temperature of 37°C, but not at 4°C. The reason for the antibody uptake is due to a rapid turnover rate of membrane-bound Hsp70. We could show that membrane Hsp70 expression is completely restored already after 15 min. after its removal by enzymatic digestion (data not shown). As expected, tumour cells with an initially low Hsp70 membrane expression level, such as 1048 pancreatic carcinoma cells and A20 B cell lymphoma cells, showed little cell surface staining at 4°C, nor did they internalize the cmHsp70.1-FITC mAb at 37°C (data not shown). In order to identify the endo-lysosomal compartment in which Hsp70 accumulates following endocytosis at 37°C a co-staining of Hsp70 (cmHsp70.1-FITC) with Cy3 secondary antibody labelled early (Rab4; Rab5a), late (Rab7, Rab9), recycling (Rab11) endosomal and lysosomal (LAMP1, LAMP2) markers was performed. As visualized in Figure 1D, co-localization of Hsp70 was predominantly found with Rab4, Rab5a, LAMP1 and LAMP2 at 4°C and at 37°C (yellow dots in the merged photographs, marked with a ‘+’). In summary, the data derived from three independent experiments (data not shown) indicate that Hsp70 predominantly accumulates in the early endosomal compartment and becomes degraded in lysosomes.
The concentration-dependent affinity of the full length cmHsp70.1 mAb to immobilized human Hsp70 was determined using a Biacore X system. The sensogram profiles of Figure 2 were globally fitted to a 1:1 binding model with the BIAevaluation software. The calculated Kon value was 6.99 × 104/M/s and the Koff value was 3.79 × 10−4/s with a dissociation equilibrium constant KD of 5.4 nM for Hsp70 with a ×2 of 59.4.
Fig 2.
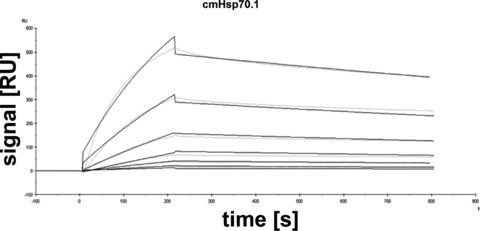
Global kinetic analysis of cmHsp70.1 mAb binding human Hsp70 using a Biacore. Purified human Hsp70 protein was diluted to final concentrations of 0.78, 1.6, 3.1, 6.3, 12.5, 25, 50 nM and injected onto a cmHsp70.1 mAb-coated gold surface. Relative response units were analysed using BIAevaluation software 4.1. Kinetic constants were Kon= 6.99 × 104/M/s, Koff= 3.79 × 10−4/s and a KD= 5.4 nM with a ×2= 59.4, respectively. Grey coloured lines contrast the measured data from the simulated fits (black).
In vivo tumour growth of intraperitoneal and subcutaneous transplanted CT26 cells
An i.p. injection of 2.5 × 104 CT26 mouse colon adenocarcinoma cells suspended in 100 μl PBS resulted in rapidly growing tumours (Fig. 3, black bars). The average weight of an individual tumour was 2.6 g ± 1.3 (n= 17) on day 19 and most animals became moribund shortly thereafter due to the large tumour weight in the abdomen. Tumour take at any tested time-point was always 100%. A comparative phenotyping of CT26 cells from tissue culture and from single cell suspensions derived from tumour-bearing mice on day 14 revealed that the amount of Hsp70 membrane-positive cells was found to be significantly elevated from 56.2 ± 9% (n= 6) up to 79.8 ± 14% (n= 7) in mouse-derived tumours (data not shown). Tumour growth was similar following s.c. injection of the same number of CT26 cells into the neck, although mice did not die until day 26 (data not shown).
Fig 3.
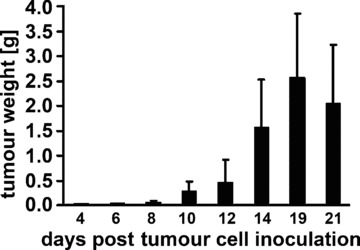
Tumour growth curve for CT26 colon adenocarcinoma cells in BALB/c mice. Following i.p. injection of tumour cells (2.5 × 104) mice were killed on days 4 (0.03 ± 0.11 g; n= 3), 6 (0.05 ± 0.2 g; n= 3), 8 (0.05 ± 0.03 g; n= 5), 10 (0.29 ± 0.2 g; n= 7), 12 (0.47 ± 0.44 g; n= 7), 14 (1.55 ± 0.9 g; n= 35), 19 (2.6 ± 1.3 g; n= 17), 21 (2.05 ± 1.18 g; n= 10) and tumour weights were determined.
Intraoperative in vivo imaging of Hsp70 in tumour-bearing mice
Fluorescence-labelled cmHsp70.1-Cy5.5 mAb or IgG1-Cy5.5 (100 μg each) were injected i.v. into the tail vein of tumour-bearing mice on day 14 after i.p. injection of CT26 cells (2.5 × 104), at which time the average tumour weight of 1.55 g ± 0.9 (n= 35, Fig. 3). In order to obtain a more detailed overview of the binding characteristics of the cmHsp70.1 mAb in vivo, an intraoperative technique was used for in vivo imaging. The upper part of Figure 4A illustrates true colour autopsy images of the CT26 tumours in mice injected either with the IgG1 isotype-matched control or the cmHsp70.1 mAb, both of which were conjugated with Cy5.5. The regions of the tumours are marked with a white dotted line. The fluorescence images of the IgG1-Cy5.5 control antibody and that of the identically labelled mAb cmHsp70.1-Cy5.5 are indicated below in false multispectral views. As indicated on the left panel, an orange spectrum represents a region of high antibody intensity, whereas a blue and green spectrum represents low antibody staining intensities. Localization of the cmHsp70.1-Cy5.5 (Fig. 4A, lower right panel), but not the IgG1-Cy5.5 isotype-matched control antibody (Fig. 4A, lower left panel), in the tumour is detectable at relatively high amounts, as early as 30 min. following i.v. injection of the antibodies in the tail vein. Kinetic studies indicated a progressive accumulation of the cmHsp70.1-Cy5.5 mAb (Fig. 4B, lower panel), but not the IgG1 isotype-matched control (Fig. 4C, lower panel), within the tumour between 2 and 8 hrs. In Figure 4B and C the Cy5.5 staining of both, cmHsp70.1-Cy5.5 mAb and isotype control is indicated in red and the antibody-free mouse tissues are represented in light blue colour spectra. It appeared that the cmHsp70.1 mAb, but not the IgG1 isotype matched control is predominantly located within the tumour. An overall inspection of different mouse organs and the tumour revealed that apart from the CT26 tumours no other mouse tissues were positively stained for the Cy5.5-labelled mAb cmHsp70.1 (data not shown). These data are confirmed by immunofluorescence studies of sections (10 μm) of the tumours (Fig. 4D, left panel) and normal tissues (lung; Fig. 4D, right panel) of the identical animals. An endo-lyso somal Cy5.5 staining pattern, as already shown for in vitro cultured CT26 tumour cells (Fig. 1D), is detectable only in the tumour sections by using the cmHsp70.1 mAb, but not in normal tissues (Fig. 4D, lower panel). The IgG1 control antibody did neither stain tumour nor normal tissues including liver, lung and heart. The lung is shown as a representative example for the normal tissue in the upper panel of Figure 4D. The cmHsp70.1-Cy5.5 mean intensity of cell area is 3.7-fold higher in the tumour compared to that in the lung tissue. In line with these findings are the results from immunofluorescence studies of sections (5 μm) derived from tumour-bearing mice that had received FITC-labelled cmHsp70.1 mAb or IgG1 immunoglobulin via the tail vein, the fluorescent intensities of which were identical, as determined by multicolour luminescence reader (data not shown). Representative images of sections of tumours (day 14 after i.p. injection of CT26 tumour cells, Fig. 5A) and normal tissues, such as the liver (Fig. 5B), lung (Fig. 5C) and kidney (Fig. 5D) of tumour-bearing mice, which had been injected either with cmHsp70.1-FITC or with IgG1-FITC, clearly demonstrate a time-dependent (from 3 to 72 hrs) and specific up-take of the Hsp70-specific antibody into the tumours. In contrast, the identically-labelled IgG1 isotype-matched control antibody was only found in the liver 3 hrs after i.v. injection. A weak staining of the liver was also detectable 3 hrs after i.v. injection of the cmHsp70.1 mAb, but this had completely disappeared after 24 hrs. Other normal tissues of the same mice, such as lung (Fig. 5C), kidney (Fig. 5D), heart (data not shown) and spleen (data not shown) did not show any fluorescence staining at the indicated time-points. In summary these data show that irrespectively of the fluorescence label (Cy5.5, Fig.4; FITC, Fig. 5; Alexa, data not shown), the cmHsp70.1 mAb binds to Hsp70 membrane-positive tumours in vivo in a highly selective manner. Due to the time-dependent concentration of the cmHsp70.1 mAb within the tumours of the mice, we suggest that in accordance with our in vitro findings (Fig. 1C) the Hsp70 mAb becomes rapidly internalized into the endo-lysosomal compartment also in vivo. A non-specific uptake of the fluorescence dye is unlikely since identical results have been obtained using Cy5.5 (Fig. 4), FITC (Fig. 5) and Alexa (data not shown) conjugated reagents.
Fig 5.
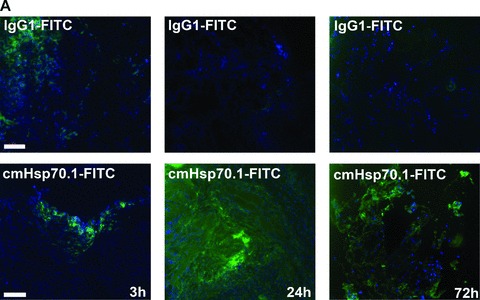
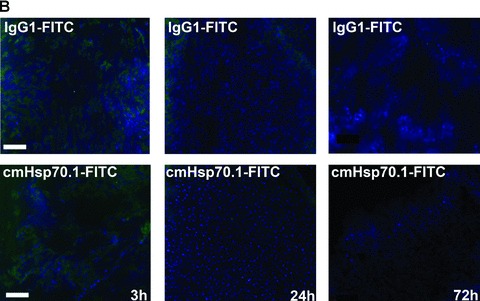
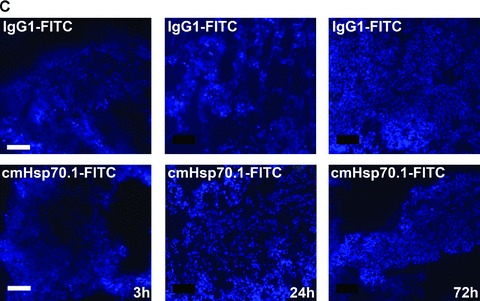
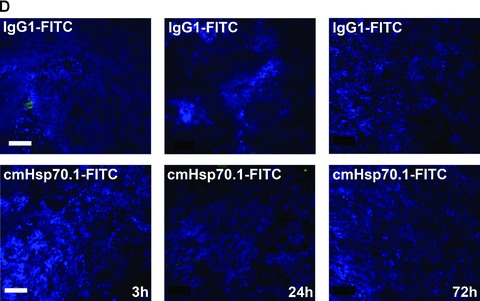
Immunofluorescence analysis of tumour and normal tissue sections. cmHsp70.1-FITC mAb (lower panel) or the identically labelled IgG1 control (upper panel, 100 μg each) was injected into the tail veins of tumour-bearing mice on day 14 after i.p. tumour cell (CT26) injection. Animals were killed 3, 24 and 72 hrs thereafter and the tumour (A), liver (B), lung (C) and kidney (D) were cryo-conserved. Representative views of sections (5 μm) of the tumours and organs were taken at the indicated time-points after the injection of the IgG1-FITC (upper panel) and cmHsp70.1-FITC mAb (lower panel). The nuclei are stained in blue (DAPI) and the localization of Hsp70 is visualized in green (FITC). The scale bar represents 100 μm.
Near-infrared fluorescence in vivo imaging of Hsp70 in tumour-bearing mice
The Optix system was used for the long-term analysis and for quantification of the fluorescence intensities in mice bearing s.c. tumours. Lifetime and fluorescence intensities were determined 0, 24, 48, 72 and 96 hrs after i.v. injection of the Cy5.5-conjugated antibodies into anaesthetized mice. The fluorescence probes were scanned by Optix in vitro in order to determine their specific fluorescent lifetimes prior to the in vivo experiments. Based on a single exponential fit to the decay of the curve, a fluorescent lifetime of 1.7 nsec. was calculated for the cmHsp70.1-Cy5.5 and the IgG1-Cy5.5.
The dye to antibody molar ratio was 0.74 for the cmHsp70.1 mAb and 1.02 for the IgG1 control. The upper panel of Figure 6A depicts fluorescence lifetimes of the scanned region in a tumour-bearing animal injected with cmHsp70.1-Cy5.5 mAb, and the lower graph in a tumour-bearing animal injected with IgG1-Cy5.5 at the indicated time-points, as determined by NIRF imaging. Lifetime images serve as specificity controls of the Cy5.5 staining and enable specific fluorescence to be distinguished from autofluorescence. The estimated fluorescence lifetime for all scanned points in both mice was approximately 1.7 nsec. This value is comparable to that of the Cy5.5 labelled antibodies measured in vitro.
Fig 6.
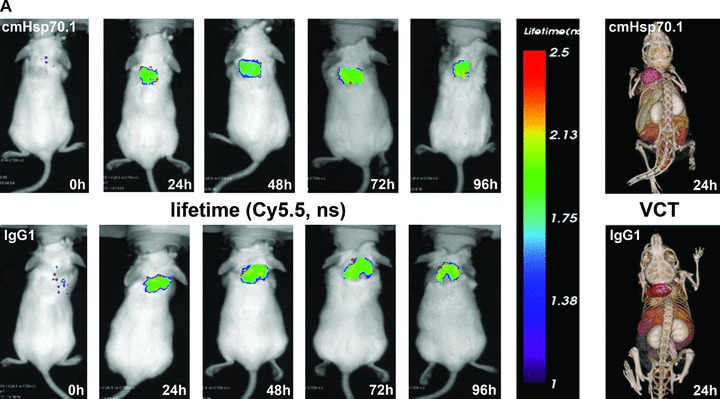
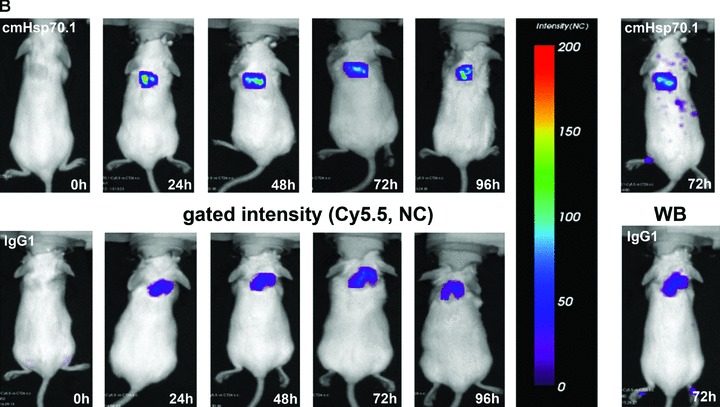
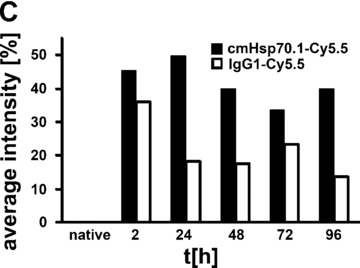
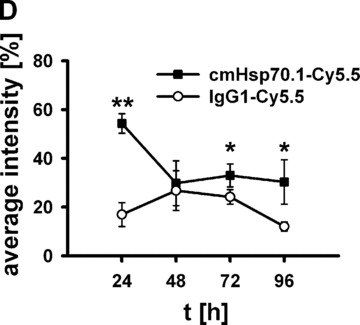
(A) Lifetime images and flat-panel VCT scans. Representative lifetime images were obtained using the Optix system. Representative fluorescence signals over the s.c. located CT26 tumour regions show lifetimes of 1.7 nsec., which are characteristic for Cy5.5-conjugated cmHsp70.1 mAb (upper panel) and the IgG1 (lower panel) isotype-matched control. Images were taken 0, 24, 48, 72 and 96 hrs after i.v. injection into the tail vein. The peak emission of Cy5.5 is at 694 nm in the bright green area. Localization of the CT26 colon adenocarcinoma in the dorsal neck region of the mice is depicted in the volume rendered flat-panel VCT scans which were taken 24 hrs after the injection of the cmHsp70.1 mAb and isotype-matched control on days 14, 15, 16 and 17 after the tumour cell injection. On day 14, the tumour size which was determined by flat-panel VCT, was 0.227 cm3 in mice injected with cmHsp70.1 mAb, and 0.211 cm3, in mice injected with the IgG1 control antibody. (B) Representative fluorescence intensity images obtained by the Optix system. Fluorescence intensity is displayed in normalized counts and is presented from two CT26 tumour-bearing mice 0, 24, 48, 72 and 96 hrs after i.v. injection of the cmHsp70.1-Cy5.5 mAb (upper panel) and an identically labelled IgG1 isotype-matched control (lower panel). Strong fluorescence signals (red outline) over the tumour of the mouse that had received the cmHsp70.1-Cy5.5 mAb, but not over the tumour in the animal that had been injected with the IgG1-Cy5.5 control were visible between 24 and 96 hrs. Whole body scans of the identical mice 72 hrs after injection of the mAb and isotype control are shown on the outer right part of the graph. Fluorescence signals were only apparent over the tumour region. (C) Quantitative analysis of the fluorescence intensity images of the tumours of mice that received either cmHsp70.1-Cy5.5 mAb (black bars) or IgG1-Cy5.5 (white bars). Average intensities of fluorescence signals in the s.c. tumour regions of the two mice shown in (B) at the indicated time-points 0, 24, 48, 72 and 96 hrs after i.v. injection of the antibodies are displayed. The data were corrected for their labelling intensities. (D) Kinetics of average fluorescence intensity of cmHsp70.1-Cy5.5 mAb (black dots) and IgG1-Cy5.5 control (white dots) in tumour-bearing mice. The data represent a summary of the average fluorescence intensity over tumour regions in mice at 24, 48, 72 and 96 hrs after i.v. injection of the antibodies. Data represent mean values of five animals; * marks values P < 0.05; ** marks values P < 0.001.
Flat-panel VCT images, which were taken 24 hrs after i.v. injection of the antibodies, revealed comparable volumes of both tumours. The tumour volume of the mouse which had been injected with cmHsp70.1 mAb was 0.227 cm3 and 0.211 cm3 for the IgG1 mAb-injected mouse. Furthermore, anatomical imaging by high-resolution 3D flat-panel VCT imaging demonstrates the localization of the tumours (Fig. 6A, right panel) in correlation to the Cy5.5 fluorescence signals (Fig. 6B).
Figure 6B represents a follow-up of the fluorescence intensity of the Cy5.5-labelled cmHsp70.1 mAb (upper panel) and IgG1 isotype-matched control (lower panel) 0, 24, 48, 72 and 96 hrs after i.v. injection of the probe. The whole body fluorescence intensity scans, which are taken 72 hrs after i.v. injection, demonstrate a selective binding of the Cy5.5 labelled cmHsp70.1 mAb to the tumour tissues (Fig. 6B, right panel). A quantitative analysis of the average fluorescence intensities in these two mice at the indicated time-points is summarized in Figure 6C. It appears that the average intensity of the cmHsp70.1 mAb was always stronger than that of the isotope-matched control at all time-points, with a maximum at 24 hrs. A summary of the average fluorescence intensities for both groups of treated animals (derived from five animals per time-point) confirmed these results and revealed significantly stronger fluorescence signals over the tumours of mice that received the cmHsp70.1 mAb in comparison to IgG1 isotype-matched control antibody at the time-points 24 (54.33 ± 3.9 versus 16.92 ± 4.9; P < 0.001), 72 (32.97 ± 4.7 versus 24.17 ± 3.0; P < 0.05) and 96 hrs (30.29 ± 9.1 versus 12.01 ± 1.9; P < 0.05) after i.v. injection (Fig. 6D).
Effects of cmHsp70.1 mAb on CT26 tumour cells in vitro
Although it is known that IgG1 mouse monoclonal antibodies in general have a low capacity to mediate ADCC, the cmHsp70.1 mAb was tested against Hsp70 membrane-positive CT26 tumour cells. As summarized in Figure 7, the cmHsp70.1 induces ADCC in CT26 at the very low concentration of 1.4 μg/ml. In contrast, lower concentrations did not affect the viability of CT26 tumour cells. Presently, experiments are ongoing which test the anti-tumoral effect of cmHsp70.1 mAb in a tumour mouse model.
Fig 7.
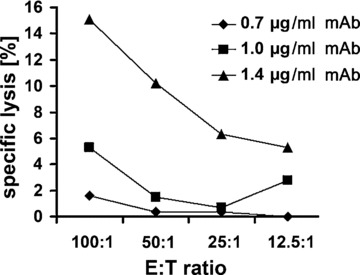
Capacity of cmHsp70.1 mAb to induce ADCC against CT26 tumour cells in vitro. In vitro ADCC of CT26 colon (containing 55% Hsp70 membrane-positive cells) carcinoma cells, using 0.7, 1 and 1.4 μg/ml cmHsp70.1 mAb and unstimulated mouse spleen cells at E:T ratios ranging from 100:1 to 12.5:1. The data show one representative experiment out of three independent experiments showing similar results.
Discussion
Tumours, but not the corresponding normal tissues, frequently present Hsp70 on their cell surface. Moreover, an Hsp70 membrane-positive phenotype was found to predict a decreased overall survival in tumour patients with an extrahepatic route of metastasis [10], and thus might act as a negative prognostic marker. In contrast, patients whose tumours metastasize into the liver have shown a better clinical outcome due to the presence of hepatic natural killer cells that may provide an immunological filter for membrane Hsp70 tumour cells [10]. These data indicate the medical need for the development of novel strategies to visualize and target highly aggressive, Hsp70 membrane-positive tumours.
Herein, the role of membrane Hsp70 as a potential tumour-specific target for in vivo imaging was evaluated. Our laboratory generated an IgG1 mouse anti-human Hsp70-specific mAb termed cmHsp70.1 which specifically detects the membrane-bound form of Hsp70 on viable human tumour cells with an intact plasma membrane [5, 6]. Human and mouse Hsp70 does not differ within the 8-mer region in the C-terminus, which is recognized by mAb cmHsp70.1 (NLLGRFEL). Therefore, it was assumed that the antibody shows cross-reactivity for Hsp70 in both species. The membrane Hsp70 phenotype was studied in several mouse tumour cell lines derived from different mouse strains. Among others, the mouse colon carcinoma cell line CT26 was found to be strongly membrane Hsp70+. Moreover, a temperature shift from 4°C to 37°C resulted in an internalization of the fluorescence-labelled cmHsp70.1 mAb. This result could be explained by a fast turnover rate of membrane-bound Hsp70 into the cytosol at physiological temperatures. The time-dependent and tumour-specific accumulation of the cmHsp70.1-FITC mAb in early endosomes and lysosomes further supports this hypothesis. In line with these findings it has been shown recently that Hsp70 associates with proteins such as MUC1 and caveolin 1 in lipid rafts of breast cancer cells [24]. These protein aggregates rapidly become endocytosed to re-enter the secretory pathway for recycling to the plasma membrane [25]. Co-staining of Hsp70 with the small GTPases Rab4 and Rab5a, which mark transport routes of proteins from the plasma membrane to early endosomes and back to the plasma membrane, also support this recycling pathway [26]. As expected, part of the intracellular located Hsp70 becomes degraded in lysosomes [26].
Given that the membrane Hsp70 positivity of CT26 tumours derived form mice autopsies was even greater than that of in vitro cultured CT26 cells, we addressed the question whether cmHsp70.1 mAb conjugated to different fluorophors also stains mouse tumours in vivo. Intraoperative and NIRF imaging techniques revealed a fast and highly specific binding of the Cy5.5-labelled cmHsp70.1 mAb to i.p. and s.c. localized tumours in living animals, as early as 30 min. after i.v. injection into the tail vein which lasts for at least 96 hrs. In contrast, an identically labelled IgG1 isotype-matched control antibody was found to be enriched in the liver at the identical time frame. A detailed macro- and microscopical inspection of tumour-free organs of the mice showed that the cmHsp70.1 mAb did not bind to any normal mouse tissues. A non-specific up-take of antibody-free fluorescence dye into the tumour is unlikely since different cmHsp70.1-fluorophor conjugates produced identical results.
The tumour-specific binding pattern of mAb cmHsp70.1 was further confirmed by NIRF imaging of s.c. located tumours. In the tumour, the fluorescence signals of an identically labelled IgG1 isotype-matched control was significantly lower than that of the cmHsp70.1 mAb. By comparing the 3D flat-panel VCT data to the 2D fluorescence maps [21], generated by NIRF imaging, we successfully matched fluorescence signals from Cy5.5-labelled cmHsp70.1 mAb to pathologic tumour structures. Co-registration of fluorescence signals obtained by Optix to flat-panel VCT data illustrating anatomical sites, as described by Dullin et al. [21], might be useful for kinetic measurements of i.p. and orthotopically localized tumours in living animals.
Since it has been shown that radiochemotherapy enhances the cell surface density of Hsp70 selectively in tumours but not in normal tissues [9, 15], the Hsp70-specific antibody might serve as a tool for measuring the therapeutic outcome. Moreover, metastases in general exhibit elevated Hsp70 levels on their cell membranes, compared to primary tumours (unpublished data), and therefore might become detectable earlier by the use of cmHsp70.1 mAb.
Despite the IgG1 isotype of the cmHsp70.1 mAb, its capacity to induce ADCC against membrane Hsp70 tumour cells has been shown. Due to the rapid and tumour-selective uptake of the Hsp70 antibody, which is most likely mediated via a high turnover rate of membrane Hsp70 [27–29], it is conceivable that the anti-tumoral activity of cmHsp70.1 mAb can be further enhanced when applied as an antibody-drug or -radionuclide conjugate.
Acknowledgments
This work was supported in part by the multimmune GmbH (Munich, Germany), by grants of the Deutsche Forschungsgemeinschaft (DFG, MU1238 7/2; SFB-824/1), the Bundesministerium für Bildung und Forschung (BMBF-MOBITUM, grant no. 01EZ0826) and the European Union (EU-STEMDIAGNOSTICS, LSHB CT 2007 037703; EU-CARDIORISK, FP7–211403). We thank Prof. Stefan Höning (University of Cologne, Germany) for providing us with the LAMP antibodies for detecting lysosomes.
References
- 1.Kampinga HH, Hagemann J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–11. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrarini M, Heltai S, Zocchi MR, et al. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613–9. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- 3.Shin BK, Wang H, Yim AM, et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–16. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 4.Multhoff G, Botzler C, Wiesnet M, et al. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–9. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 5.Botzler C, Li G, Issels RD, et al. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones. 1998;3:6–11. doi: 10.1379/1466-1268(1998)003<0006:doeleo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Multhoff G, Pfister K, Gehrmann M, et al. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6:337–44. doi: 10.1379/1466-1268(2001)006<0337:amhpsn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hantschel M, Pfister K, Jordan A, et al. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones. 2000;5:438–42. doi: 10.1379/1466-1268(2000)005<0438:hpmeop>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinjung T, Arndt O, Feldmann HJ, et al. Heat shock protein 70 (Hsp70) membrane expression on head-and-neck cancer biopsy-a target for natural killer (NK) cells. Int J Radiat Oncol Biol Phys. 2003;57:820–6. doi: 10.1016/s0360-3016(03)00629-1. [DOI] [PubMed] [Google Scholar]
- 9.Farkas B, Hantschel M, Magyarlaki M, et al. Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res. 2003;13:147–52. doi: 10.1097/00008390-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Pfister K, Radons J, Busch R, et al. Patient survival by Hsp70 membrane phenotype: association with different routes of metastasis. Cancer. 2007;110:926–35. doi: 10.1002/cncr.22864. [DOI] [PubMed] [Google Scholar]
- 11.Gehrmann M, Radons J, Molls M, et al. The therapeutic implications of clinically applied modifiers of heat shock protein 70 (Hsp70) expression by tumor cells. Cell Stress Chaperones. 2008;13:1–10. doi: 10.1007/s12192-007-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehrmann M, Liebisch G, Schmitz G, et al. Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. Plos One. 2008 doi: 10.1371/journal.pone.0001925. 10.1371/j.0001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falguieres T, Maak M, von Weyhern C, et al. Human colorectal tumors and metastases express Gb3 and can be targeted by an intestinal pathogen-based delivery tool. Mol Cancer Ther. 2008;7:2498–508. doi: 10.1158/1535-7163.MCT-08-0430. [DOI] [PubMed] [Google Scholar]
- 14.Schilling D, Gehrmann M, Steinem C, et al. Binding of Hsp70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J. 2009;23:2467–77. doi: 10.1096/fj.08-125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vega V, Rodriguez-Silva M, Frey T, et al. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- 16.Arispe N, Doh M, Simakova O, et al. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18:1636–45. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- 17.Gehrmann M, Marienhagen J, Eichholtz-Wirth H, et al. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 2005;12:38–51. doi: 10.1038/sj.cdd.4401510. [DOI] [PubMed] [Google Scholar]
- 18.Dullin C, Zientkowska M, Napp J, et al. Semiautomatic landmark-based two-dimensional-three-dimensional image fusion in living mice: correlation of near-infrared fluorescence imaging of Cy5.5-labelled antibodies with flat-panel volume computed tomography. Mol Imaging. 2009;8:2–14. [PubMed] [Google Scholar]
- 19.Wang M, Bronte V, Chen PW, et al. Active immunotherapy of cancer with a non-replicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, et al. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–54. [PubMed] [Google Scholar]
- 21.Missbach-Guentner J, Dullin C, Kimmina S, et al. Morphological changes of mammary carcinomas in mice over time as monitored by flat-panel detector volume computed tomography. Neoplasia. 2008;10:663–73. doi: 10.1593/neo.08270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Liu R, Huang W. A 14-mer peptide from HSP70 protein is the critical epitope which enhances NK activity against tumor cells in vivo. Immunol Invest. 2007;36:233–46. doi: 10.1080/08820130600992073. [DOI] [PubMed] [Google Scholar]
- 23.Broquet AH, Thomas G, Masliah J, et al. Expression of the molecular chaperone Hsp70 in detergent resistant microdomains correlates with its membrane expression and release. J Biol Chem. 2003;278:21601–6. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- 24.Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics. 2009;9:2820–35. doi: 10.1002/pmic.200800793. [DOI] [PubMed] [Google Scholar]
- 25.Gastpar R, Gehrmann M, Bausero M, et al. Hsp70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of NK cells. Can Res. 2005;65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 27.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 28.Scallon BJ, Snyder LA, Anderson GM, et al. A review of antibody therapeutics and antibody-related technologies for oncology. J Immunother. 2006;29:351–64. doi: 10.1097/01.cji.0000199196.97845.c3. [DOI] [PubMed] [Google Scholar]
- 29.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]


