Abstract
Inflammatory pathways are involved in the development of atherosclerosis. Interaction of vessel wall cells and invading monocytes by cytokines may trigger local inflammatory processes. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are standard medications used in cardiovascular diseases. They are thought to have anti-inflammatory capacities, in addition to their lipid-lowering effects. We investigated the anti-inflammatory effect of statins in the cytokine-mediated-interaction-model of human vascular smooth muscle cells (SMC) and human mononuclear cells (MNC). In this atherosclerosis-related inflammatory model LPS (lipopolysaccharide, endotoxin), as well as high mobility group box 1 stimulation resulted in synergistic (i.e. over-additive) IL-6 (interleukin-6) production as measured in ELISA. Recombinant IL-1, tumour necrosis factor-α and IL-6 mediated the synergistic IL-6 production. The standard anti-inflammatory drugs aspirin and indomethacin (Indo) reduced the synergistic IL-6 production by 60%. Simvastatin, atorvastatin, fluvastatin or pravastatin reduced the IL-6 production by 53%, 50%, 64% and 60%, respectively. The inhibition by the statins was dose dependent. Combination of statins with aspirin and/or Indo resulted in complete inhibition of the synergistic IL-6 production. The same inhibitors blocked STAT3 phosphorylation, providing evidence for an autocrine role of IL-6 in the synergism. MNC from volunteers after 5 day aspirin or simvastatin administration showed no decreased IL-6 production, probably due to drug removal during MNC isolation. Taken together, the data show that anti-inflammatory functions (here shown for statins) can be sensitively and reproducibly determined in this novel SMC/MNC coculture model. These data implicate that statins have the capacity to affect atherosclerosis by regulating cytokine-mediated innate inflammatory pathways in the vessel wall.
Keywords: atherogenesis, early atherosclerosis, cell-interaction, cytokines, immunovascular activity, innate immunity, inflammation, interleukin, monocytes, statins
Introduction
Atherosclerosis is a complex vascular disease. Inflammatory processes play a key role in progression of the atherosclerotic process [1]. The development of the atherosclerotic plaque may result to a large degree from local cell interaction(s) in the vessel wall and the inflammatory force accelerated by these interactions. In early atherogenesis, during the interaction of vascular smooth muscle cells (SMC) and invading monocytes in the vessel wall, cytokines, such as interleukin-1 (IL-1), IL-6, tumour necrosis factor-α (TNF-α) or monocyte chemoattractant protein (MCP-1) may be produced and secreted, subsequently contributing to the regulation of local inflammatory processes [2, 3]. Elevated levels of IL-6 have been reported under chronic inflammatory conditions, and inflammatory markers, such as the acute phase protein C-reactive protein (CRP) are taken as markers of cardiovascular disease [4]. IL-6, a potent pro-inflammatory molecule activating the acute phase response, is copiously produced by SMC [5]. IL-6 may also contribute to cardiovascular diseases by vascular remodelling, such as observed in pulmonary hypertension [6].
Interaction of vessel wall and blood cells is important for development of atherosclerosis [1]. Interaction of SMC and endothelial cells, endothelial cells and monocytes, or SMC and monocytes has been investigated in vitro (for summary see [3]). We observed recently that the IL-6 and MCP-1 production was up-regulated synergistically upon SMC-MNC interaction [7]. This novel coculture model constitutes a highly sensitive, atherosclerosis-related pro-inflammatory system, useful for detection of anti-inflammatory effects of drugs used in cardiovascular diseases, including statins.
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are commonly used as lipid-lowering drugs. The beneficial effects of statins may also be related to plaque stabilization or lowering of inflammatory parameters [8]. Clinical studies have shown that coronary events are associated with enhanced CRP and that statins reduced this pro-inflammatory marker [9]. Animal studies showed that simvastatin significantly attenuated P-selectin expression in an in vivo model resulting in reduced rolling and migration [10]. In cell culture, statins inhibited the expression of adhesion molecules on endothelial cells [11], and reduced the production of pro-inflammatory cytokines in THP-1-cells or SMC [12, 13].
However, little is known about the effect of statins on the interaction of SMC and monocytes. Anti-inflammatory drugs may reduce the inflammatory load in the vessel wall tissue. Thus, we used a novel SMC-MNC coculture system, in order to quantify the anti-inflammatory effects of four statins on the inflammatory potential resulting from the interaction of SMC and MNC. This system is of particular interest, since the IL-6 production in this model is enormously enhanced, i.e. the level of IL-6 in the coculture is 10 to 30-fold higher than the sum of the IL-6 present in supernatants of separately cultured SMC and MNC. This synergistically enhanced cytokine production may be of importance for atherogenesis and may constitute a more sensitive system for determination of anti-inflammatory capacities of compounds than other in vitro systems. The anti-inflammatory activity in the coculture was determined as reduction of the synergistic IL-6 production. The data showed that the standard anti-inflammatory drugs aspirin and indomethacin (Indo), as well as the tested statins reduced the inflammatory activity. In combination, these drugs completely abolished IL-6 production. Taken together, the measurement of the anti-inflammatory capacity of drugs in the present coculture system was sensitive and reproducible with the tested statins. The data show that statins have the capacity to interfere with the activation of inflammatory pathways during interaction of vascular cells and blood cells. The data also support the hypothesis that statins may reduce the pro-inflammatory status in the atherosclerotic vessel wall, thereby retarding atherosclerosis.
Materials and methods
Materials
Dulbecco’s MEM (DMEM), Biocoll separating solution, Hank’s balanced salt solution, sodium bicarbonat and foetal calf serum (FCS) were obtained from Biochrom AG (Berlin, Germany). Lipopolysaccharide (LPS) of Salmonella enterica serovar Friedenau was a kind gift of Prof. Dr. H. Brade (Forschungszentrum Borstel, Germany). The HMG-CoA reductase inhibitors simvastatin, atorvastatin and pravastatin were from HEXAL AG (Holzkirchen, Germany), Parke Davis (Karlsruhe, Germany) and Bristol Myers-Squibb (Munich, Germany), respectively. Simvastatin sodium salt and fluvastatin sodium salt (active forms) were purchased from Calbiochem (Darmstadt, Germany). Acetylsalicylic acid (Asa), Indo and dimethyl sulfoxide (DMSO) were purchased from Sigma (Deisenhofen, Germany). Antibodies to unphosphorylated STAT3 and phosphorylated STAT3 were from Cell Signalling Technology (New England Biolabs, Frankfurt a.M., Germany).
Isolation and culture of vascular smooth muscle cells
Human vascular SMC were isolated from saphenous veins obtained following bypass surgery. The use of these tissues was approved by the local ethics committee. The work performed in this study conforms with the declaration of Helsinki. The cells were cultured and characterized as described before [5, 7, 14]. Briefly, SMC were isolated from the media of the vascular tissue by the outgrowth technique, and characterized by the typical ‘hill and valley’ growth pattern observed in phase contrast microscopy, as described earlier [15, 16]. The outgrowing cells were removed from the culture dishes by trypsin treatment and subsequently incubated in DMEM-medium (DMEM with 1 g/L D-glucose, supplemented with 10% FCS, 1% antibiotics and 1% L-glutamine).
Isolation and culture of human mononuclear cells
Mononuclear cells (MNC) were isolated from heparinized whole blood, obtained from healthy volunteers, recruited at the transfusion medicine of the university clinics, using Biocoll separating solution. The use of the blood was approved by the local ethics committee. Whole blood (heparinized) was taken from healthy donors and MNC were isolated as described earlier [7]. Cell number and viability were determined in a Neubauer chamber after staining with Trypan blue and Tuerk’s solution.
Coculture of SMC with MNC
For coculture experiments, SMC were incubated for 24 hrs in 24-well plates (10,000 cells/cm2) in DMEM-medium. Subsequently, the cells were washed twice and MNC were seeded into the wells at a ratio of 1 MNC per 1 SMC. Simvastatin, pravastatin and atorvastatin tablets were ground to fine powder. Pravastatin and atorvastatin powder, or commercially available Asa, were dissolved in cell culture medium. Indo was dissolved in ethanol. Simvastatin powder, as well as the active simvastatin salt were dissolved in DMSO, the active fluvastatin salt was dissolved in water. The solutions were sterilized by filtration. Solvent controls, consisting of the solvents without drugs, did not alter the IL-6 production. LPS (100 pg/ml) was added immediately after the drugs. The coculture assay consisted of separately cultured SMC, separately cultured MNC and cocultured SMC-MNC. The cultures were incubated for 24 hrs (37°C, 5% CO2), the supernatants were harvested and stored at −20°C until IL-6 analysis. The IL-6 levels in the supernatants were determined with an IL-6 ELISA (BD Biosciences Pharmingen, Heidelberg, Germany) according to the manufacturer’s instructions. The synergism of the IL-6 production was determined from the IL-6 values detected in the supernatants of the SMC cultured alone (IL-6SMC), the MNC cultured alone (IL-6MNC) and the cocultured cells (IL-6Coculture) by the following calculation:
We defined a synergistic (over-additive) IL-6 production to be present, if IL-6 in the coculture was more than the IL-6 added up from the separate cultures (IL-6Coculture > IL-6SMC+ IL-6MNC).
The lactate dehydrogenase (LDH) level in the supernatants was investigated with the ‘Cytotoxicity Detection KitPLUS (LDH)’ (Roche, Mannheim, Germany) as suggested by the manufacturer.
Short-term drug application to healthy volunteers
The effect of short term aspirin and simvastatin treatment was tested by intake of aspirin (100 mg/day; Aspirin protect 100, Bayer) or simvastatin (40 mg/day; Simvahexal 40, Hexal AG) by four healthy volunteers. For this experiment four senior authors of the manuscript (HL, MB, AS, UMW) donated blood prior to medication and after 5 days of medication. At both time-points MNC were isolated and cocultured with SMC of the same isolate and IL-6 was measured in ELISA. The MNC isolated after the aspirin treatment were used in a standard coculture experiment (i.e. SMC alone, MNC alone, coculture of both) in the absence or presence of endotoxin as a stimulus. The MNC isolated after the simvastatin administration were also cultured in a standard coculture experiment. However, a parallel coculture was incubated in vitro with simvastatin (50 μg/ml), in order to analyse whether or not a refractory effect was observed.
Statistical analysis
The cell culture experiments were performed in duplicate in 24-well culture plates and each supernatant was measured in duplicate in ELISA. Mean and standard deviation, as well as t-test were calculated in SPSS. Multi-group-analysis was performed by one-way anova. Briefly, One-way ANOVA was performed including Brown–Forsythe and Welch test, as well as multiple group analysis by Bonferroni analysis.
Results
The inflammatory stimuli high mobility group box 1 (HMGB1) and endotoxin (LPS) activate the synergistic IL-6 production in cocultures of SMC and MNC
We have previously shown that endotoxin induces synergistic IL-6 production in cocultures of SMC and MNC. Other substances (besides LPS) involved in inflammatory responses, may activate the cells also. HMGB1 has inflammatory functions and is present in the atherosclerotic vessel wall. Thus, we investigated the activation of the cells in the coculture by HMGB1 (Table 1). In the present experiment endotoxin-stimulated cocultures expressed a 4.43-fold synergism. The two concentrations of HMGB1 showed a 1.17- and 2.14-fold synergism, reflecting 26.4% and 48.3% of the endotoxin-induced synergism, respectively. Thus, the tested HMGB1 concentrations were almost half as potent as LPS in the induction of the synergism. On the other hand, the incubation of the coculture with the mitogen platelet-derived growth factor (PDGF) did not cause a synergism, whereas stimulation with Gram– and Gram+ bacteria, interferon (IFN)-α, as well as advanced glycation end products (AGEs) activated synergistic IL-6 production (data not shown). These data, as well as unpublished data, indicate that, although LPS is used as the standard activator in the coculture, other inflammatory mediators also can trigger the synergism.
Table 1.
HMGB1 and LPS induce synergistic IL-6 production
| IL-6 production (pg/ml)* | ||||
|---|---|---|---|---|
| None | LPS | HMGB1 0.5 | HMGB1 2.0 | |
| SMC | 2411 ± 137 | 5315 ± 855 | 2516 ± 50 | 1992 ± 45 |
| MNC | 1 ± 1 | 226 ± 5 | 10 ± 7 | 106 ± 3 |
| Coculture | 1547 ± 136 | 24,528 ± 4369 | 2951 ± 334 | 4485 ± 100 |
| Calculated values | ||||
| Synergism† | 0.64 | 4.43 | 1.17 | 2.14 |
| Percentage‡ | 100 | 26.4 | 48.3 | |
Vascular SMC and MNC were cultured separately or in coculture, without stimulus (None) or with the indicated stimulus (LPS, 100 ng/ml; HMGB1, in μg/ml) for 24 hrs. The supernatants were harvested and IL-6 was determined in ELISA.
Synergism = IL-6Coculture/ (IL-6MNC+ IL-6SMC).
The ‘Synergism’ in the LPS-stimulated culture was defined to be 100%.
The exogenous recombinant cytokines IL-1, TNF-α and hyper IL-6 mimic the synergistic IL-6 production
In order to demonstrate the mechanism of the synergistic IL-6 production, we analysed whether IL-1, TNF and IL-6 (hyper IL-6) are involved in the generation of the synergism. Hyper IL-6 is a fusion protein of IL-6 and the soluble-IL-6-receptor which is thought to activate SMC by trans-signalling [17], an activation pathway supposed to be involved in the induction of the synergism. Figure 1 shows that, depending on the combination of the three cytokines, endotoxin and serum, a synergistic IL-6 production was measured. The stacked columns (grey, green, red) show the IL-6 level induced by the single cytokines, the black columns show the IL-6 level upon simultaneous stimulation. The data show that the synergism was stronger in the LPS-stimulated (+) than in the unstimulated cultures (–). This difference was much more pronounced under serum-free culture conditions than under serum-containing conditions, due to enhanced basal IL-6 levels in serum-containing cultures. The synergism was stronger at the lower IL-1 concentrations, and at the higher TNF and hyper IL-6 concentrations. The maximal synergism obtained in the present experiment was 5.8-fold, which represents a moderate coculture result. The data show that IL-1 concentrations, as low as 5 or 50 pg/ml, can contribute potently to the synergism.
Fig 1.
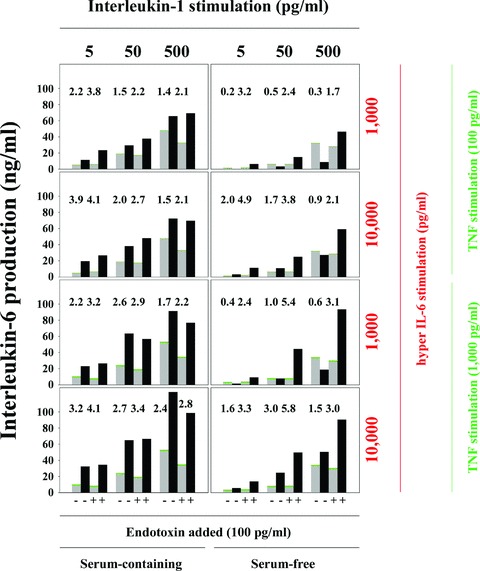
Exogenous IL-1, TNF and IL-6 are sufficient to initiate synergistic IL-6 production. SMC (10,000 SMC/cm2) were cultured in 96-well plates for 24 hrs. Medium was changed and recombinant IL-1β, TNF-α or hyperIL-6, as well as endotoxin (100 pg/ml) were added. Parallel cultures were incubated in the absence (Serum-free) or presence (Serum-containing) of 10% FCS. IL-6 production was measured in ELISA. The numbers above the columns present the x-fold increase (synergism) following combined stimulation by all cytokines (black columns) compared with the sum of the IL-6 production in the individually stimulated cultures (stacked columns: grey, IL-1; green, TNF; red, hyper IL-6). Four experiments showed similar results.
Asa and Indo inhibit the synergistic IL-6 production in SMC and MNC cocultures
The system characterized above represents a candidate for a sensitive screening tool for anti-inflammatory capacities of substances. In order to verify this suggestion we first investigated the anti-inflammatory capacity of the two well-known anti-inflammatory drugs Asa and Indo. Figure 2 shows that in LPS-stimulated cultures (yellow columns), addition of Asa decreased the IL-6 production in SMC, coculture, and MNC by 32%, 74% and 58%, respectively. Addition of Indo decreased the IL-6 production by 66%, 63% and 20% in SMC, coculture and MNC, respectively. A dose-response investigation with Asa (not presented as a figure) showed that much less material, i.e. 20, 200 and 2000 μg/ml, reduced the IL-6 production in LPS-stimulated cocultures by 32%, 44% and 66%, respectively. The above results indicated that the coculture system indeed has the potential to detect anti-inflammatory capacities of compounds.
Fig 2.
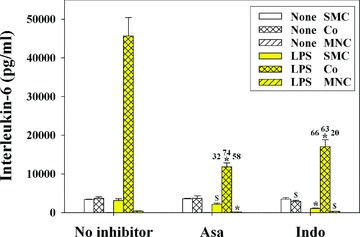
Asa and Indo inhibit the IL-6 production in LPS-stimulated cocultures of SMC/MNC. SMC (1 × 104 cell/cm2) and MNC (1 × 104 cell/cm2) were cocultured in 24-well plates for 24 hrs with or without anti-inflammatory drugs (Asa; 2 mg/ml; Indo; 20 μg/ml), in the absence or presence of LPS (100 pg/ml). Asa was dissolved in culture medium. Indo was pre-diluted in ethanol (0.15%). The used ethanol concentration did not alter the IL-6 production in control experiments (not included in the figure). The supernatants were harvested and IL-6 determined in human IL-6 ELISA. The numbers above the columns describe the inhibition (%). Five experiments showed similar results. The significance of the inhibition of the respective conditions (i.e. None versus Asa; None versus Indo) are given above the columns: #, P < 0.05; §, P < 0.01; *, P < 0.001).
Statins inhibit the synergistic IL-6 production in the cocultures in a dose-dependent manner
Next, we investigated the anti-inflammatory capacity of four statins in dose-response experiments in the coculture system. Figure 3 presents four independent experiments. The figure shows the effect of the various statins on SMC, coculture and MNC, both, stimulated with LPS or unstimulated. The effect of the different statins on the separately cultured SMC and the MNC was not consistent, whereas all statins consistently inhibited the IL-6 production in the cocultures. In detail, atorvastatin and fluvastatin inhibited both, the SMC and the MNC, whereas pravastatin inhibited the MNC, but not the SMC, and simvastatin inhibited the SMC, but not the MNC. However, in the coculture all statins inhibited both, the unstimulated and LPS-stimulated cocultures. The inhibition by all statin conditions was significant. Except pravastatin, all drugs inhibited most potently at the highest concentration. At a concentration of 50 μg/ml atorvastatin and simvastatin still inhibited the synergism by approximately 50%. Inhibition of IL-6 production by pravastatin and fluvastatin was slightly higher than with atorvastatin and simvastatin, still inhibiting more than 30% at 10 μg/ml. Independently, we tested two forms of simvastatin, the active compound, which does not need to be hydrolysed, and the drug form, which has to be hydrolysed in the body, in order to become the active lipid-lowering compound. Both forms inhibited the IL-6 production in the coculture to the same degree (data not shown). In order to prove that the inhibitory effect was not caused by cytotoxicity we measured the LDH activity in all cultures. The measurement showed that no statin-treated culture expressed enhanced levels of LDH (data not shown).
Fig 3.
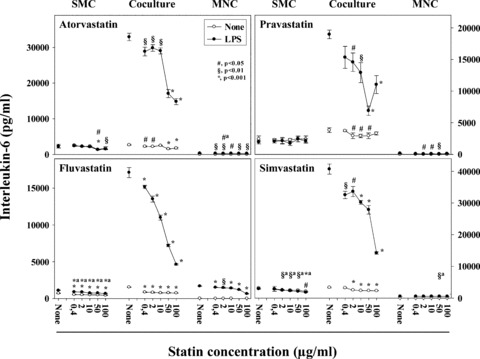
Statins inhibit the synergistic IL-6 production in LPS-induced SMC/MNC cocultures dose dependently. SMC (1 × 104 cell/cm2), MNC (1 × 104 cell/cm2) and cocultures thereof were cultured with or without LPS (100 pg/ml) and statins (0.4, 2, 10, 50 and 100 μg/ml). Simvastatin was dissolved in DMSO (0.2%), the other statins were dissolved in culture medium. The used DMSO concentration did not alter the IL-6 production in control experiments (not included in the figure). The supernatants were harvested and IL-6 determined in human IL-6 ELISA. Significant differences to culture supernatants incubated without statins are indicated by the symbols given in the figure. Significances were determined by one-way ANOVA. a, because of space limitations the significances of the unstimulated condition were written above the significance values of the LPS-stimulated cultures. Two dose–response experiments for each statin were performed.
Combination of statins with Asa and/or Indo enhances the inhibitory effect
The inhibitory pathways of the statins and the classical anti-inflammatory drugs Asa and Indo may be different and, therefore, may result in additive inhibition. Thus, we analysed the effect of various combinations of Asa, Indo and the statins. Figure 4 shows that all individual inhibitors, compared to the cultures without inhibitor (None), reduced the IL-6 production in the cocultures. The inhibition was significant in all cases; however, due to space limitations we did not include the significance symbols. Combinations of the inhibitors further reduced the IL-6 production. The inhibitory effect of fluvastatin with aspirin was similar to that of a combination of aspirin and Indo. A combination of aspirin, Indo and the respective statin resulted in an almost complete reduction (by ≥90%) of the IL-6 production. These data indeed indicated that independent pathways, such as COX-1 and HMG-CoA-reductase, the latter by influencing the prenylation process, may contribute to the observed inhibition of the inflammatory marker IL-6.
Fig 4.
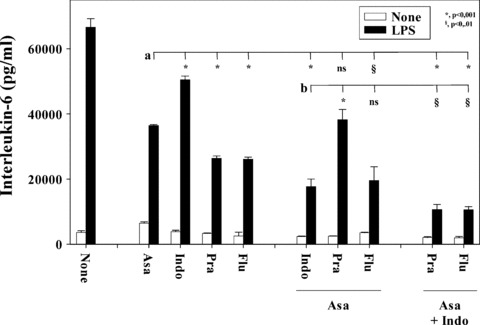
Combination of Asa and Indo with statins increases the inhibition of the IL-6 production. SMC (1 × 104 cell/cm2) and MNC (1 × 104 cell/cm2) were cocultured in 24-well plates for 24 hrs with various combinations of statins (pravastatin, Pra; fluvastatin, Flu; both 50 μg/ml), Asa (2 mg/ml) and Indo (20 μg/ml), in the presence of LPS (100 pg/ml). The statins and Asa were dissolved in culture medium, Indo was pre-diluted in ethanol (0.15%; compare Fig. 2). The supernatants were harvested and IL-6 determined in ELISA. All cocultures containing inhibitors were significantly lower (P < 0.001) than the coculture without drug (None; the symbols for the significances are not included in the figure). The significances of other comparisons are indicated by the horizontal lines, a: Asa versus the others and b: Asa with Indo versus the others. Two experiments showed similar results.
STAT3 phosphorylation is abolished following addition of aspirin, Indo and simvastatin
The synergistic IL-6 production in the coculture may partially result from trans-signalling [17] caused by endogenously produced IL-6, which would be illustrated by STAT3 phosphorylation. Figure 5A shows in the upper panel that SMC and the respective cocultures expressed similar amounts of unphosphorylated STAT3. MNC expressed much less unphosphorylated STAT3, only poorly detectable under the experimental conditions. Quantification of total protein showed that SMC and cocultures contained similar protein concentrations, whereas MNC expressed only a third of the protein present in SMC or cocultures. On the other hand, the expression of phosphorylated STAT3 was differentially regulated. Already unstimulated SMC and cocultures contained phosphorylated STAT3 to some extent, but stimulation with LPS enhanced the STAT3 phosphorylation potently. In the coculture, STAT3 phosphorylation was stronger than in the SMC cultured alone. A combination of simvastatin, aspirin and Indo abolished STAT3 phosphorylation. The quantification of the band densities of the specific STAT3 and phosphoSTAT3 bands in the Western blot visualizes these data more clearly (Fig. 5B). The inhibitors also potently reduced the IL-6 production measured in parallel in the supernatants of the cultures (Table 2).
Fig 5.
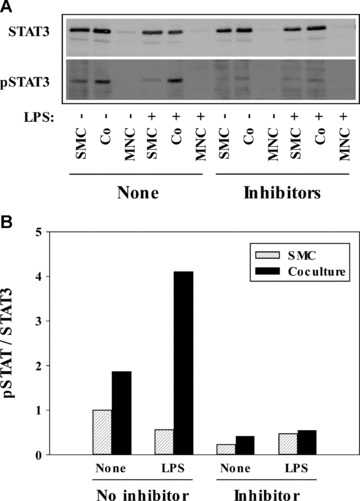
Combined application of simvastatin, Asa and Indo inhibits the phosphorylation of STAT3. (A) Western blot analysis. SMC (2 × 104 cell/cm2), MNC (2 × 104 cell/cm2) and cocultures (Co) thereof were cultured in 6-well plates. After 24 hrs LPS (100 pg/ml) or medium (+ and −, respectively), as well as simvastatin (50 μg/ml), Asa (1 mg/ml) and Indo (20 μg/ml) were applied (Inhibitor). The cells were harvested after 24 hrs. STAT3 and pSTAT3 were determined by Western blot. Two experiments showed similar results. The supernatants were analysed in IL-6 ELISA (compare Table 2). (B) Quantification of the specific bands in the Western blot. The STAT3 bands and the pSTAT3 bands of the Western blot presented above were quantified using the program AIDA and the values of the phosphorylated STAT3 were normalized to the bands of the respective unphosphorylated STAT3.
Table 2.
Combined application of aspirin, Indo and simvastatin potently reduces the synergistic IL-6 production*
| IL-6 production (pg IL-6/ml)† | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| No Inhibitors | Inhibitors added | |||||
| None | LPS | None | LPS | None | LPS | |
| MNC | 1 ± 0 | 864 ± 18 | 1 ± 0 | 90 ± 1 | 0 | 90 |
| SMC | 3418 ± 76 | 3660 ± 333 | 1823 ± 53 | 1626 ± 8 | 47 | 56 |
| Coculture | 4008 ± 76 | 240,079 ± 2496 | 1376 ± 30 | 13,197 ± 353 | 67 | 95 |
SMC (20,000) and MNC were cultured separately or in coculture at a ratio of 1 to 1 in 6-well culture plates. Cultures were incubated with a combination of aspirin (1 mg/ml), Indo (20 μg/ml) and simvastatin (50 μg/ml). Subsequently, the cultures were stimulated with LPS (100 pg/ml), incubated for 24 hrs and the supernatants harvested and stored at −20°C.
IL-6 in the supernatant was measured in ELISA. The IL-6 data represent a measurement of the supernatants of the cultures analysed in the Western blot shown in Fig. 5A.
Short term drug administration did not reduce the ex vivo synergism of isolated MNC
Finally, we analysed the function of MNC isolated from healthy probands, treated for 5 days with aspirin or simvastatin, in the coculture system. MNC isolated before simvastatin intake (Table 3; Exp. 1) induced a 17.4-fold synergism. A similar synergistic effect was present in the cocultures with MNC isolated after the 5-day statin treatment, documenting a lack of inhibition in isolated MNC under ex vivo conditions. In order to analyse a possible development of a simvastatin-refractory state, we also incubated parallel-cultures of the MNC, isolated after the simvastatin-treatment, with simvastatin (50 μg/ml) in vitro. This experiment showed a clear inhibition (17.4 versus 7.4, and 16.8 versus 10.5, respectively) of the IL-6 production, similar to that described above (compare Figs. 3 and 4). This result indicated that the anti-inflammatory activity of the statin is not detectable ex vivo and that the isolated cells are not refractory to simvastatin. In order to analyse whether the lack of anti-inflammatory activity ex vivo is a general phenomenon we administered aspirin to the same volunteers 4 weeks after the simvastatin exposure (Table 3; Exp. 2). As observed above for simvastatin, aspirin administration did not result in a reduction of the IL-6 synergism ex vivo. The lack of detectable ex vivo effects of both compounds is probably due to removal of the drugs during the isolation of the MNC from the blood, in combination with the short half-life and the reversibility of binding of the compounds to the respective binding molecule.
Table 3.
Short-term administration (5 days) of aspirin or simvastatin to healthy volunteers does not reduce the IL-6 synergism ex vivo*
| IL-6 production (pg/ml) | |||||||
|---|---|---|---|---|---|---|---|
| MNC isolated before drug administration† | MNC isolated after drug administration | ||||||
| Drug | Exp‡ | In vitro Simva§ | None | LPS | None | LPS | |
| Simvastatin | 1 | − | MNC | 1.2 ± 1.1# | 468 ± 149 | 1.3 ± 1.0 | 666 ± 114 |
| 1 | − | SMC | 2158 ± 430 | 2994 ± 293 | 3014 ± 274 | 4276 ± 492 | |
| 1 | − | Coculture | 2313 ± 377 | 60,195 ± 4060 | 3254 ± 462 | 82,962 ± 11,831 | |
| Synergism$ | 1.1 | 17.4 | 1.1 | 16.8 | |||
| Simvastatin | 1 | +§ | MNC | 1.3 ± 0.8 | 167 ± 58 | 1.4 ± 0.6 | 357 ± 150 |
| 1 | +§ | SMC | 1188 ± 126 | 1213 ± 141 | 1588 ± 249 | 1813 ± 190 | |
| 1 | +§ | Coculture | 1369 ± 558 | 10,167 ± 3355 | 1585 ± 352 | 22,750 ± 11,991 | |
| Synergism$ | 1.2 | 7.4 | 1.0 | 10.5 | |||
| Aspirin | 2 | − | MNC | 1.2 ± 0.9 | 335 ± 91 | 2.8 ± 2.4 | 387 ± 89 |
| 2 | − | SMC | 4694 ± 292 | 5547 ± 527 | 4478 ± 548 | 5653 ± 731 | |
| 2 | − | Coculture | 5984 ± 2164 | 53,612 ± 12,068 | 5045 ± 315 | 59.469 ± 4678 | |
| Synergism$ | 1.3 | 9.1 | 1.1 | 9.9 | |||
Four healthy volunteers (HL, MB, AS, UMW) took simvastatin (40 mg/day) or, 4 weeks later, aspirin (100 mg/day) for 5 days.
MNC were isolated by Biocoll gradient separation before and after the 5-day administration period. The cells were incubated alone or in coculture with SMC (10,000/cm2) incubated on 24-well plates for 24 hrs. The same isolate of SMC was used at the ‘before’ and ‘after time-point.
Exp, experiment number.
The MNC isolated from the volunteers before and after simvastatin intake, as well as SMC and cocultures, were additionally incubated in parallel with simvastatin (20 μg/ml) in vitro.
The data are presented as mean ± SD of all four volunteers.
Synergism = IL-6Co/ (IL-6SMC+ IL-6MNC).
Discussion
Inflammatory processes play a key role in atherogenesis [1]. Interaction of vascular SMC and monocytes may contribute to the regulation of local inflammatory processes [2, 3]. Reduction of the inflammatory load in the vessel wall may reduce the risk of cardiovascular diseases. For analysis of the anti-inflammatory capacity of drugs we used a pro-inflammatory model system related to atheroslerosis. This experimental system consists of a coculture system of human SMC and MNC (i.e. cells essential for atherosclerosis). This novel model illustrates the inflammatory capacity of vessel wall-leucocyte interaction by an enormously enhanced IL-6 production [7]. We show that HMGB1, in addition to LPS, increased the synergistic inflammatory capacity in the coculture. IL-1, TNF and IL-6 are the mechanisms responsible for the synergism. The synergistic IL-6 production was potently reduced by the standard anti-inflammatory drugs Asa (aspirin) and Indo (indomethacin). Importantly, four statins reduced the inflammatory marker IL-6 to a similar degree. Combination of statins with the antiphlogistic drugs further extended the reduction. Prevention of STAT3 phosphorylation by the drugs verified a potent contribution of autocrine IL-6 to the synergistic IL-6 production. The data suggest that measurement of IL-6 in the coculture is a pro-inflammatory model useful for the analysis of anti-inflammatory capacities of drugs. The coculture system is of advantage over measurement with separately cultured cells, since it can determine the anti-inflammatory capacity of the drug more sensitively and consistently. It has to be emphasized also that this system may replace the use of animals to test pro- and anti-inflammatory capacities of substances.
IL-6 is a cytokine produced by a variety of cells including monocytes [18], epithelial cells [19], vascular endothelial cells [20] and vascular SMC [5]. Although its role in atherosclerosis is not completely understood, and examples for both pro- and anti-atherogenic activities of IL-6 have been described [21–26], IL-6 is of importance for the regulation of monocyte-vascular cell interaction [2, 3]. This suggestion is underlined by the high amount of IL-6 produced by SMC upon stimulation with exogenous cytokines or in the coculture [5, 7]. The latter featuring synergistic (i.e. over-additive) production of this mediator. The synergism in the present system was originally described with LPS as an activator [7]. Microorganisms may be involved in atherogenesis, since bacteria and viruses have been detected in the plaque [27]. However, other cell activators, such as PDGF, IFNs, AGEs and HMGB1, may be of importance, too [3]. While PDGF did not promote the synergism, IFN-α and AGE (data not shown), as well as HMGB1 activated the synergism potently. IFN, AGE and HMGB1 are probably more related to inflammatory pathways important for atherogenesis than PDGF [1]. For example, HMGB1 can activate monocytes [28], and SMC appear to be both a target and a producer of HMGB1 [29]. PDGF may increase the IL-6 production due to an enhanced cell number, caused by its mitogenic function [5]. However, there is (to our knowledge) no report of IL-1, TNF or IL-6 production caused by PDGF stimulation or PDGF receptor expression in monocytes. In conclusion, the coculture system is not only activated by LPS, but also by other pro-inflammatory molecules relevant for atherosclerosis.
Previous research has shown that SMC can produce mediators relevant for atherosclerosis, such as IL-6 [5], as well as chemokines, such as MCP-1 [30] or fractalkine [31]. Interaction of cells related to atherogenesis, such as endothelial cells, SMC and monocytes, results in the activation of a variety of functions (for summary see [3]), including IL-6 and MCP-1 production, recently shown by us [7]. IL-1, TNF and autocrine (SMC-derived) IL-6 may be involved in the induction of the synergism. In this paper we show that various combinations of recombinant cytokines indeed synergistically enhanced the IL-6 production of SMC. Enhanced IL-6 production upon IL-1 and TNF stimulation, although not synergistic (0.7 to 1.3-fold), has been observed in intestine epithelial cells and SMC [32]. Furthermore, the production of IL-11 was 4-fold enhanced upon stimulation of vascular SMC with IL-1α, TNF and TGF-β[33]. The synergistic enhancement in the cocultures described here was frequently higher than the 5.8-fold enhancement observed after stimulation with the recombinant cytokines. These data are in favour of a role for additional mediators, besides IL-1, TNF and IL-6, in the induction of the synergism in the coculture. Although inhibition experiments with combined IL-1, TNF and IL-6 blockade showed a completely reduced synergism [7], other cytokines still may contribute to the synergism: in view of the cooperative effect of many mediators, elimination of the major player(s) may result in complete loss of the cooperative effect(s).
Asa and Indo are two well-known anti-inflammatory drugs. Thus, we used these substances as prototypic anti-inflammatory drugs in the coculture system. They decreased the IL-6 production by up to 71%, indicating that the coculture reflects a useful model for detection of anti-inflammatory effects of drugs. It has been proposed that HMG-CoA reductase inhibitors (statins), usually renowned as lipid-lowering drugs, may exert their beneficial effects by additional, inflammation-related effects, although the reports are not consistent [8, 9, 34, 35]. In hypercholesteremic patients simvastatin reduced IL-6, IL-8 and MCP-1 plasma levels, and reduced IL-6, IL-8 and MCP-1 mRNA expression in isolated endothelial cells and monocytes [36]. It was noted that fluvastatin reduced the IL-6 production of THP1 cells [12], whereas other authors showed that the IL-6 production of monocytes was not altered by various statins [37]. On the other hand, statins reduced the IL-6 production in vascular SMC [13]. In our experiments, fluvastatin and simvastatin reduced the IL-6 production of SMC, whereas atorvastatin and fluvastatin reduced the IL-6 production of MNC. However, all four statins tested by us reduced the IL-6 production in the coculture. These data may explain the inconsistent effects described in the literature. This inconsistency is an important disadvantage of systems using either monocytes or SMC separately. One cause of this inconsistency may be the lower cytokine levels, as compared with the coculture. In our hands the coculture did not show such inconsistency, it reproducibly showed an anti-inflammatory effect of both, the antiphlogistic drugs and the statins. This result may be enabled by the high cytokine (IL-6) level present under the experimental conditions. In other words, the ‘amplifier’ effect observed in the coculture appears to make the system more sensitive to anti-inflammatory effects than the (linear) effect present in the monocellular-cultures of SMC or MNC. On the other hand, the range of activity of the statins may depend on their lipo-/hydrophilicity. In our hands, fluvastatin and pravastatin are a little more active than simvastatin or atorvastatin. Depending on the definition of the lipo-/hydrophilicity range of the compounds (provided by Stancu and Sima or White, respectively), the range of activity in the coculture shows a dependence [38] or an incomplete correlation [39].
Similar to the tested statins, Asa and Indo, if applied separately, did not block the IL-6 synthesis completely, suggesting that different pathways (i.e. COX-1 or HMG-CoA-reductase) may contribute to the cell-activation in the synergism. The anti-inflammatory effects of statins, although their molecular basis is not completely defined, are linked to prenylation processes, which can be influenced by HMG-CoA inhibitors [40]. Thus, parallel usage of separate inhibitor classes appears to be necessary for complete inhibition of the synergism. Indeed, we observed that the IL-6 production was decreased by up to 95% by statins combined with Asa and Indo. Combined therapy with statins and COX inhibitors has been proposed earlier [41]. The results of the STAT3 phosphorylation experiments also supported the suggestion of combined usage of independent inhibitor classes, since the combination of Asa, Indo and statin completely reduced STAT3 phosphorylation.
We compared the anti-inflammatory effect of the statins observed in vitro with the ex vivo IL-6 production in cocultures using MNC isolated from statin-treated volunteers. However, after a short-term (5 days) administration of aspirin or simvastatin we did not observe any reduction of the synergism in the cocultures with MNC isolated from the volunteers. This may, at the first glance, appear to be a limitation of the study, however, this lack of effect may be explained by the following. First, although anti-inflammatory effects are thought to be present much faster than lipid lowering effects, the 5-day administration time may still be too short for ex vivo measurements. On the other hand, the removal of the drugs during and after the isolation of the MNC, involving a couple of washing steps, may have caused the recovery of the cells from the anti-inflammatory status present in vivo. This may also be fostered by the short half-lives of the substances. On the other hand, the in vitro inhibition by the statins was similar before and after statin administration, indicating that the statins did not induce a refractory state or tolerance to the statin in the isolated cells.
Taken together, the SMC/MNC coculture system provides a potent pro-inflammatory system. The detected synergism can largely be mimicked by the exogenous cytokines IL-1, TNF and IL-6, and it is present also upon activation with inflammatory activators, other than LPS. This system can be used to detect anti-inflammatory capacities of drugs. Statins dose dependently reduced the pro-inflammatory activity and combination of statins with anti-phlogistica further reduced it. The data indicate that the coculture system, rather than the ex vivo measurement, can be used as a sensitive assay for identification of anti-inflammatory drugs. The tested drugs, in addition to their ‘classical’ effects, may provide beneficial effects by reduction of pro-inflammatory pathways, such as cell interaction of SMC and monocytes in the vessel wall. The present data also support the proposal that the combination of drugs may be useful in primary and/or secondary prevention in cardiovascular diseases, by regulating inflammatory functions of cells.
Acknowledgments
We thank Prof. Dr. H. Brade for providing us with endotoxin of Salmonella enterica Serovar friedenau. We are grateful to Drs. Julian Hering and Jörg Harth (Transfusion Medicine, University Clinics Halle) for providing us with heparinized whole blood. The excellent technical assistance of Mrs. Claudia Pilowski, Mrs. Susanne Koch and Mrs. Thekla Wangemann (Universitätsklinik und Poliklinik für Herz- und Thoraxchirurgie) is gratefully acknowledged.
Conflict of interest
None declared.
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 3.Loppnow H, Werdan K, Buerke M. Vascular cells contribute to atherosclerosis by cytokine- and innate-immunity-related inflammatory mechanisms. Innate Immunity. 2008;14:63–87. doi: 10.1177/1753425908091246. [DOI] [PubMed] [Google Scholar]
- 4.Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC (Fragmin during Instability in Coronary Artery Disease) Study Group. N Engl J Med. 2000;343:1139–47. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 5.Loppnow H, Libby P. Proliferating or interleukin-1-activated human vascular smooth muscle cells secrete copious interleukin-6. J Clin Invest. 1990;85:731–8. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–44. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Frister A, Wang S, et al. Interaction of vascular smooth muscle cells and monocytes by soluble factors synergistically enhances interleukin-6 and MCP-1 production. Am J Physiol. 2009;296:H987–96. doi: 10.1152/ajpheart.01158.2008. [DOI] [PubMed] [Google Scholar]
- 8.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 10.Pruefer D, Scalia R, Lefer AM. Simvastatin inhibits leukocyte-endothelial cell interactions and protects against inflammatory processes in normocholesterolemic rats. Arterioscler Thromb Vasc Biol. 1999;19:2894–900. doi: 10.1161/01.atv.19.12.2894. [DOI] [PubMed] [Google Scholar]
- 11.Chen YH, Lin SJ, Chen YL, et al. Anti-inflammatory effects of different drugs/agents with antioxidant property on endothelial expression of adhesion molecules. Cardiovasc Hematol Disord Drug Targets. 2006;6:279–304. doi: 10.2174/187152906779010737. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda U, Shimada K. Statins and monocytes. Lancet. 1999;353:2070. doi: 10.1016/S0140-6736(05)77885-5. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, Ikeda U, Shimpo M, et al. HMG-CoA reductase inhibitors reduce interleukin-6 synthesis in human vascular smooth muscle cells. Cardiovasc Drugs Ther. 2002;16:121–6. doi: 10.1023/a:1015701415588. [DOI] [PubMed] [Google Scholar]
- 14.Schönbeck U, Herzberg M, Petersen A, et al. Human vascular smooth muscle cells express interleukin-1β-converting enzyme (ICE), but inhibit processing of the interleukin-1β precursor by ICE. J Exp Med. 1997;185:1287–94. doi: 10.1084/jem.185.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross R, Kariya B. Morphogenesis of vascular smooth muscle in atherosclerosis and cell structure. In: Bohr DF, Somlyo AP, Sparks HY, editors. Handbook of physiology: the cardiovascular system, Section 2. Bethesda: American Physiological Society; 1980. pp. 69–91. [Google Scholar]
- 16.Warner SJC, Auger KR, Libby P. Human interleukin-1 induces interleukin-1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987;165:1316–31. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose-John S, Scheller J, Elson G, et al. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 18.Gauldie J, Richards C, Harnish D, et al. Interferon-β2/BSF-2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–5. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Patay B, Loppnow H, Feindt J, et al. Catecholamines and lipopolysaccharide synergistically induce the release of interleukin-6 from thymic epithelial cells. J Neuroimmunol. 1997;86:182–9. doi: 10.1016/s0165-5728(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 20.Loppnow H, Libby P. Adult human vascular endothelial cells express the IL-6 gene differentially in response to LPS or IL-1. Cell Immunol. 1989;122:493–503. doi: 10.1016/0008-8749(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 21.Takeda N, Manabe I, Shindo T, et al. Synthetic retinoid Am80 reduces scavenger receptor expression and atherosclerosis in mice by inhibiting IL-6. Arterioscler Thromb Vasc Biol. 2006;26:1177–83. doi: 10.1161/01.ATV.0000214296.94849.1c. [DOI] [PubMed] [Google Scholar]
- 22.Elhage R, Clamens S, Besnard S, et al. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–20. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- 23.Schieffer B, Selle T, Hilfiker A, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 24.Rus HG, Vlaicu R, Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis. 1996;127:263–71. doi: 10.1016/s0021-9150(96)05968-0. [DOI] [PubMed] [Google Scholar]
- 25.Huber SA, Sakkinen P, Conze D, et al. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–7. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–24. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Moroi M, Yamamoto M, et al. Presence and severity of Chlamydia pneumoniae and Cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J. 2006;47:511–9. doi: 10.1536/ihj.47.511. [DOI] [PubMed] [Google Scholar]
- 28.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porto A, Palumbo R, Pieroni M, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–6. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 30.Dragomir E, Manduteanu I, Calin M, et al. High glucose conditions induce upregulation of fractalkine and monocyte chemotactic protein-1 in human smooth muscle cells. Thromb Haemost. 2008;100:1155–65. [PubMed] [Google Scholar]
- 31.Ludwig A, Berkhout T, Moores K, et al. Fractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-alpha and is modulated by metalloproteinase activity. J Immunol. 2002;168:604–12. doi: 10.4049/jimmunol.168.2.604. [DOI] [PubMed] [Google Scholar]
- 32.Ng EK, Panesar N, Longo WE, et al. Human intestinal epithelial and smooth muscle cells are potent producers of IL-6. Mediators Inflamm. 2003;12:3–8. doi: 10.1080/0962935031000096917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taki H, Sakai T, Sugiyama E, et al. Monokine stimulation of interleukin-11 production by human vascular smooth muscle cells in vitro. Atherosclerosis. 1999;144:375–80. doi: 10.1016/s0021-9150(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 34.Smith C, Halvorsen B, Otterdal K, et al. High levels and inflammatory effects of soluble CXC ligand 16 (CXCL16) in coronary artery disease: down-regulatory effects of statins. Cardiovasc Res. 2008;79:195–203. doi: 10.1093/cvr/cvn071. [DOI] [PubMed] [Google Scholar]
- 35.Romano M, Diomede L, Sironi M, et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest. 2000;80:1095–100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- 36.Rezaie-Majd A, Maca T, Bucek RA, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–9. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- 37.Bessler H, Salman H, Bergman M, et al. In vitro effect of statins on cytokine production and mitogen response of human peripheral blood mononuclear cells. Clin Immunol. 2005;117:73–7. doi: 10.1016/j.clim.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–87. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42:963–70. [PubMed] [Google Scholar]
- 40.Jasinska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59:483–99. [PubMed] [Google Scholar]
- 41.Chyrchel M, Dudek D, Bartus S, et al. High-dose statin and COX-2 inhibitor therapy rapidly decreases C-reactive protein level in patients with unstable angina. Kardiol Pol. 2004;61:213–21. [PubMed] [Google Scholar]


