Abstract
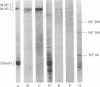
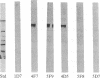
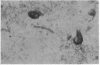
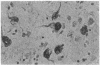
Neurofibrillary tangles (NFT) are the principal structural alteration of neuronal cell bodies in Alzheimer disease as well as in normal aging of the human brain. While the ultrastructure of these intraneuronal lesions has been extensively studied, the biochemical composition of the fibers comprising the NFT is unknown. We report the production of three monoclonal antibodies against the microtubule-associated protein 2 (MAP-2), one of which intensely labels Alzheimer NFT. All three antibodies specifically recognize MAP-2 on immunoblots and stain brain tissue in a characteristic dendritic pattern. The three antibodies are directed against at least two different antigenic sites on the MAP-2 molecule, and one appears to recognize a phosphorylation site on MAP-2. That only one of the three antibodies immunolabels NFT suggests that the formation of the tangle involves some modification of the MAP-2 molecule. Our findings suggest that one aspect of Alzheimer-type neurofibrillary pathology is an aggregation of MAP-2 or MAP-2 fragments with altered neurofilamentous elements present in NFT. Normal interactive function, which putatively occurs between neurofilaments and MAP-2, may thus be disrupted in Alzheimer disease.
Full text
PDF
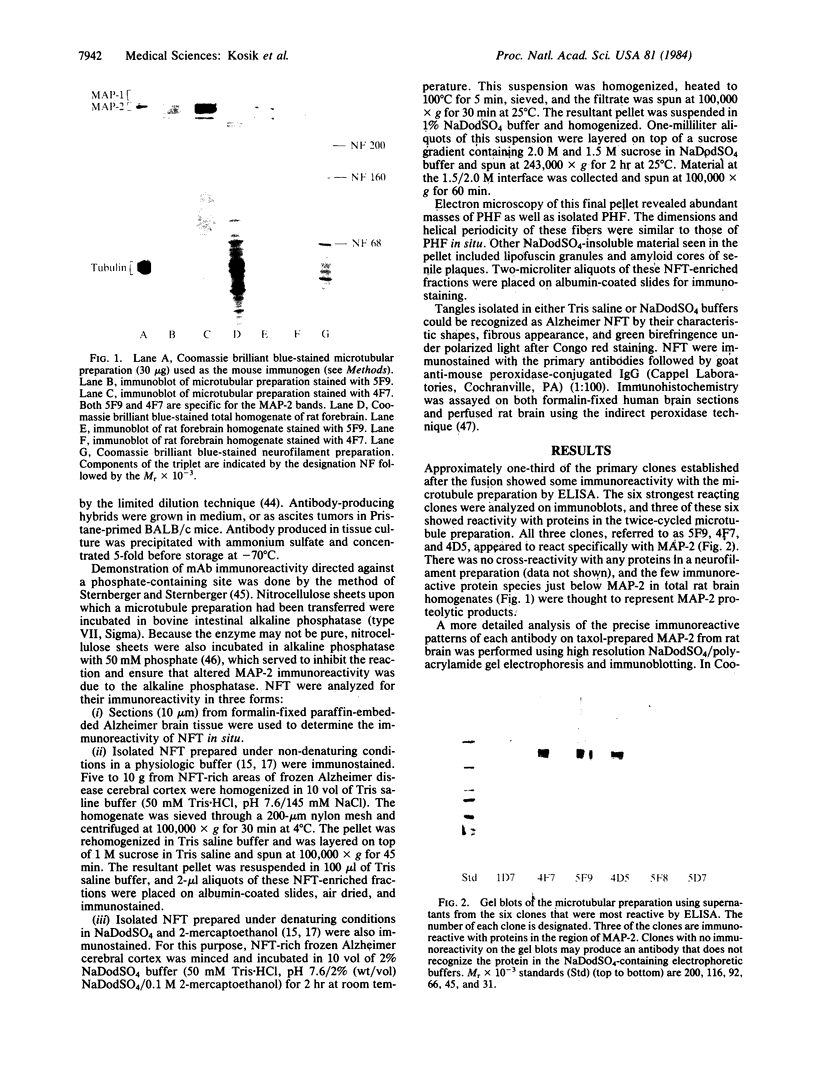



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. A., Katagiri J., Binder H. K., Williams R. C., Jr Separation and characterization of microtubule proteins from calf brain. Biochemistry. 1977 Dec 13;16(25):5610–5617. doi: 10.1021/bi00644a035. [DOI] [PubMed] [Google Scholar]
- Bloom G. S., Vallee R. B. Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J Cell Biol. 1983 Jun;96(6):1523–1531. doi: 10.1083/jcb.96.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger P. C., Vogel F. S. The development of the pathologic changes of Alzheimer's disease and senile dementia in patients with Down's syndrome. Am J Pathol. 1973 Nov;73(2):457–476. [PMC free article] [PubMed] [Google Scholar]
- Burton P. R., Fernandez H. L. Delineation by lanthanum staining of filamentous elements associated with the surfaces of axonal microtubules. J Cell Sci. 1973 Mar;12(2):567–583. doi: 10.1242/jcs.12.2.567. [DOI] [PubMed] [Google Scholar]
- Caceres A., Payne M. R., Binder L. I., Steward O. Immunocytochemical localization of actin and microtubule-associated protein MAP2 in dendritic spines. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1738–1742. doi: 10.1073/pnas.80.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dahl D., Selkoe D. J., Pero R. T., Bignami A. Immunostaining of neurofibrillary tangles in Alzheimer's senile dementia with a neurofilament antiserum. J Neurosci. 1982 Jan;2(1):113–119. doi: 10.1523/JNEUROSCI.02-01-00113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis W. G., McCulloch J. R., Corley C. L. Presenile dementia in Down's syndrome. Ultrastructural identity with Alzheimer's disease. Neurology. 1974 Feb;24(2):101–106. doi: 10.1212/wnl.24.2.101. [DOI] [PubMed] [Google Scholar]
- Ghatak N. R., Nochlin D., Hadfield M. G. Neurofibrillary pathology in progressive supranuclear palsy. Acta Neuropathol. 1980;52(1):73–76. doi: 10.1007/BF00687231. [DOI] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. Evidence for actin filament-microtubule interaction mediated by microtubule-associated proteins. J Cell Biol. 1978 Sep;78(3):958–965. doi: 10.1083/jcb.78.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982 Aug 10;257(15):9143–9151. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Johnson A. B., Wisniewski H. M., Terry R. D., Iqbal K. Evidence that Alzheimer neurofibrillary tangles originate from neurotubules. Lancet. 1979 Mar 17;1(8116):578–580. doi: 10.1016/s0140-6736(79)91006-7. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Ishii T., Haga S., Tokutake S. Presence of neurofilament protein in Alzheimer's neurofibrillary tangles (ANT). An immunofluorescent study. Acta Neuropathol. 1979 Nov;48(2):105–112. doi: 10.1007/BF00691151. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem. 1982 Sep 10;257(17):10467–10470. [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambre C., Kasturi K. N. A microplate immunoenzyme assay for anti-influenza antibodies. J Immunol Methods. 1979;26(1):61–67. doi: 10.1016/0022-1759(79)90041-3. [DOI] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Preferential phosphorylation of the 150,000 molecular weight component of neurofilaments by a cyclic AMP-dependent, microtubule-associated protein kinase. J Cell Biol. 1981 Sep;90(3):755–760. doi: 10.1083/jcb.90.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A., Bernhardt R., Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci U S A. 1981 May;78(5):3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P., Walter U., Theurkauf W. E., Vallee R. B., De Camilli P. Frozen tissue sections as an experimental system to reveal specific binding sites for the regulatory subunit of type II cAMP-dependent protein kinase in neurons. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5562–5566. doi: 10.1073/pnas.79.18.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Hirano A., Yamaguchi H., Hirai S. [The fine structure of eosinophilic stages of Alzheimer's neurofibrillary tangles]. Rinsho Shinkeigaku. 1982 Sep;22(9):840–846. [PubMed] [Google Scholar]
- Rewcastle N. B., Ball M. J. Electron microscopic structure of the "inclusion bodies" in Pick's disease. Neurology. 1968 Dec;18(12):1205–1213. doi: 10.1212/wnl.18.12.1205. [DOI] [PubMed] [Google Scholar]
- Rice R. V., Roslansky P. F., Pascoe N., Houghton S. M. Bridges between microtubules and neurofilaments visualized by stereoelectron microscopy. J Ultrastruct Res. 1980 Jun;71(3):303–310. doi: 10.1016/s0022-5320(80)90081-7. [DOI] [PubMed] [Google Scholar]
- Runge M. S., Laue T. M., Yphantis D. A., Lifsics M. R., Saito A., Altin M., Reinke K., Williams R. C., Jr ATP-induced formation of an associated complex between microtubules and neurofilaments. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1431–1435. doi: 10.1073/pnas.78.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattilaro R. F., Dentler W. L., LeCluyse E. L. Microtubule-associated proteins (MAPs) and the organization of actin filaments in vitro. J Cell Biol. 1981 Aug;90(2):467–473. doi: 10.1083/jcb.90.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherline P., Lee Y. C., Jacobs L. S. Binding of microtubules to pituitary secretory granules and secretory granule membranes. J Cell Biol. 1977 Feb;72(2):380–389. doi: 10.1083/jcb.72.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama H., Kitoh J. Electron microscopic structure of the Alzheimer's neurofibrillary changes in case of atypical senile dementia. Acta Neuropathol. 1978 Mar 15;41(3):229–234. doi: 10.1007/BF00690441. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRY R. D. THE FINE STRUCTURE OF NEUROFIBRILLARY TANGLES IN ALZHEIMER'S DISEASE. J Neuropathol Exp Neurol. 1963 Oct;22:629–642. doi: 10.1097/00005072-196310000-00005. [DOI] [PubMed] [Google Scholar]
- Tellez-Nagel I., Wiśniewski H. M. Ultrastructure of neurofibrillary tangles in Steele-Richardson-Olszewski syndrome. Arch Neurol. 1973 Nov;29(5):324–327. doi: 10.1001/archneur.1973.00490290064007. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. Structure and phosphorylation of microtubule-associated protein 2 (MAP 2). Proc Natl Acad Sci U S A. 1980 Jun;77(6):3206–3210. doi: 10.1073/pnas.77.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voter W. A., Erickson H. P. Electron microscopy of MAP 2 (microtubule-associated protein 2). J Ultrastruct Res. 1982 Sep;80(3):374–382. doi: 10.1016/s0022-5320(82)80051-8. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Narang H. K., Terry R. D. Neurofibrillary tangles of paired helical filaments. J Neurol Sci. 1976 Feb;27(2):173–181. doi: 10.1016/0022-510x(76)90059-9. [DOI] [PubMed] [Google Scholar]
- Yagishita S., Itoh Y., Nan W., Amano N. Reappraisal of the fine structure of Alzheimer's neurofibrillary tangles. Acta Neuropathol. 1981;54(3):239–246. doi: 10.1007/BF00687747. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Gaskin F., Terry R. D. Immunocytochemical studies of neurofibrillary tangles. Am J Pathol. 1981 Jul;104(1):77–89. [PMC free article] [PubMed] [Google Scholar]
- Zingsheim H. P., Herzog W., Weber K. Differences in surface morphology of microtubules reconstituted from pure brain tubulin using two different microtubule-associated proteins: the high molecular weight MAP 2 proteins and tau proteins. Eur J Cell Biol. 1979 Jun;19(2):175–183. [PubMed] [Google Scholar]