Abstract
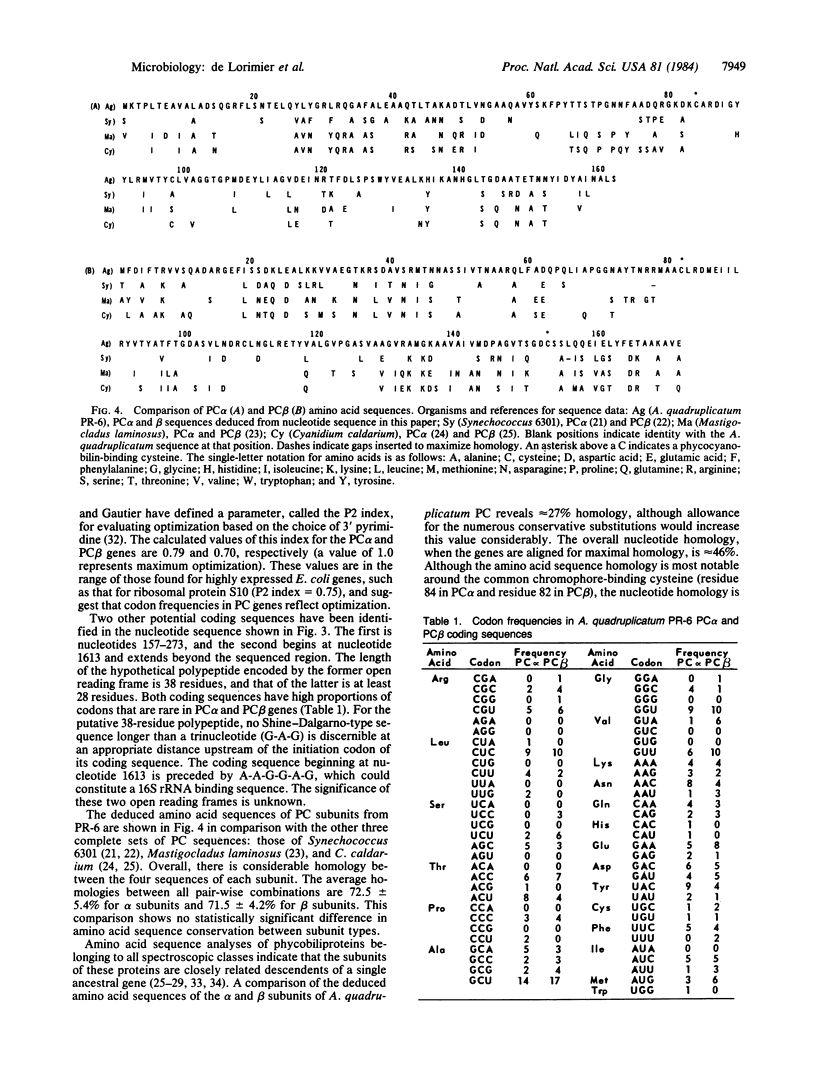
Phycocyanin (PC) is a light-harvesting protein common to blue-green and red algae. We have isolated the genes for the two apoprotein subunits, alpha and beta, of PC from the blue-green alga Agmenellum quadruplicatum PR-6. We synthesized eight sense-strand tetradecameric oligonucleotide probes that could encode a particular pentapeptide in PC alpha from A. quadruplicatum. Only one probe hybridized with total RNA from this organism. This oligonucleotide showed homology to a unique restriction fragment when used to probe Southern blots of A. quadruplicatum DNA. The probe-homologous 3.1-kilobase pair HindIII fragment was cloned. The nucleotide sequence of a 1.7-kilobase pair segment of this clone was determined. Two open reading frames are contained in this region, which correspond in deduced amino acid sequence to PC alpha and PC beta subunits. Both coding sequences are in the same orientation, separated by 105 base pairs, with the PC beta gene 5' to the PC alpha gene. Each gene has a Shine-Dalgarno-type sequence near the initiation codon. Codon frequencies in the two genes may be correlated with the abundance of their products. The deduced amino acid sequences of the gene products show considerable homology with the alpha and beta PC subunits from other species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belford H. S., Offner G. D., Troxler R. F. Phycobiliprotein synthesis in the unicellular rhodophyte, Cyanidium caldarium. Cell-free translation of the mRNAs for the alpha and beta subunit polypeptides of phycocyanin. J Biol Chem. 1983 Apr 10;258(7):4503–4510. [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Plasmid transformation in Agmenellum quadruplicatum PR-6: construction of biphasic plasmids and characterization of their transformation properties. J Bacteriol. 1983 Jun;154(3):1446–1450. doi: 10.1128/jb.154.3.1446-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad B., Mount D. W. Microcomputer programs for DNA sequence analysis. Nucleic Acids Res. 1982 Jan 11;10(1):31–38. doi: 10.1093/nar/10.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G., Sidler W., Widmer H., Zuber H. The complete amino acid sequence of both subunits of C-phycocyanin from the cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1491–1507. doi: 10.1515/bchm2.1978.359.2.1491. [DOI] [PubMed] [Google Scholar]
- Freidenreich P., Apell G. S., Glazer A. N. Structural studies on phycobiliproteins II. C-phycocyanin: amino acid sequence of the beta subunit. Specific cleavage of the alpha subunit. J Biol Chem. 1978 Jan 10;253(1):212–219. [PubMed] [Google Scholar]
- Gardner E. E., Stevens S. E., Jr, Fox J. L. Purification and characterization of the C-phycocyanin from Agmenellum quadruplicatum. Biochim Biophys Acta. 1980 Jul 24;624(1):187–195. doi: 10.1016/0005-2795(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Giddings T. H., Wasmann C., Staehelin L. A. Structure of the Thylakoids and Envelope Membranes of the Cyanelles of Cyanophora paradoxa. Plant Physiol. 1983 Feb;71(2):409–419. doi: 10.1104/pp.71.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Phycobilisomes: structure and dynamics. Annu Rev Microbiol. 1982;36:173–198. doi: 10.1146/annurev.mi.36.100182.001133. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Jay E., MacKnight D., Lutze-Wallace C., Harrison D., Wishart P., Liu W. Y., Asundi V., Pomeroy-Cloney L., Rommens J., Eglington L. Chemical synthesis of a biologically active gene for human immune interferon-gamma. Prospect for site-specific mutagenesis and structure-function studies. J Biol Chem. 1984 May 25;259(10):6311–6317. [PubMed] [Google Scholar]
- Lau R. H., MacKenzie M. M., Doolittle W. F. Phycocyanin synthesis and degradation in the blue-green bacterium Anacystis nidulans. J Bacteriol. 1977 Dec;132(3):771–778. doi: 10.1128/jb.132.3.771-778.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega F. G., Dieckmann C. L., Tzagoloff A. A rapid method for detecting specific RNA transcripts by hybridization to DNA probes in solution. Anal Biochem. 1983 May;131(1):141–145. doi: 10.1016/0003-2697(83)90145-8. [DOI] [PubMed] [Google Scholar]
- Offner G. D., Brown-Mason A. S., Ehrhardt M. M., Troxler R. F. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. I. Complete amino acid sequence of the alpha subunit. J Biol Chem. 1981 Dec 10;256(23):12167–12175. [PubMed] [Google Scholar]
- Offner G. D., Troxler R. F. Primary structure of allophycocyanin from the unicellular rhodophyte, Cyanidium caldarium. The complete amino acid sequences of the alpha and beta subunits. J Biol Chem. 1983 Aug 25;258(16):9931–9940. [PubMed] [Google Scholar]
- Paone D. A., Stevens S. E. Nitrogen Starvation and the Regulation of Glutamine Synthetase in Agmenellum quadruplicatum. Plant Physiol. 1981 Jun;67(6):1097–1100. doi: 10.1104/pp.67.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Koths K. E. The blue-green alga agmenellum quadruplicatum contains covalently closed DNA circles. Cell. 1976 Dec;9(4 Pt 1):551–557. doi: 10.1016/0092-8674(76)90037-4. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidler W., Gysi J., Isker E., Zuber H. The complete amino acid sequence of both subunits of allophycocyanin, a light harvesting protein-pigment complex from the cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1981 Jun;362(6):611–628. doi: 10.1515/bchm2.1981.362.1.611. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Porter R. D. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Ehrhardt M. M., Brown-Mason A. S., Offner G. D. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. II. Complete amino acid sequence of the beta subunit. J Biol Chem. 1981 Dec 10;256(23):12176–12184. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Williamson S. E., Doolittle W. F. Genes for tRNAIle and tRNAAla in the spacer between the 16S and 23S rRNA genes of a blue-green alga: strong homology to chloroplast tRNA genes and tRNA genes of the E. coli rrnD gene cluster. Nucleic Acids Res. 1983 Jan 11;11(1):225–235. doi: 10.1093/nar/11.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N. Cyanobacterial phycobilisomes. Role of the linker polypeptides in the assembly of phycocyanin. J Biol Chem. 1982 Apr 10;257(7):3429–3433. [PubMed] [Google Scholar]