Abstract
Background
Horizontal transfer of transposable elements (HTT) is increasingly appreciated as an important source of genome and species evolution in eukaryotes. However, our understanding of HTT dynamics is still poor in eukaryotes because the diversity of species for which whole genome sequences are available is biased and does not reflect the global eukaryote diversity.
Results
In this study we characterized two Mariner transposable elements (TEs) in the genome of several terrestrial crustacean isopods, a group of animals particularly underrepresented in genome databases. The two elements have a patchy distribution in the arthropod tree and they are highly similar (>93% over the entire length of the element) to insect TEs (Diptera and Hymenoptera), some of which were previously described in Ceratitis rosa (Crmar2) and Drosophila biarmipes (Mariner-5_Dbi). In addition, phylogenetic analyses and comparisons of TE versus orthologous gene distances at various phylogenetic levels revealed that the taxonomic distribution of the two elements is incompatible with vertical inheritance.
Conclusions
We conclude that the two Mariner TEs each underwent at least three HTT events. Both elements were transferred once between isopod crustaceans and insects and at least once between isopod crustacean species. Crmar2 was also transferred between tephritid and drosophilid flies and Mariner-5 underwent HT between hymenopterans and dipterans. We demonstrate that these various HTTs took place recently (most likely within the last 3 million years), and propose iridoviruses and/or Wolbachia endosymbionts as potential vectors of these transfers.
Keywords: Horizontal transfer, Transposable elements, Isopod crustaceans, Hexapods
Background
Horizontal transfer (HT) of genetic material is the transmission of DNA between non-mating organisms [1]. Most known eukaryote-to-eukaryote HT events are transfers of transposable elements (TEs) [2]. Given the profound impact TEs have on the genome architecture of their hosts, HT of TEs (HTT) is increasingly recognized as an important force in eukaryote genome evolution [3]. On the TE side, spreading between genomes via HT may be viewed as a strategy to escape vertical extinction due to purifying selection, mutational decay and/or host defense mechanisms. Among the over 330 cases of eukaryote-to-eukaryote HTT events characterized so far, the vast majority involve DNA transposons (n = 188 cases) and LTR retrotransposons (n = 118 cases) [4], indicating that the long-term survival of these TEs may rely more on HT than that of non-LTR retrotransposons. Yet, while whole genomes are sequenced at an exponential pace, the global diversity of eukaryote genomes is still poorly represented, precluding any strong generalization on HTT dynamics. Even in animals, whole genome sequencing efforts are biased towards species closely related to model organisms or species of economic interest, and whole genome sequences are lacking for many large taxonomic groups. Our current understanding of the global HTT dynamics and impacts is therefore incomplete, both at the host and TE level.
With only one genome fully sequenced [5] out of over 50,000 species described [6], crustaceans are particularly underrepresented in genome databases. The order Isopoda (Vericrustacea clade according to [7]) is unique among crustaceans in that the colonization of landmasses by one of its lineages (belonging to the suborder Oniscidea) during the Mesozoic yielded a large diversity of terrestrial species (>3,600 [8]) now distributed all over the world in every biotope (except for the poles) [9]. In this study we report new cases of HTT involving terrestrial isopod crustaceans and hexapods. We used a combination of cross-species PCR screening of TEs, phylogenetic and other evolutionary analyses to characterize in detail these HTT and to shed light on the evolutionary dynamics of the first two TEs described in isopod crustaceans.
Results and discussion
Characterization of two Mariner elements in the isopod crustacean Armadillidium vulgare
In order to detect TEs that underwent horizontal transfer between isopod crustaceans and other taxa, we used all consensus sequences deposited in Repbase [10] as of May 2013 as queries to perform BLASTn searches on draft genomic contigs and on a transcriptome of the pill bug Armadillidium vulgare that have been generated in our lab as part of other ongoing projects. Importantly, the contigs generated by these projects are too short to carry out a comprehensive de novo mining of A. vulgare TEs. The BLASTn searches yielded two TEs belonging to the Tc1/Mariner superfamily of Class II DNA transposons that show more than 90% identity over more than 500 bp to A. vulgare sequences. The first one (Crmar2) was originally characterized in the tephritid fly Ceratitis rosa based on a PCR/sequencing screening [11], and the second one (Mariner-5_Dbi) was described by Kojima and Jurka [12] in Drosophila biarmipes based on whole genome sequence data mining. We reconstructed an A. vulgare consensus sequence of both elements (named Crmar2_Avul and Mariner-5_Avul) using 100 to 1300 bp-long fragments resulting from our various BLAST outputs, such that the entire sequence of the consensus was covered by at least five different copies. Crmar2_Avul is 1304 bp in length, has 39-bp terminal inverted repeats (TIRs) and encodes a 361 amino acid (aa) transposase while Mariner-5_Avul is 1013 bp in length, has 28-bp TIRs and encodes a 200 aa transposase. Both elements are flanked by TA target site duplications, which is characteristic of the Tc1/Mariner superfamily [13]. The evolution of this superfamily has yielded a large number of elements which have colonized the genome of many eukaryote taxa [14,15] and have been classified in various subfamilies (for example, [16]). Crmar2 belongs to the rosa subfamily [11] and our phylogenetic analysis of the transposase revealed that Mariner-5_Dbi belongs to the irritans subfamily [see Additional file 1: Figure S1].
Taxonomic distribution of the two Mariner elements in eukaryotes
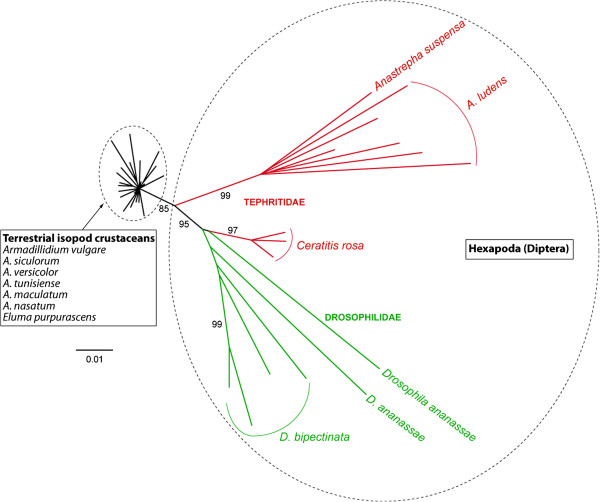
Next we sought to assess the taxonomic distribution of Crmar2_Avul and Mariner-5_Avul in eukaryotes by performing BLASTn searches on all eukaryotic genomes that were available in Genbank as of May 2013. In addition to the species in which the two elements had previously been described (C. rosa, Anastrepha ludens and Anastrepha suspensa for Crmar2; D. biarmipes for Mariner-5_Dbi) we found TEs highly similar (>90% identity over >500 bp) to Crmar2_Avul in Drosophila ananassae and Drosophila bipectinata, and to Mariner-5_Avul in the ant Harpegnathos saltator. Interestingly, the taxonomic distribution of the two elements is patchy, not only at the level of the arthropod phylogeny, but also within the lower level taxa in which we found them (Figure 1), a pattern likely indicative of horizontal transfer [17]. For example, Crmar2_Avul is only present in two closely related Drosophila species out of the 13 that we searched, and Mariner-5_Avul was identified only in one of the three hymenopteran genomes available.
Figure 1.
Timetree of Arthropoda. The tree includes all species in which Crmar2 and Mariner-5 were found as well as most closely related species for which whole genome sequences are available in Genbank. Phylogenetic relationships are taken from Tamura et al.[18], Regier et al. [19], Sharkey [20] and Regier et al. [7]. Divergence times are taken from Hedges et al.[21]. Divergence times between D. bipectinata and D. ananassae, between D. biarmipes and other Drosophila species, between Dendroctonus ponderosae and Tribolium castaneum, between A. vulgare and Daphnia pulex, and between H. saltator and Nasonia vitripennis are unknown and have therefore been set at arbitrary values for illustrative purposes.
Horizontal transfer of the two Mariner elements between hexapods and isopods and within hexapods
To formally assess whether the distribution of the two elements in arthropods is the result of HT, we compared TE genetic distances calculated between hexapod species and A. vulgare to distances calculated for 46 orthologous genes available for both A. vulgare and Drosophila melanogaster[7]. As illustrated in Figure 2, distances between orthologous genes (average = 35%, min = 21%, and max = 49%) are much higher than distances between TEs (average = 6%, min = 4.9%, and max = 7.4%). Under vertical transmission of the TEs, TE distances between taxa are expected to be higher than distances between orthologous genes because TEs are known to evolve neutrally after insertion in a given genome [22], that is, faster than host genes that evolve under purifying selection due to functional constraints. The high similarity between A. vulgare and hexapoda TEs coupled with the deep divergence time between these two arthropod taxa (>400 million years) and to the patchy distribution of the two elements in arthropods allows us to confidently conclude that the presence of both Crmar2 and Mariner-5 in isopod crustaceans and hexapods results from HT. Interestingly, we found that gene distances between tephritid (Ceratitis capitata) and drosophilid (D. melanogaster) flies on one hand (average = 27%, min = 16%, and max = 45%) and between Drosophila and the ant H. saltator on the other hand (average = 34%, min = 18%, and max = 56%) are also much higher than TE distances (4% for both elements; Figure 2). This pattern suggests that in addition to transferring horizontally between hexapods and isopods, Crmar2 also underwent HT between the two dipteran lineages and Mariner-5 also transferred horizontally between dipterans and hymenopterans.
Figure 2.
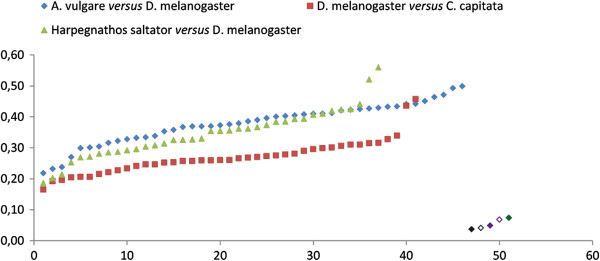
Graph illustrating the pairwise corrected distances between arthropod orthologous genes and betweenCrmar2andMariner-5consensus sequences. Orthologous gene distances between A. vulgare and D. melanogaster, between D. melanogaster and C. capitata, and between H. saltator and D. melanogaster are illustrated with blue lozenges, red squares, and green triangles, respectively. Other symbols correspond to transposable element (TE) distances: Mariner-5 between H. saltator and D. biarmipes (filled black lozenge), Crmar2 between C. rosa and D. bipectinata (empty black lozenge), Mariner-5 between A. vulgare and D. biarmipes (filled purple lozenge), Crmar2 between A. vulgare and C. rosa (empty purple lozenge), and Crmar2 between A. vulgare and D. bipectinata (filled green lozenge). A detailed list of genes and distances is provided in Additional file 8: Table S3. Before the distance values were plotted, they were sorted by ascending order.
Recent horizontal transfer of the two Mariner elements within isopods
To shed light on the evolutionary dynamics of Crmar2_Avul and Mariner-5_Avul in terrestrial isopod crustaceans we carried out two sets of PCR screenings in 14 species. The first screening involved primer pairs designed in the internal region of the elements in order to check for the presence of each TE in the various species (type 1 primers in Additional file 2: Figure S2). The second screening aimed at finding specific copies of the two elements that would be shared at orthologous loci between the various isopod species. For the latter screen we used primer pairs for which one primer was designed in the 5’ or 3’ end of Crmar2_Avul and Mariner-5_Avul and the other primer was designed in the flanking region of several copies of each element (type 2 primers in Additional file 2: Figure S2; n = 4 for Crmar2_Avul and 3 for Mariner-5_Avul). The first screening (internal primers) uncovered Crmar2_Avul and Mariner-5_Avul, respectively, in six and nine of the 14 species (Figure 3). The second screening (search for orthologous copies) did not reveal any shared copies between A. vulgare and any of the other 13 isopod species. This absence of amplification could be due to a lack of conservation of the regions flanking the various copies in the different species. But perhaps more interestingly, we also noticed that two of the four Crmar2 copies failed to amplify in one of the two A. vulgare individuals in which we searched them, suggesting that they are polymorphic in terms of presence/absence in A. vulgare populations. Overall, these data indicate that both elements likely underwent recent HT in isopods and may still be active.
Figure 3.
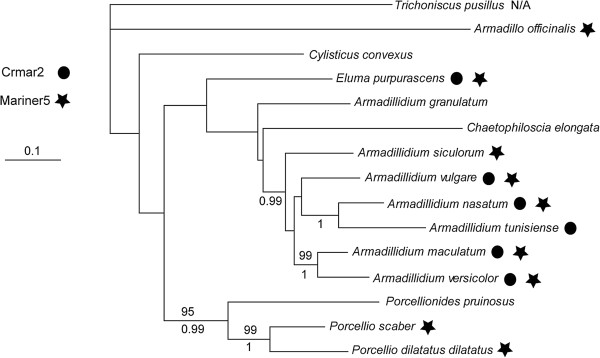
Phylogenetic tree of the various terrestrial isopod crustacean species included in this study. The topology of the tree corresponds to the consensus of the bootstrap analysis performed under the maximum likelihood criteria. Bootstrap values above 70% and Bayesian posterior probabilities above 0.9 are shown above and below branches respectively. Species in which Crmar2 and/or Mariner-5 were uncovered by PCR are marked with a filled circle and/or a star. Trichoniscus pusillus was used as an outgroup to root the present phylogeny based on the topology obtained in Michel-Salzat and Bouchon [23] and was not screened for the presence of the two TEs (N/A: not applicable). The other species were PCR screened but none of the two elements were amplified.
To further test the possibility that both elements invaded isopod genomes recently via HT and are still actively transposing, we cloned and sequenced two to five different copies of Crmar2_Avul and Mariner-5_Avul in all isopod species in which we found them (except for Eluma purpurascens in which all ten clones that we sequenced contained an identical copy of Crmar2). Pairwise genetic distances between these copies within each genome are all very low (average = 1.2%, min = 0.9%, and max = 2% for Crmar2_Avul and average = 4.5%, min = 2%, and max = 6% for Mariner-5_Avul). In addition, the between-species distances for both TEs are also much lower than distances calculated for the androgenic gland hormone gene (average = 34%; Figure 4). Following the same reasoning as for the comparisons between TE and orthologous gene distances discussed above, we believe these results strongly suggest that both Crmar2_Avul and Mariner-5_Avul underwent one or more recent HTs within isopods. Interestingly, using RT-PCR, we verified that Crmar2_Avul and Mariner-5_Avul are transcribed both in somatic and germ cells in A. vulgare [see Additional file 3: Figure S3]. Furthermore, seven of the eight Crmar2_Avul transcripts that we uncovered in the A. vulgare transcriptome contain a full-length and intact (devoid of non-sense mutations) ORF (sequences provided in Additional file 4: Dataset 1), suggesting that at least one source of functional transposase is transcribed for this element in A. vulgare.
Figure 4.
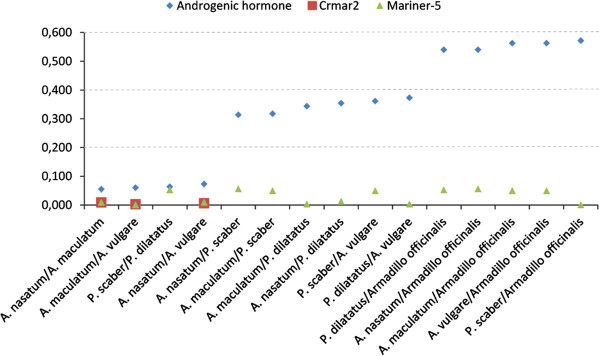
Graph illustrating the pairwise corrected distances between the androgenic gland hormone gene and between Crmar2 and Mariner - 5 consensus sequences within terrestrial isopods.
To provide an estimate of the absolute age of the activity burst of Mariner-5 and Crmar2, we divided the average copy/consensus distance calculated for each element in D. biarmipes (11%) and D. bipectinata (6.9%) by the experimentally derived neutral substitution rate of D. melanogaster (0.0346 substitutions per base per million years (myr); [24-26]). This yielded a burst age of 3.2 myrs for Mariner-5 in D. biarmipes and 2 myrs for Crmar2 in D. bipectinata. The age of Mariner-5_Avul and Crmar2_Avul cannot be precisely estimated because nuclear substitution rates are not available for isopods. Together with the absence of shared orthologous copies of both elements between the various isopods species and the seemingly polymorphic state of Crmar2 in A. vulgare, the fact that an intact Crmar2_Avul transposase is transcribed in this species is consistent with a recent invasion of isopod genomes by Mariner-5_Avul and Crmar2_Avul and suggest both elements are active sources of genomic variation in this major crustacean group. In addition, given that isopod Crmar2 and Mariner-5 are highly similar to Drosophila Crmar2 and Mariner-5 (95% and 93% identity, respectively; Figure 2), we believe these HTTs most likely took place within the past few million years at most.
Number of horizontal transfer of transposon events
To assess the number of HTTs that occurred between hexapods and crustacean isopods and within each of the two taxa, we reconstructed a phylogeny of both elements based on an alignment including all copies of Crmar2 and Mariner-5 that we sequenced from the various isopod species and those that we found in the other sequenced arthropod genomes. In the resulting Crmar2 tree both hexapod and isopod Crmar2 elements are monophyletic (Figure 5). Therefore, a single HTT event between isopods and hexapods needs to be inferred to explain the taxonomic distribution of this element in arthropods. Within hexapods, Crmar2 TEs from the tephritid C. rosa are more closely related to Drosophila elements than they are to the other tephritid elements found in A. ludens and A. suspensa by Gomulski et al.[11]. This topology upholds our TE versus orthologous gene distance analysis (see above), indicating that Crmar2 also underwent HT within dipterans. In the Mariner-5 tree (Figure 6), isopod elements fall within two relatively distantly related clusters. However, given that the tree is unrooted, we cannot conclude on whether Mariner-5 was transferred once or more than once between hexapods and isopods. Interestingly, the topology of isopod Mariner-5 copies (Figure 6) is clearly incongruent with that of the isopod tree (Figure 3). For example, Porcellio dilatatus dilatatus and Porcellio scaber form a strongly supported clade in the species phylogeny, but Crmar2 copies from the former fall within the cluster that groups all Armadillidium and Eluma purpurascens copies and those of P. scaber group with Crmar2 copies from Armadillo officinalis. This phylogenetic incongruence between host and TE phylogenies, together with the general lack of phylogenetic resolution within isopod Crmar2 and Mariner-5 clusters (Figures 5 and 6), and the fact that copies from each isopod species do not form monophyletic groups, further supports the HT of both elements between the various isopod species.
Figure 5.
Phylogenetic tree ofCrmar2copies. Maximum likelihood bootstrap values above 70% are shown on branches. Given the absence of phylogenetic support for the branching of Crmar2 copies within isopods, the name of the species is shown only once in the black rectangle to facilitate the reading of the figure.
Figure 6.

Phylogenetic tree ofMariner-5copies. Maximum likelihood bootstrap values above 70% are shown on branches. Given the absence of phylogenetic support for the branching of Mariner-5 copies within the two clusters of isopod sequences, the name of the species is shown only once in the two black rectangles to facilitate the reading of the figure.
Potential vectors of horizontal transfer
Though the question of the mechanisms and vectors underlying HTT between multicellular eukaryotes remains largely open, growing evidence suggests that host-parasite relationships likely facilitate such transfers [27-31]. In particular, viruses are often cited as ideal HTT vectors due to their capacity to inject DNA/RNA into host cells [32-35]. Though the viral fauna infecting the various species involved in Crmar2 and Mariner-5 HTT is poorly known, it is noteworthy that members of the Iridoviridae have been found in several species of dipterans, hymenopterans and terrestrial isopods [36,37]. In addition, a recent study identified two TEs inserted in the genome of an iridovirus infecting dipteran [38], emphasizing the potential of this type of viruses to shuttle transposons between their hosts. Another possible route for HTT to occur in arthropods is via transfers of endosymbiotic bacteria. Several species of isopods as well as D. ananassae, D. bipectinata and A. suspensa are known to bear intracellular, maternally transmitted alphaproteobacteria called Wolbachia[39-43]. The fact that isopod Wolbachia strains are known to have undergone several HT [44] and that several genes of eukaryotic origin have been found integrated in Wolbachia genomes [45-47] suggest that endosymbionts could also facilitate HT of DNA between hosts.
Conclusions
In this study, we have characterized the evolutionary dynamics of two Tc1/Mariner elements in isopod crustaceans and shown that their current taxonomic distribution in arthropods results from at least one HT between hexapods and isopods as well as one or more HTs within isopods. Furthermore, we have demonstrated that Crmar2 transferred horizontally between drosophilid and tephritid flies and that Mariner-5 underwent HT between Diptera and Hymenoptera. Conservatively, and assuming that Crmar2 and Mariner-5 transferred horizontally only once within the isopods, we have uncovered a total of six new HTT events in this study. Together with 70 previously known cases (for example, [48-50]; reviewed in [4]) our results bring to 76 the number of HT events described in metazoans for the Tc1/Mariner superfamily, further emphasizing the indifference of these elements to host factors to transpose [51]. Of note, HT of two other Mariner elements have previously been characterized in marine crustaceans [52] (one between two decapods and one between a decapod and an amphipod), but our study is the first to report HTT involving terrestrial crustaceans. Finally, we have shown that these newly described HTT events most likely took place within the last 3 myrs, and we propose iridoviruses and/or Wolbachia endosymbionts as the potential vectors of transfer, a hypothesis that will be interesting to test in future studies.
Methods
Mining of available eukaryote genomes
In order to identify transposable elements similar to Crmar2 and Mariner-5_Dbi that could have been horizontally transferred between isopods and other taxa we used the nucleotide sequence of the two elements to carry out BLASTn searches against the nr (non-redundant nucleotide), EST (expressed sequence tag) and WGS (whole genome sequence) databases available on the NCBI website. We considered only those elements that showed more than 90% nucleotide identity over more than 500 bp of our query sequences.
DNA extraction, PCR, cloning, and sequencing
Genomic DNA was extracted from 14 species of terrestrial isopods (Figure 3) using the Qiagen™ DNeasy blood and tissue extraction kit (Hilden, Germany). PCRs were carried out using four types of primer pairs: 1) one pair designed on the internal region of Mariner-5_Avul and Crmar2_Avul, 2) three and four pairs designed to screen specific copies of the two elements at orthologous position in the 14 isopod species, 3) one pair designed to amplify the mitochondrial cytochrome oxidase I (Co1) for the six species for which this gene is not available in Genbank, and 4) one pair designed to amplify the mitochondrial 16S gene for all species included in this study except for T. pusillus (taken from Genbank). The list of PCR primers used in this study, together with their respective melting temperatures, is given in Additional file 5: Table S1. PCR reactions were conducted using the following temperature cycling: initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 52 to 58°C (depending on the primer pair) for 30 s, and elongation at 72°C for 45 sec, ending with a 10 min elongation step at 72°C. PCR products obtained with the Co1 primers were purified and directly sequenced using ABI BigDye sequencing mix (1.4 μl template PCR product, 0.4 μl BigDye, 2 μl manufacturer supplied buffer, 0.3 μl primer, and 6 μl H2O). Sequencing reactions were ethanol precipitated and run on an ABI 3730 sequencer. PCR products obtained with the primers internal to Crmar2_Avul and Mariner-5_Avul were cloned into pGEM-T easy vector (Promega, USA, Madison, WI) and several clones were Sanger-sequenced as described above until we obtained five different copies of each element in the various species in which we found them.
Sequencing of androgenic gland hormone (Agh) cDNA
Total RNA was isolated from androgenic glands of fifteen males (6 glands per individual) using the RNeasy Mini kit (Qiagen, Hilden, Germany). The cDNA was synthesized using the M-MLV-RT kit (Promega). PCR amplification was performed using several degenerated primer pairs designed on the consensus sequences of Agh cDNAs of A. vulgare, P. scaber and P.dilatatus [see Additional file 6: Table S2] [53]. PCR and direct sequencing were performed as described above.
RT-PCR
The expression of Crmar2_Avul and Mariner-5_Avul was assessed in both dissected ovaries and somatic tissues (head + nervous chain) of A. vulgare females using the SuperScript™ III First-Strand Synthesis System for RT-PCR» (Invitrogen, Eugene, OR, USA).
Transposon distances versus gene distances
All Crmar2 and Mariner-5 consensus sequences reconstructed in this study are provided in Additional file 7: Dataset 2. In order to test whether Crmar2 and Mariner-5 TEs were inherited vertically or horizontally we compared the distances calculated between TE consensus sequences and between several orthologous genes for several pairs of taxa. To calculate gene distances between Isopoda and Hexapoda, we used the 57 A. vulgare genes sequenced by Regier et al. [7] as queries to perform BLASTn searches against the D. melanogaster genome. We chose the D. melanogaster genome rather than the genome of Drosophila species involved in the HTT characterized in this study because it is the most completely sequenced and best assembled Drosophila genome available. We found 46 A. vulgare orthologs in D. melanogaster, which we aligned at the nucleotide level between the two species. For the Ceratitis/Drosophila gene distances we used the 46 genes resulting from the above search as queries to perform BLASTn searches against the whole genome sequence of C. capitata. This search yielded 41 genes that we aligned at the nucleotide level with those of D. melanogaster. The same approach was used to find genes orthologous between D. melanogaster and H. saltator which resulted in the alignment of 37 genes. Genetic distances between Crmar2 and Mariner-5 consensus sequences as well as between each pair of orthologous genes were calculated using the Jukes Cantor model in MEGA 5 [54]. The name of all genes and the distances between them are provided in Additional file 8: Table S3. Jukes Cantor distances were also calculated between the various copies of both TEs found within each genome in which we found them.
Phylogenetic analyses
The phylogeny of the 14 terrestrial isopod species understudy was reconstructed using 16S, Co1, and Agh sequences. All sequences produced in this study were deposited in Genbank under accession numbers KF957774-KF957833. Each gene was aligned manually using BioEdit 7.0.5.3 [55], and ambiguous regions were removed. All alignments are provided in Additional file 9: Dataset 3. A bootstrapped neighbor joining phylogeny was first reconstructed using MEGA 5 for each alignment with the maximum likelihood distance option. Given that no incongruence supported by bootstrap values >50% was observed between the three resulting trees (not shown), we then concatenated the three alignments and reconstructed a bootstrapped maximum likelihood phylogeny of the three combined markers using PhyML 3 [56]. A bayesian analysis of this alignment was also performed using MrBayes [57] in order to obtain posterior probabilities for each node of the tree. The model of nucleotide evolution best fitting the combined alignment (GTR + I + G) and used for the phylogenetic analyses was chosen based on the Akaike information criterion (AIC) in jModeltest 2 [58].
The phylogeny of Crmar2 and Mariner-5 was reconstructed based on alignments of all different copies of each element from each species in which we found them. Ambiguous regions and regions absent in more than 25% of the sequences were removed. Alignments are provided in Additional file 9: Dataset 3. The model of nucleotide evolution best fitting each alignment (TPM1uf + G for both elements) was chosen based on the Akaike information criterion (AIC) in jModeltest 2 and each alignment was analyzed using PhyML 3. In order to assess the phylogenetic position of Mariner-5_Dbi in the mariner tree, we have aligned the amino acid sequence of a transposase representative of most described mariner subfamily and performed a neighbor joining analysis using MEGA 5 (JTT model, 1000 bootstrap replicates). The accession numbers of the sequences we have used are provided in Additional file 1: Figure S1.
Abbreviations
HTT: horizontal transfer of transposons; LTR: long terminal repeat; myr: million year; RT-PCR: reverse-transcription polymerase chain reaction; TE: transposable element.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MD and CG designed the study, carried out the experiments and analyses and drafted the manuscript. SL generated the draft assembly of the Armadillidium vulgare genome and contributed to the writing of the manuscript. NC sequenced the androgenic gland hormone gene in the various isopod species and contributed to the writing of the manuscript. DB provided the draft transcriptome assembly of A. vulgare and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Phylogenetic relationships between various mariner transposases showing that Mariner-5_Dbi belongs to the irritans subfamily.
Illustration of the position of the two types of primer sets we used to screen for Mariner-5_Avul and Crmar2_Avul elements in the various isopod species. The sequence of the primers is provided in Additional file 5: Table S1. TIR: Terminal inverted repeat.
Pictures of agarose gels showing the results of the reverse transcription PCR experiments on Crmar2 (A) and Mariner-5(B) in Armadillidium vulgare ovaries and somatic tissues (head + nervous chain). A band of the expected size was obtained for all reactions showing that both elements are transcribed in A. vulgare soma and germ line. RT, reverse transcriptase; L, size ladder.
Sequences of the Crmar2 transcripts encoding a full length, intact, and thus potentially functional transposase aligned together with the Crmar2_Avul consensus sequence (fasta format).
List of primers used to amplify and sequence Crmar2 and Mariner-5 elements.
List of primers used to amplify and sequence the androgenic gland hormone.
Consensus sequences of Crmar2 and Mariner-5 elements reconstructed in this study (fasta format).
List of genes sequenced by Regier et al. [7] used to calculate genetic distances between the various species included in this study.
Sequence alignments used to reconstruct the phylogeny of crustacean isopod species and Crmar2 and Mariner-5 copies (fasta format).
Contributor Information
Mathilde Dupeyron, Email: ma.dupeyron@orange.fr.
Sébastien Leclercq, Email: sebastien.leclercq@univ-poitiers.fr.
Nicolas Cerveau, Email: nicolas.cerveau@geo.uni-goettingen.de.
Didier Bouchon, Email: didier.bouchon@univ-poitiers.fr.
Clément Gilbert, Email: clement.gilbert@univ-poitiers.fr.
Acknowledgements
We thank all the technical staff of the UMR CNRS 7267. We acknowledge Catherine Debenest for assistance with the dissection of terrestrial isopods and Richard Cordaux and Pierre Grève for insightful comments on an earlier version of the manuscript. This work was supported by the CNRS, the Agence Nationale de la Recherche (ANR ImmunSymbArt 10-BLAN-1701), and a European Research Council Starting Grant (FP7/2007-2013, grant 260729 EndoSexDet) to Richard Cordaux.
References
- Keeling PJ, Palmer JD. Horizontal gene transfer in eukaryotic evolution. Nat Rev Genet. 2008;9:605–618. doi: 10.1038/nrg2386. [DOI] [PubMed] [Google Scholar]
- Schaack S, Gilbert C, Feschotte C. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol Evol. 2010;25:537–546. doi: 10.1016/j.tree.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivancevic AM, Walsh AM, Kortschak RD, Adelson DL. Jumping the fine LINE between species: Horizontal transfer of transposable elements in animals catalyses genome evolution. Bioessays. 2013;35:1071–1082. doi: 10.1002/bies.201300072. [DOI] [PubMed] [Google Scholar]
- Wallau GL, Ortiz MF, Loreto EL. Horizontal transposon transfer in eukarya: detection, bias, and perspectives. Genome Biol Evol. 2012;4:689–699. doi: 10.1093/gbe/evs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Cáceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, Fröhlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kültz D, Laforsch C, Lindquist E, Lopez J. et al. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JW, Davis GE. An updated classification of the recent Crustacea. Science Series 39. Los Angeles, CA: Natural History Museum of Los Angeles County; 2001. [Google Scholar]
- Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature. 2010;463:1079–1083. doi: 10.1038/nature08742. [DOI] [PubMed] [Google Scholar]
- Schmalfuss H. World catalog of terrestrial isopods (Isopoda: Oniscidea) Stuttgart; 2003. p. 341. (Stuttgarter Beitrage zur Naturkunde Serie A). Serie A, Nr. 654. [Google Scholar]
- Broly P, Deville P, Maillet S. The origin of terrestrial isopods (Crustacea: Isopoda: Oniscidea) Evol Ecol. 2013;27:461–476. doi: 10.1007/s10682-012-9625-8. [DOI] [Google Scholar]
- Jurka J. Repbase update: a database and an electronic journal of repetitive elements. Trends Genet. 2000;16:418–420. doi: 10.1016/S0168-9525(00)02093-X. [DOI] [PubMed] [Google Scholar]
- Gomulski LM, Torti C, Bonizzoni M, Moralli D, Raimondi E, Capy P, Gasperi G, Malacrida AR. A new basal subfamily of Mariner elements in Ceratitis rosa and other tephritid flies. J Mol Evol. 2001;53:597–606. doi: 10.1007/s002390010246. [DOI] [PubMed] [Google Scholar]
- Kojima KK, Jurka J. DNA transposons from the Drosophila biarmipes genome. Repbase Reports. 2012;12:740. [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, Paux E, SanMiguel P, Schulman AH. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8:973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Hartl DL, Lohe AR, Lozovskaya ER. Modern thoughts on an ancyent marinere: function, evolution, regulation. Annu Rev Genet. 1997;31:337–358. doi: 10.1146/annurev.genet.31.1.337. [DOI] [PubMed] [Google Scholar]
- Plasterk RH, Izsvák Z, Ivics Z. Resident aliens: the Tc1/mariner superfamily of transposable elements. Trends Genet. 1999;15:326–332. doi: 10.1016/S0168-9525(99)01777-1. [DOI] [PubMed] [Google Scholar]
- Rouault JD, Casse N, Chénais B, Hua-Van A, Filée J, Capy P. Automatic classification within families of transposable elements: application to the mariner Family. Gene. 2009;448:227–232. doi: 10.1016/j.gene.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Silva JC, Loreto EL, Clark JB. Factors that affect the horizontal transfer of transposable elements. Curr Issues Mol Biol. 2004;6:57–71. [PubMed] [Google Scholar]
- Tamura K, Subramanian S, Kumar S. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol. 2004;21:36–44. doi: 10.1093/molbev/msg236. [DOI] [PubMed] [Google Scholar]
- Regier JC, Shultz JW, Kambic RE. Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc Biol Sci. 2005;272:395–401. doi: 10.1098/rspb.2004.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey MJ. Phylogeny and classification of hymenoptera. Zootaxa. 2007;1668:521–548. [Google Scholar]
- Hedges SB, Dudley J, Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- Lampe DJ, Witherspoon DJ, Soto-Adames FN, Robertson HM. Recent horizontal transfer of mellifera subfamily Mariner transposons into insect lineages representing four different orders shows that selection acts only during horizontal transfer. Mol Biol Evol. 2003;20:554–562. doi: 10.1093/molbev/msg069. [DOI] [PubMed] [Google Scholar]
- Michel-Salzat A, Bouchon D. Phylogenetic analysis of mitochondrial LSU rRNA in oniscids. C R Acad Sci III. 2000;323:827–837. doi: 10.1016/S0764-4469(00)01221-X. [DOI] [PubMed] [Google Scholar]
- Cutter AD. Divergence times in Caenorhabditis and Drosophila inferred from direct estimates of the neutral mutation rate. Mol Biol Evol. 2008;25:778–786. doi: 10.1093/molbev/msn024. [DOI] [PubMed] [Google Scholar]
- Keightley PD, Trivedi U, Thomson M, Oliver F, Kumar S, Blaxter ML. Analysis of the genome sequences of three Drosophila melanogaster spontaneous mutation accumulation lines. Genome Res. 2009;19:1195–1201. doi: 10.1101/gr.091231.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obbard DJ, Maclennan J, Kim KW, Rambaut A, O’Grady PM, Jiggins FM. Estimating divergence dates and substitution rates in the Drosophila phylogeny. Mol Biol Evol. 2012;29:3459–3473. doi: 10.1093/molbev/mss150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck MA, Clark JB, Peterson KR, Kidwell MG. Possible horizontal transfer of Drosophila genes by the mite Proctolaelaps regalis. Science. 1991;253:1125–1128. doi: 10.1126/science.1653453. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Tu Z, Kainoh Y, Honda H, Shono T, Kimura K. Possible horizontal transfer of a transposable element from host to parasitoid. Mol Biol Evol. 2001;18:1952–1958. doi: 10.1093/oxfordjournals.molbev.a003735. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Schaack S, Pace JK II, Brindley PJ, Feschotte C. A role for host-parasite interactions in the horizontal transfer of transposons across phyla. Nature. 2010;464:1347–1350. doi: 10.1038/nature08939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraku S, Qiu H, Meyer A. Horizontal transfers of Tc1 elements between teleost fishes and their vertebrate parasites, lampreys. Genome Biol Evol. 2012;4:929–936. doi: 10.1093/gbe/evs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardena BK, Minchella DJ, DeWoody JA. Hosts, parasites, and horizontal gene transfer. Trends Parasitol. 2013;29:329–338. doi: 10.1016/j.pt.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Routh A, Domitrovic T, Johnson JE. Host RNAs, including transposons, are encapsidated by a eukaryotic single-stranded RNA virus. Proc Natl Acad Sci U S A. 2012;109:1907–1912. doi: 10.1073/pnas.1116168109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle JA, Fritsch E, Nickel A, Huber J, Backhaus H. TCl4.7: a novel lepidopteran transposon found in Cydia pomonella granulosis virus. Virology. 1995;207:369–379. doi: 10.1006/viro.1995.1096. [DOI] [PubMed] [Google Scholar]
- Jehle JA, Nickel A, Vlak JM, Backhaus H. Horizontal escape of the novel Tc1-like lepidopteran transposon TCp3.2 into Cydia pomonella granulovirus. J Mol Evol. 1998;46:215–224. doi: 10.1007/PL00006296. [DOI] [PubMed] [Google Scholar]
- Piskurek O, Okada N. Poxviruses as possible vectors for horizontal transfer of retroposons from reptiles to mammals. Proc Natl Acad Sci U S A. 2007;104:12046–12051. doi: 10.1073/pnas.0700531104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. Natural invertebrate hosts of iridoviruses (Iridoviridae) Neotrop Entomol. 2008;37:615–632. doi: 10.1590/S1519-566X2008000600001. [DOI] [PubMed] [Google Scholar]
- Lupetti P, Montesanto G, Ciolfi S, Marri L, Gentile M, Paccagnini E, Lombardo BM. Iridovirus infection in terrestrial isopods from Sicily (Italy) Tissue Cell. 2013;45:321–327. doi: 10.1016/j.tice.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Piégu B, Guizard S, Spears T, Cruaud C, Couloux A, Bideshi DK, Federici BA, Bigot Y. Complete genome sequence of invertebrate iridescent virus 22 isolated from a blackfly larva. J Gen Virol. 2013;94:2112–2116. doi: 10.1099/vir.0.054213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn K, Conlon C, Jones M, Quail MA, Holroyd NE, Parkhill J, Blaxter M. Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathog. 2006;2:e94. doi: 10.1371/journal.ppat.0020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon D, Cordaux R, Grève P. In: Insect Symbiosis. 3. Bourtzis K, Miller T, editor. Boca Raton, FL: Taylor and Francis Group LLC; 2008. Feminizing Wolbachia and the evolution of sex determination in isopods; pp. 273–294. [Google Scholar]
- Coscrato VE, Braz AS, Perondini ALP, Selivon D, Marino CL. Wolbachia in Anastrepha fruit flies (Diptera: Tephritidae) Curr Microbiol. 2009;59:295–301. doi: 10.1007/s00284-009-9433-8. [DOI] [PubMed] [Google Scholar]
- Cordaux R, Bouchon D, Grève P. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 2011;27:332–341. doi: 10.1016/j.tig.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Ravikumar H, Prakash BM, Sampathkumar S, Puttaraju HP. Molecular subgrouping of Wolbachia and bacteriophage WO infection among some Indian Drosophila species. J Genet. 2011;90:507–510. doi: 10.1007/s12041-011-0117-3. [DOI] [PubMed] [Google Scholar]
- Cordaux R, Michel-Salzat A, Bouchon D. Wolbachia infection in crustaceans: novel hosts and potential routes for horizontal transmission. J Evol Biol. 2001;14:237–243. doi: 10.1046/j.1420-9101.2001.00279.x. [DOI] [Google Scholar]
- Klasson L, Kambris Z, Cook PE, Walker T, Sinkins SP. Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics. 2009;10:33. doi: 10.1186/1471-2164-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfit M, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL. An ancient horizontal gene transfer between mosquito and the endosymbiotic bacterium Wolbachia pipientis. Mol Biol Evol. 2009;26:367–374. doi: 10.1093/molbev/msn253. [DOI] [PubMed] [Google Scholar]
- Duplouy A, Iturbe-Ormaetxe I, Beatson SA, Szubert JM, Brownlie JC, McMeniman CJ, McGraw EA, Hurst GD, Charlat S, O’Neill SL, Woolfit M. Draft genome sequence of the male-killing Wolbachia strain wBol1 reveals recent horizontal gene transfers from diverse sources. BMC Genomics. 2013;14:20. doi: 10.1186/1471-2164-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM. The mariner transposable element is widespread in insects. Nature. 1993;362:241–245. doi: 10.1038/362241a0. [DOI] [PubMed] [Google Scholar]
- Robertson HM. In: Mobile DNA II. Craig NL, Robert Craigie R, Gellert M, Lambowitz A, editor. Washington, DC: ASM Press; 2002. Evolution of DNA transposons in eukaryotes; pp. 1093–1110. [Google Scholar]
- Lorite P, Maside X, Sanllorente O, Torres MI, Periquet G, Palomeque T. The ant genomes have been invaded by several types of Mariner transposable elements. Naturwissenschaften. 2012;99:1007–1020. doi: 10.1007/s00114-012-0982-5. [DOI] [PubMed] [Google Scholar]
- Kidwell MG. Evolutionary biology, voyage of an ancient mariner. Nature. 1993;362:202. doi: 10.1038/362202a0. [DOI] [PubMed] [Google Scholar]
- Casse N, Bui QT, Nicolas V, Renault S, Bigot Y, Laulier M. Species sympatry and horizontal transfers of Mariner transposons in marine crustacean genomes. Mol Phylogenet Evol. 2006;40:609–619. doi: 10.1016/j.ympev.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Greve P, Braquart-Varnier C, Strub JM, Felix C, Van Dorsselaer A, Martin G. The glycosylated androgenic hormone of the terrestrial isopod Porcellio scaber (Crustacea) Gen Comp Endocrinol. 2004;136:389–397. doi: 10.1016/j.ygcen.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. BioEdit version 5.0.6. http://www.mbio.ncsu.edu/BioEdit/bioedit.html.
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic relationships between various mariner transposases showing that Mariner-5_Dbi belongs to the irritans subfamily.
Illustration of the position of the two types of primer sets we used to screen for Mariner-5_Avul and Crmar2_Avul elements in the various isopod species. The sequence of the primers is provided in Additional file 5: Table S1. TIR: Terminal inverted repeat.
Pictures of agarose gels showing the results of the reverse transcription PCR experiments on Crmar2 (A) and Mariner-5(B) in Armadillidium vulgare ovaries and somatic tissues (head + nervous chain). A band of the expected size was obtained for all reactions showing that both elements are transcribed in A. vulgare soma and germ line. RT, reverse transcriptase; L, size ladder.
Sequences of the Crmar2 transcripts encoding a full length, intact, and thus potentially functional transposase aligned together with the Crmar2_Avul consensus sequence (fasta format).
List of primers used to amplify and sequence Crmar2 and Mariner-5 elements.
List of primers used to amplify and sequence the androgenic gland hormone.
Consensus sequences of Crmar2 and Mariner-5 elements reconstructed in this study (fasta format).
List of genes sequenced by Regier et al. [7] used to calculate genetic distances between the various species included in this study.
Sequence alignments used to reconstruct the phylogeny of crustacean isopod species and Crmar2 and Mariner-5 copies (fasta format).