Abstract
Background
Endogenous or iatrogenic antitumor immune responses can improve the course of follicular lymphoma (FL), but may be diminished by immune checkpoints in the tumor microenvironment. These may include effects of programmed death (PD)-1, a co-inhibitory receptor that impairs T-cell function and is highly expressed on intratumoral T cells. In a Phase II trial, we determined the activity of pidilizumab, a humanized anti-PD-1 monoclonal antibody, with rituximab in patients with relapsed FL.
Methods
FL patients with rituximab-sensitive disease relapsing after 1–4 prior therapies were eligible. Pidilizumab was administered at 3 mg/kg every 4 weeks for 4 infusions, plus 8 optional infusions every 4 weeks for patients with stable disease or better. Starting 2 weeks after the first infusion of pidilizumab, rituximab was given at 375 mg/m2 weekly for 4 weeks. The primary endpoint was to assess the overall response rate. Analysis was by intention to treat. Peripheral blood and tumor biopsies were studied to assess immunological effects of pidilizumab. This trial has been completed and was registered at www.clinicaltrials.gov as NCT00904722.
Findings
The combination was well-tolerated, with no autoimmune or therapy-related grade 3/4 toxicities. The most common grade 1 adverse events were anemia (14 patients) and fatigue (13 patients), and the most common grade 2 adverse event was respiratory infection (5 patients). Overall 19/29 (66%) and complete 15/29 (52%) response rates in 29 evaluable patients were high, with tumor regression in 25/29 (86%) of patients. Median progression-free survival was 18.8 months (95% CI: 14.7 months to not reached). The median response duration for the 19 responders was 20.2 months (95% CI: 13.9 months to not reached). Correlative studies of blood and tumor provided insights into predicting response and understanding mechanisms involved.
Interpretation
Pidilizumab with rituximab is well-tolerated and its activity compared favorably to historical retreatment with rituximab monotherapy in patients with relapsed FL. Our results establish that immune checkpoint blockade is worthy of further study in FL.
Funding
National Institutes of Health, Leukemia and Lymphoma Society, Cure Tech Ltd, and UT MD Anderson Cancer Center.
Introduction
The natural history of follicular lymphoma (FL), the most common indolent non-Hodgkin lymphoma worldwide, is characterized by stable disease or even spontaneous remissions, lasting months to years prior to progression.1 This suggests a transition from immune surveillance and equilibrium to escape,2 and is supported by numerous studies characterizing the influence of the immune system on FL. In a landmark study, Dave and colleagues demonstrated that survival duration of patients with FL correlated with gene expression signatures of infiltrating nonmalignant immune cells.3 An immunosurveillance pattern (CD8+ T cells) or an immune-escape pattern (CD57+ T cells) correlated with good or poor prognosis, respectively, in other FL studies.4, 5 Tumor-specific T cells can also be isolated from the peripheral blood (PB) and tumor microenvironment in FL.6, 7 Together, these results suggest that endogenous antitumor immune responses are naturally induced in patients with FL but eventually rendered ineffective, possibly due to immune escape or immune checkpoints in the tumor microenvironment.8, 9 Blocking immune checkpoints may promote or unleash an endogenous antitumor immune response and augment the efficacy of immunotherapeutic interventions.
Programmed death (PD)-1 is an inhibitory receptor expressed by activated T cells, activated B cells, NK cells, and myeloid cells. PD-1 inhibits T-cell activation when engaged by its ligands PD-L1 or PD-L2, expressed on tumor cells and/or stromal cells.10 PD-1 is markedly upregulated on CD4+ and CD8+ T cells after chronic antigenic stimulation by viral infection or tumor exposure. High PD-1 expression is associated with T-cell exhaustion, and blockade of the PD-1/PD-ligand pathway with antibodies against PD-L1 and/or PD-1 augmented and/or restored the function of viral and tumor-specific CD4+ and CD8+ T cells in mouse and human studies.11 In FL patients, PD-1 is also highly expressed on intratumoral and PB CD4+ and CD8+ T cells, and associated with impaired T-cell function.12, 13 Therefore, targeting the PD-1/PD-ligand pathway may enhance endogenous antitumor immune responses in FL.
Pidilizumab (formerly CT-011) is a humanized IgG-1 kappa recombinant monoclonal antibody that targets PD-1. In preclinical studies, CT-011 and BAT, the mouse monoclonal antibody from which CT-011 was derived, inhibited growth of melanoma, lymphoma, lung, colon, and breast tumors and extended the survival of mice.14–17 Selective depletion of T or NK cells in tumor-bearing mice reduced the efficacy of BAT, suggesting that both T cells and NK cells are necessary for the in vivo antitumor effect of this antibody.15 In a phase I clinical trial in patients with advanced hematological malignancies, CT-011 was found to be safe and well tolerated with no observed treatment- or infusion-related serious adverse events. Evidence of activity included a patient with FL who achieved durable complete remission.18
The monoclonal antibody rituximab, directed against the B cell antigen CD20, is utilized alone and in combination to treat FL, in both the frontline and relapse setting. Rituximab has improved response rates, progression-free survival (PFS), and overall survival (OS) of patients with FL.19–22 Patients previously treated with single-agent rituximab have been successfully retreated after relapse.23, 24 Rituximab acts in part via activation of NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC). Therefore, we reasoned that the combination of pidilizumab and rituximab would have additive and/or synergistic effects via activation of both the innate (NK cells) and adaptive (T cells) arms of the immune system, enhancing clinical efficacy without increasing toxicity. Here, we report safety, activity, and correlative studies of pidilizumab and rituximab from a single-arm phase II trial in patients with relapsed FL.
Methods
Patients
In this institutional review board-approved single-institution, open-label, nonrandomized phase II trial, grade 1–2 adult FL patients relapsing after 1–4 prior therapies with rituximab-sensitive disease, defined as complete or partial response lasting ≥6 months were enrolled after written informed consent in accordance with the Declaration of Helsinki. Other inclusion criteria included measurable disease, performance status <2, absolute neutrophil count ≥1.5×109/L, absolute lymphocyte count (ALC) ≥0.6×109/L, platelets ≥50×109/L, and adequate organ function. Patients with active infection, central nervous system lymphoma, autoimmune diseases or allogeneic stem cell transplantation were excluded. This trial was registered at www.clinicaltrials.gov as NCT00904722.
Treatment
Pidilizumab was dosed at 3 mg/kg intravenously every 4 weeks for 4 infusions. Rituximab was dosed at 375 mg/m2 intravenously weekly for 4 weeks starting day 17 after the first infusion of pidilizumab. Patients with stable disease (SD) or better received 8 additional optional infusions of pidilizumab every 4 weeks for a total of 12 doses. Dose modifications were not permitted for pidilizumab or rituximab. Dose interruption of up to 3 weeks was permitted for pidilizumab for grade 3 or higher toxicity.
Assessment of response and toxicity
The primary objective was to determine the overall response rate (ORR). The secondary objectives were to determine the safety and toxicity, complete (CR) and partial (PR) response rates, PFS, and immunological effects of pidilizumab. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 3.0. Response was determined according to Revised Response Criteria for Malignant Lymphoma using computed tomography (CT) scans and bone marrow biopsy.25 Positron emission tomography (PET)-CT scan was performed at the discretion of the treating physician and was also used to assess response when performed. Assessments were performed after completion of the second and fourth infusions of pidilizumab, and every 12 weeks thereafter for 2 years or until relapse.
Flow cytometric analysis
Immunophenotyping was performed on PB mononuclear cells (PBMC) by flow cytometry prior to and on day 14 after the first infusion of pidilizumab. Data were acquired using a BD LSRFortessa™ Cell Analyzer (BD Biosciences) and analyzed using FlowJo v9.5.2 software (Tree Star, Inc.).
Gene expression profiling (GEP)
Core needle biopsies from involved lymph nodes, obtained before and 14 days after the first infusion of pidilizumab, were collected in RNAlater fixative and stored at −80°C until RNA isolation. For samples with adequate RNA quantity and quality, determined with an Agilent 2100 Bioanalyzer, 300 ng of total RNA was amplified and biotin-labeled by the Eberwine method for hybridization to HT-12 version 4 BeadArrays from Illumina. Data processing was performed as previously described,26 and genes were excluded if expression levels were not significantly above background in at least 25% of samples. Gene Set Enrichment Analysis (GSEA; http://www.broadinstitute.org/gsea) was performed with assigned gene ranking. To find gene signatures that correlate significantly with PFS, we used a novel method (manuscript in preparation). In brief, for each specific signature and sample, a signature score was based on the expression level of signature genes, then used in a univariate Cox proportional hazards model test of correlation with PFS. The predictive power of the signature, defined as the negative logarithm of the p value from the Cox test, was then compared to a null distribution of the similarly-determined predictive power of 1000 signatures of randomly-selected genes. The significance p value of the signature’s predictive power was then based on its relative rank in the null distribution.
Statistical analysis
At the time of trial design, pidilizumab and rituximab combination therapy was expected to induce an ORR of 60% as compared with ORR of 40% expected with rituximab monotherapy when used as retreatment in FL patients.23 PFS was measured from enrollment to disease progression or recurrence or death from any cause. Patients were censored at the last disease evaluation if progression has not occurred. Paired student’s t-test was used to determine the significance of change in biomarkers between time points. Fisher’s exact test was used to evaluate the association between response status and other patient characteristics. Wilcoxon rank-sum test was used to evaluate differences in marker expression between responders and non-responders. Kaplan-Meier method was used for time-to-event analysis. The log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups. Univariate Cox proportional hazards models were fitted to evaluate the correlation with PFS of biomarkers, including tumor shrinkage, and GEP signatures. Statistical software SAS 9.1.3 (SAS, Cary, NC) and S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA) were used for the analyses.
Role of the funding source
This was an investigator-initiated study. Pidilizumab was provided free of cost by Cure Tech Ltd, Yavne, Israel. National Institutes of Health, Bethesda, Maryland, USA; Leukemia and Lymphoma Society; Cure Tech Ltd; and UT MD Anderson Cancer Center, Houston, Texas, USA provided funding to conduct the study and correlative studies. The sponsors had no role in study design, data collection, data analysis, data interpretation, or writing. The authors (JRW, FC, RED, and SSN) had full access to all raw data and final responsibility for the decision to write and submit this for publication.
Results
Thirty-two patients were enrolled between January 13, 2010 and January 20, 2012. Two patients were ineligible and not treated, and one patient was withdrawn after one infusion of pidilizumab and received alternative treatment as per the treating physician’s decision. Thus, 30 patients were evaluable for toxicity and 29 patients were eligible for efficacy analysis. Characteristics of the 30 treated patients are summarized in Table 1. Patients were fairly well distributed among the three risk groups of FL International Prognostic Index (FLIPI) 127 and FLIPI2.28 All patients had received rituximab previously either as monotherapy or in combination and 21/30 (70%) received combination chemotherapy or chemoimmunotherapy. The median number of prior treatments was 1 (range, 1–4).
Table 1.
Patient Characteristics at Enrollment (n = 30)
| Characteristic | n (%/Range) | |
|---|---|---|
| Age | Median (Range) | 61 (35–79) |
|
| ||
| Sex | Male | 17 (57) |
| Female | 13 (43) | |
|
| ||
| FLIPI 1 | Low | 13 (43) |
| Intermediate | 7 (23) | |
| High | 10 (33) | |
|
| ||
| FLIPI 2 | Low | 7 (34) |
| Intermediate | 15 (50) | |
| High | 8 (27) | |
|
| ||
| Prior Therapies | Median (Range) number of treatment regimens | 1 (1–4) |
| Median (Range) time (months) from last therapy | 23.8 (9.8–76.1) | |
| Chemotherapy combination | 21 (70) | |
| Biologic therapy combination | 11 (37) | |
| Radioimmunotherapy | 2 (7) | |
| Rituximab maintenance | 7 (23) | |
| Rituximab monotherapy | 1 (3) | |
| Any Rituximab | 30 (100) | |
| Median (Range) number of prior rituximab doses | 7 (2–22) | |
The median number of pidilizumab infusions administered for the 30 patients was 10 (range, 1–12) and 29 patients received the four infusions of rituximab as per protocol. The treatment was well tolerated with no autoimmune or therapy-related grade 3 or 4 adverse events. No patient had dose interruption or discontinued therapy due to toxicity. Grade 1 and 2 adverse events observed in ≥10% of patients regardless of attribution are summarized in Table 2.
Table 2.
Summary of Grades 1 and 2 Adverse Events (n = 30)
| Adverse Event | Grade 1, n (%) | Grade 2, n (%) | Grade 3/4, n |
|---|---|---|---|
|
| |||
| Anemia | 14 (47) | 0 | 0 |
| Fatigue | 13 (43) | 2 (7) | 0 |
| Leukopenia | 11 (37) | 0 | 0 |
| Thrombocytopenia | 8 (27) | 2 (7) | 0 |
| Dyspnea | 6 (20) | 0 | 0 |
| Neutropenia | 5 (17) | 1 (3) | 0 |
| Nausea | 5 (17) | 0 | 0 |
| Sweating | 4 (13) | 0 | 0 |
| Neuropathy | 4 (13) | 0 | 0 |
| Cough | 4 (13) | 0 | 0 |
| Pain | 3 (10) | 2 (7) | 0 |
| Edema | 3 (10) | 1 (3) | 0 |
| Pruritus | 3 (10) | 0 | 0 |
| Diarrhea | 3 (10) | 0 | 0 |
| Anorexia | 3 (10) | 0 | 0 |
| Hypotension | 2 (7) | 1 (3) | 0 |
| Respiratory Infection | 1 (3) | 5 (17) | 0 |
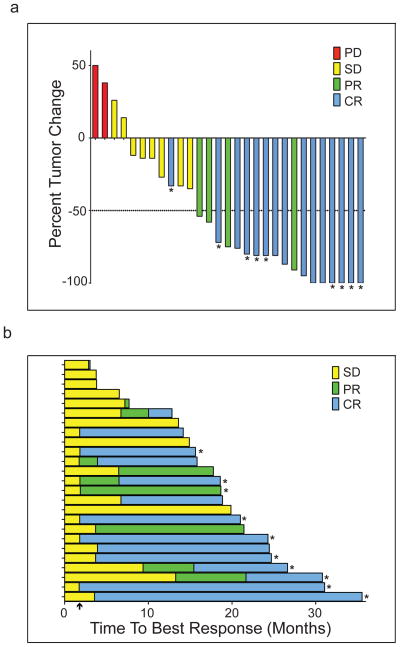
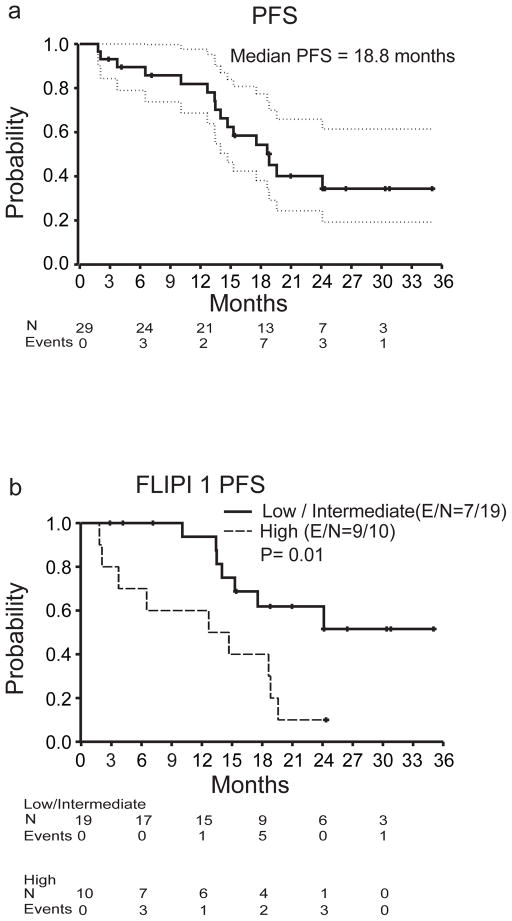
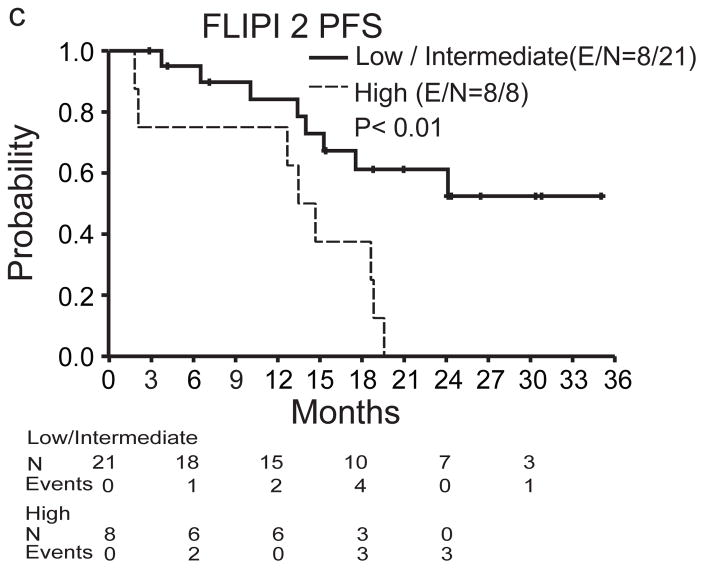
Of the 29 patients evaluable for efficacy analysis, 19 achieved an objective response for an ORR of 66%. CR was observed in 15/29 (52%) and PR in 4/29 patients (14%). Follow-up PET-CT scan was performed in 9 patients and confirmed CR. Altogether, 25/29 (86%) patients had measurable tumor regression (Figure 1a). The median time to observed response was 88 days (range, 53–392). Six/29 (21%) patients had considerably delayed response with the initial response >4 months after first pidilizumab infusion (Figure 1b). The median follow-up was 15.4 months, ranging from 1.8–35.0 months (IQR 10.1–21.0 months). The median PFS for all patients was 18.8 months (95% confidence interval (CI): 14.7, NA) (Figure 2a) but was not reached (95% CI: 18.8, NA) for the 19 responders, and was 19.6 months (95% CI: 17.5, NA) for the 25 patients with measurable tumor regression. The median response duration for the 19 responders was 20.2 months (95% CI: 13.9, NA) and only seven responders progressed to date. Clinical response was not significantly associated with FLIPI1, FLIPI2, prior therapy, number of prior rituximab doses, or duration of response to prior therapy (p>0.05). However, PFS was significantly associated with both FLIPI1 (median PFS for low/intermediate versus high risk, not reached (95% CI: 15.3, NA) versus 13.7 months (95% CI: 3.7, NA; p=0.01) (Figure 2b) and FLIPI2 (median PFS for low/intermediate versus high, not reached (95% CI: 15.3, NA) versus 14.1 months (95% CI: 12.7, NA); p<0.01) (Figure 2c). There were no deaths on the trial.
Figure 1. Clinical response after pidilizumab and rituximab therapy.
a) Best response after pidilizumab and rituximab therapy. Percent change in tumors size from baseline was determined by measuring the sum of the product of the diameters of up to six tumors on CT scans. Asterisk (*) indicates patients whose complete response (CR) was confirmed by PET-CT scan. PR, partial response; SD, stable disease; PD, progressive disease. b) Time to best response is shown for each of the 25 patients that had tumor reduction. Arrow on x-axis indicates the first time point at which tumor response was assessed after start of therapy. Asterisks (*) indicate patients with ongoing response at last assessment.
Figure 2. Progression-free survival (PFS) after pidilizumab and rituximab therapy.
a) Kaplan-Meier curve of PFS with 95% confidence intervals (CI). b and c) Kaplan-Meier curves of PFS for low/intermediate vs high risk groups for FLIPI 1 (b) and FLIPI 2 (c). The number of events (E) and the total number of patients at risk (N) over time and the p values by log-rank test are shown.
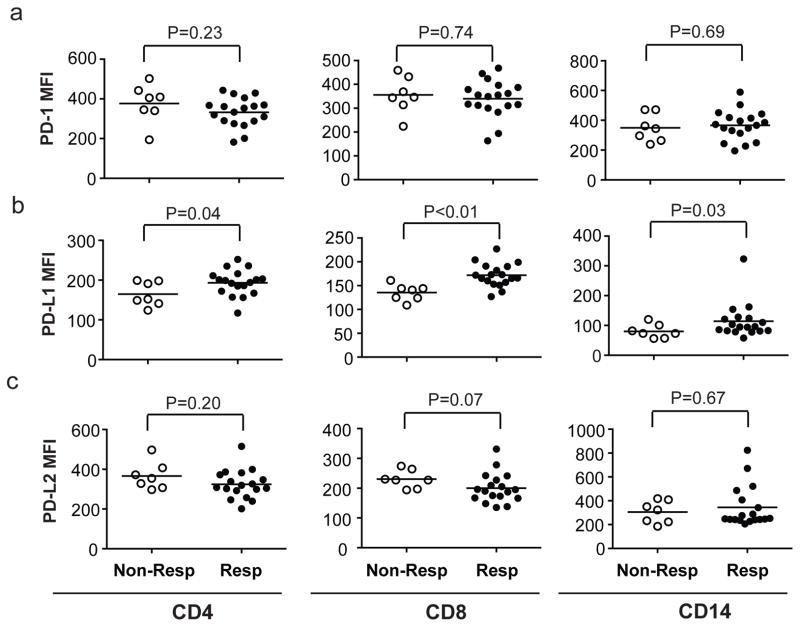
Correlative studies on baseline samples implicated factors predictive and/or explanatory of response to PD-1 blocking. Expression of PD-L1 (but not PD-1 or PD-L2), a marker of endogenous antitumor immunity,11, 29 was significantly higher in PB CD4+, CD8+, and CD14+ cells among responders compared with non-responders (Figures 3a–c), but was not associated with PFS (p>0.05). GEP data of baseline tumor biopsies from 18 patients were analyzed to find multi-gene signatures correlating with PFS, after assigning to each patient a score based on the expression of signature genes. Among publicly-curated signatures significantly predictive of longer PFS in our dataset (Table 3), prominent were signatures of genes upregulated during T-cell activation, or repressed in regulatory T cells, often with considerable overlap, suggesting that endogenous antitumor immunity at baseline predicted better response to pidilizumab. Significant positive correlation with PFS was also observed for a signature created by GEP studies we did on CD4+ T cells sorted from a separate group of banked FL tumor biopsies. In brief, we identified 41 genes more highly expressed in effector T cells (Teffs, PD-1intCXCR5int/PD-1loCXCR5lo), as compared to follicular helper T cells (TFH, PD-1hiCXCR5hi) (Supplementary Figures 1a and 1b). Consistent with our expectation that Teffs are likely to have antitumor effects, whereas TFH are likely to have protumor effects,30, 31 we found that low expression of this signature, suggesting more TFH and fewer PD-1+ Teffs within the tumor, predicted less tumor shrinkage and shorter PFS: median of 12.7 months (95% CI: 6.5, 21.6) for signature-low patients vs. not reached (95% CI: NA, NA) for signature-high patients (Supplementary Figures 2a–c). In contrast, dichotomization by this signature did not show a significant difference in OS in an external dataset of 191 FL patients treated largely with chemotherapy alone3 (Supplementary Figures 2d and 2e). This difference suggests that the predictive power of the 41-gene signature, and the process that it represents, may require the therapeutic context of anti-PD-1 antibody and/or rituximab in order to be relevant, and not be features of the “natural history” of FL. However, this implication should be regarded as highly speculative, in need of much more documentation.
Figure 3. Expression of PD-1, PD-L1, and PD-L2 on peripheral blood T cells and monocytes.
a–c) The mean fluorescence intensity (MFI) of PD-1 (a), PD-L1 (b), and PD-L2 (c) on peripheral blood CD4+ and CD8+ T cells and monocytes was determined by flow cytometry on 25 patients (18 responders and 7 non-responders) with available PBMC samples at baseline. The horizontal line indicates the mean for each group. Wilcoxon rank-sum test was used to evaluate differences in marker expression between the two patient groups.
Table 3.
T-cell activation signatures in tumors at baseline that correlated with PFS.*
| Gene Signature Name | Genes | Category | Original P value | PPV | FDR Q value |
|---|---|---|---|---|---|
|
| |||||
| GSE26928_EFF_MEMORY_VS_CXCR5_POS_CD4_TCELL_DN | 153 | C7 | 0.001 | 0 | 0 |
| LYMPHOCYTE_DIFFERENTIATION | 19 | C5 | 0.001 | 0.001 | 0.06 |
| GSE22045_TREG_VS_TCONV_DN | 159 | C7 | 0.001 | 0.002 | 0.08 |
| GSE14308_TH1_VS_INDUCED_TREG_UP | 178 | C7 | 0.003 | 0.01 | 0.08 |
| ZHENG_FOXP3_TARGETS_IN_T_LYMPHOCYTE_DN | 32 | C2 | 0.004 | 0.01 | 0.09 |
| BIOCARTA_IL7_PATHWAY | 16 | C2 | 0.004 | 0.01 | 0.09 |
| IMMUNE_SYSTEM_DEVELOPMENT | 58 | C5 | 0.004 | 0.01 | 0.09 |
| T_CELL_DIFFERENTIATION | 11 | C5 | 0.004 | 0.01 | 0.09 |
| GSE13306_TREG_VS_TCONV_SPLEEN_DN | 156 | C7 | 0.01 | 0.01 | 0.08 |
| POSITIVE_REGULATION_OF_IMMUNE_SYSTEM_PROCESS | 39 | C5 | 0.01 | 0.02 | 0.09 |
| GSE3982_MEMORY_CD4_TCELL_VS_TH1_UP | 173 | C7 | 0.01 | 0.02 | 0.08 |
| GSE7852_TREG_VS_TCONV_LN_DN | 140 | C7 | 0.01 | 0.03 | 0.08 |
| LYMPHOCYTE_ACTIVATION | 49 | C5 | 0.01 | 0.03 | 0.09 |
| BIOCARTA_TCR_PATHWAY | 42 | C2 | 0.01 | 0.03 | 0.09 |
| GSE10239_MEMORY_VS_KLRG1HIGH_EFF_CD8_TCELL_UP | 143 | C7 | 0.02 | 0.03 | 0.08 |
| GSE22886_NAIVE_CD8_TCELL_VS_MEMORY_TCELL_DN | 191 | C7 | 0.01 | 0.03 | 0.08 |
| GSE17580_TREG_VS_TEFF_S_MANSONI_INF_DN | 172 | C7 | 0.02 | 0.03 | 0.08 |
| GSE7852_TREG_VS_TCONV_DN | 166 | C7 | 0.02 | 0.03 | 0.08 |
| POSITIVE_REGULATION_OF_IMMUNE_RESPONSE | 20 | C5 | 0.02 | 0.03 | 0.09 |
| GSE7460_TREG_VS_TCONV_ACT_DN | 145 | C7 | 0.02 | 0.04 | 0.08 |
| GSE20366_TREG_VS_TCONV_DN | 163 | C7 | 0.02 | 0.04 | 0.08 |
| MARSON_FOXP3_TARGETS_DN | 49 | C2 | 0.02 | 0.05 | 0.10 |
Listed are selected gene signatures whose expression levels in gene expression profiles of pretreatment tumor biopsies from 18 patients were found to correlate significantly and positively with PFS by univariate Cox regression. Candidate signatures were drawn from the C2, C5, and C7 categories of the Molecular Signatures Database (http://www.broadinstitute.org/gsea/msigdb). The p value for each signature tested in isolation is shown, as well as its position (PPV, permutation p value) in the distribution of p values of 1000 signatures of randomly-selected genes similarly tested for correlation with PFS. A false discovery rate (FDR) control for multiple comparisons is used, based on the number of candidate signatures with isolated p values ≤ 0.05.
We also examined the effects of pidilizumab by comparing samples taken 14 days after the first pidilizumab infusion to baseline samples. In PB samples, there were significant increases in ALC, and CD3+ and CD4+ T cells, but not in CD8+ T cells (Supplementary Figures 3a and b). Naïve, effector memory, and central memory CD4+ T cells were significantly increased post-treatment (Supplementary Figure 3c). Among CD8+ T-cells, terminally differentiated cells were decreased but other subsets were not significantly altered (Supplementary Figure 3d). Expression of the activating receptor NKG2D on NK cells was significantly increased (p<0.05; data not shown). To analyze GEP data from paired core needle biopsies from 8 patients, the change in expression after treatment was correlated with outcome. Increased expression of T-cell activation signatures (Table 4) after pidilizumab treatment was associated with longer PFS, suggesting that endogenous antitumor immune responses were enhanced by pidilizumab. We also performed GSEA, based on ranking all genes by the slope of Pearson’s correlation between the pidilizumab-induced change in expression and the FTC of these 8 patients. Signatures of processes significantly associated with favorable FTC were related to CD4+ and CD8+ T-cell activation, proliferation or to changes in mitochondria, particularly in genes involved in oxidative phosphorylation; these are processes upregulated in T cells during an acute immune response,32 again suggesting that favorable responses to pidilizumab were due to enhanced T-cell immunity (Supplementary Figures 4a–d).
Table 4.
Change in T-cell activation signatures in tumors after pidilizumab treatment that correlated with PFS.*
| Gene Signature Name | Genes | Category | OPV | PPV | FDR Q value | Prognosis |
|---|---|---|---|---|---|---|
|
| ||||||
| GSE15930_NAIVE_VS_72H_IN_VITRO_STIM_CD8_TCELL_DN | 182 | C7 | 0.03 | 0.002 | 0.03 | Positive |
| GSE19825_NAIVE_VS_DAY3_EFF_CD8_TCELL_UP | 96 | C7 | 0.03 | 0.003 | 0.03 | Negative |
| GSE15930_NAIVE_VS_24H_IN_VITRO_STIM_INFAB_CD8_TCELL_UP | 154 | C7 | 0.03 | 0.01 | 0.04 | Negative |
| GOLDRATH_EFF_VS_MEMORY_CD8_TCELL_UP | 183 | C7 | 0.04 | 0.02 | 0.06 | Positive |
| GSE11057_NAIVE_VS_MEMORY_CD4_TCELL_UP | 133 | C7 | 0.04 | 0.03 | 0.06 | Negative |
| GSE10239_NAIVE_VS_DAY4.5_EFF_CD8_TCELL_DN | 180 | C7 | 0.04 | 0.03 | 0.06 | Positive |
| GSE15930_NAIVE_VS_24H_IN_VITRO_STIM_CD8_TCELL_DN | 188 | C7 | 0.04 | 0.03 | 0.06 | Positive |
| GSE15930_NAIVE_VS_48H_IN_VITRO_STIM_CD8_TCELL_DN | 190 | C7 | 0.04 | 0.05 | 0.07 | Positive |
| GSE10239_NAIVE_VS_KLRG1INT_EFF_CD8_TCELL_DN | 171 | C7 | 0.04 | 0.05 | 0.07 | Positive |
Gene expression profiles from 8 patients with paired biopsies (pretreatment, and 14 days after the first pidilizumab infusion) were compared to generate a “subtracted” profile of relative fold-change for each gene after pidilizumab. Subtracted profiles were interrogated, as described for Table 3, to find signatures significantly correlated with PFS, and those related to T-cell activation are shown. “Prognosis” refers to the direction of correlation. OPV, Original p value; PPV, Permutation p value; FDR, False discovery rate.
Discussion
The combination of pidilizumab and rituximab was active and well-tolerated in patients with relapsed FL. The ORR (66%) and CR rate (52%) compare favorably with the previously-reported ORR (40%) and CR rate (11%) in patients retreated with rituximab monotherapy.23 The median PFS for responders in this trial (not reached) also compares favorably with the estimated median time to progression of 17.8 months reported with rituximab monotherapy retreatment in the Davis et al and other studies.23, 33 These encouraging clinical results, together with the extremely low toxicity profile of this combination, make it especially appealing for FL patients, most of whom are elderly. Although selection bias or disease assessment methods may influence the results of single arm trials, we think this is unlikely as our patient characteristics (Table 1) are typical of patients with relapsed FL and our disease assessment methods followed international standards. However, a randomized study is necessary to definitively compare the efficacy of this combination relative to rituximab monotherapy. Pidilizumab administration after autologous stem cell transplantation in diffuse large B-cell lymphoma patients was also safe and showed potential clinical benefit.34 The absence of autoimmune adverse events in our study stands in contrast to the immune-related adverse events reported with other anti-PD-1 antibodies, nivolumab and lambrolizumab.29, 35 This difference may be due to the higher dose and more frequent administration used for nivolumab and lambrolizumab; prior exposure to ipilimumab, an anti-CTLA-4 antibody associated with autoimmune adverse events, in the nivolumab and lambrolizumab studies; B-cell depletion induced by rituximab in our study; and possibly a more immunocompromised state of FL patients. Randomized studies are needed to directly compare both safety and efficacy of these anti-PD-1 antibodies. Furthermore, the safety and efficacy of long-term therapy with pidilizumab with and without rituximab maintenance needs to be explored.
Therapeutic agents that target immune checkpoints are expected to enhance endogenous antitumor immune responses and therefore, benefit patients with preexisting antitumor immunity.11 Consistent with this notion, longer PFS was observed in patients with higher levels of T-cell activation signatures, and/or the 41-gene Teff vs. TFH signature, in their tumors at baseline (Table 3 and Supplementary Figure 2). Since the Teff signature did not correlate with OS in an external dataset of FL patients treated largely with chemotherapy alone, this signature may be specific for predicting outcome after anti-PD-1 antibody therapy. However, evaluation of this signature in additional external datasets of FL patients treated with rituximab alone or confirmation in randomized studies is necessary to definitively make this association. Another marker of preexisting antitumor immunity is PD-L1, due to the phenomenon of adaptive resistance.11, 29, 36 In concordance with this, responders expressed higher levels of PD-L1 on PB T cells and monocytes at baseline relative to non-responders. Moreover, comparison of baseline to day 14 samples showed that pidilizumab increased expression of activation-associated genes by T and NK cells in the PB and/or the tumor microenvironment, signatures of processes associated with T-cell immune responses, and absolute numbers of effector and memory CD4+ T cells in the PB, suggesting that pidilizumab enhanced endogenous antitumor immune responses. Due to the small sample size, the results of our correlative studies need to be interpreted with caution and should be tested in larger studies. In addition, the results of our GEP studies need to be confirmed by flow cytometry and immunohistochemistry studies of tumor samples. Inability to perform these studies due to inadequate samples and inability to compare these results to blood and tissue samples at initial diagnosis are potential limitations of this study. Nevertheless, multiple predictors associated with clinical outcome from pre-treatment samples, and multiple effects observed in post-treatment samples, were consistent with the expected mechanism of action of pidilizumab.
Although our analysis suggested that signatures of T-cell activation and/or Teffs at baseline are associated with PFS, the FL tumor microenvironment has multiple T-cell subsets that express PD-1: antitumor Teffs such as CD4+ T helper 1 and cytotoxic CD8+ T cells; protumor TFH: and subsets of regulatory T cells, including recently-described follicular regulatory T cells (TFR) that may suppress tumor B cells and TFH.12, 13, 30, 31, 37 Inconsistency of reports on the prognostic effect of PD-1+ lymphocytes in FL as assessed by immunohistochemistry38–42 might therefore be due to the multiplicity of PD-1+ T-cell subsets. While PD-1 blockade enhances the function of antitumor Teffs,10, 11 its effects on other PD-1+ T-cell subsets are unclear. Future studies of anti-PD-1 antibody in FL should incorporate strategies to enumerate these subsets in tumor samples, as it is likely that the net effect of PD-1 blockade may depend on the relative proportion of the various PD-1+ T-cell subsets.
Animal and human studies suggest that PD-1+ T cells may also express other inhibitory receptors such as CTLA-4, TIM-3, LAG-3, BTLA, CD160, CD244, and others.11, 43, 44 Therefore, blocking PD-1 alone may not fully restore the function of antitumor T cells. Indeed, CTLA-4 and TIM-3 are expressed on intratumoral T cells in FL and ipilimumab therapy has been associated with clinical responses in FL.8, 12, 45–47 Thus, combining pidilizumab with other immune checkpoint inhibitors48 may further enhance the endogenous antitumor T-cell responses and improve clinical outcome in these patients. Combining pidilizumab with immunostimulatory agents such as vaccines,49 Toll-like receptor ligands,50 lenalidomide and/or agonists of OX-4051 and 4-1BB52 may also be a rational approach.
In summary, our results suggest that pidilizumab and rituximab therapy is active and well-tolerated in patients with relapsed, rituximab-sensitive FL. Pidilizumab appears to activate T and NK cells and enhance endogenous antitumor immune responses. Further evaluation of pidilizumab with rituximab and/or other immunomodulatory agents is warranted.
Supplementary Material
Research in context.
Systematic review
The design of our study was informed by strong evidence that immunotherapy could induce meaningful and durable clinical remissions in FL. The critical interaction between the intact immune system and FL, and the role of modulating this interaction, is summarized in published works cited in the Introduction and Discussion. We did a comprehensive scientific literature search consisting of structured searches of PubMed when writing our report in July, 2013. We placed no date or language restrictions on the searches. We did four specific searches: (1) (“follicular lymphoma” AND “immune”), (2) (“follicular lymphoma” AND “rituximab” AND “immune”), (3) (“follicular lymphoma” AND “PD-1”), and (4) (“follicular lymphoma” AND “rituximab” AND “PD-1”). We did not identify any other clinical trials targeting PD-1 combined with rituximab, although several papers with in vitro data suggested the concept to be worthy of exploration.
Interpretation
Our findings suggest that the combination of rituximab and pidilizumab is an active and well-tolerated therapy, and establish that immune checkpoint blockade is worthy of further study in FL. These results also support further investigation of pidilizumab combined with other treatments, perhaps chemotherapy or additional immune-modulating therapies.
Acknowledgments
This work was supported by grants from the National Institutes of Health R21 CA143785 (SSN) and R01 CA155143 (SSN and RED), Leukemia and Lymphoma Society Specialized Center of Research grant 7262-08 (SSN, LMV, LR, and LWK), the University of Texas MD Anderson Cancer Center, and Cure Tech Ltd. This work was also supported by the NIH Clinical and Translational Science Award UL1 RR024148 and by the NIH Cancer Center Support Grant (CCSG) award CA16672 to MD Anderson Cancer Center (LF and VB).
Footnotes
Contributors
SSN and RRY designed the study. JRW, LEF, LWK, NF, JR, FH, MF, FS, and SSN participated in patient enrollment, treatment of patients, data collection, data analysis, and data interpretation. FC, MZ, ZW, WM, YG, LMV, LR, TM, RED, and SSN participated in performing correlative studies, data analysis, and data interpretation. LF and VB performed statistical analysis. MW obtained tumor biopsies for correlative studies. JRW, FC, RED, and SSN wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
SSN received research support from Cure Tech Ltd, Yavne, Israel. RRY is an employee of Cure Tech Ltd.
References
- 1.Horning SJ, Rosenberg SA. The Natural History of Initially Untreated Low-Grade Non-Hodgkin’s Lymphomas. New England Journal of Medicine. 1984;311(23):1471–5. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 3.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159–69. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 4.Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13(2 Pt 1):388–97. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro T, Lejeune M, Salvado MT, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24(34):5350–7. doi: 10.1200/JCO.2006.06.4766. [DOI] [PubMed] [Google Scholar]
- 6.Schultze JL, Seamon MJ, Michalak S, Gribben JG, Nadler LM. Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro. Blood. 1997;89(10):3806–16. [PubMed] [Google Scholar]
- 7.Lee ST, Liu S, Radvanyi L, et al. A novel strategy for rapid and efficient isolation of human tumor-specific CD4(+) and CD8(+) T-cell clones. J Immunol Methods. 2008;331(1–2):13–26. doi: 10.1016/j.jim.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang ZZ, Ansell SM. The tumor microenvironment in follicular lymphoma. Clin Adv Hematol Oncol. 2012;10(12):810–8. [PubMed] [Google Scholar]
- 9.Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest. 2012;122(10):3424–31. doi: 10.1172/JCI63186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107(9):3639–46. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myklebust JH, Irish JM, Brody J, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121(8):1367–76. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy B, Indjiia L, Rodionov G, Raiter A, Inbal A. Treatment with BAT monoclonal antibody decreases tumor burden in a murine model of leukemia/lymphoma. Int J Oncol. 2001;19(5):897–902. doi: 10.3892/ijo.19.5.897. [DOI] [PubMed] [Google Scholar]
- 15.Hardy B, Niv Y, Fadaeev L, Raiter A. BAT mAb induces lymphopoiesis in nude mice. Int Immunol. 2005;17(5):615–9. doi: 10.1093/intimm/dxh244. [DOI] [PubMed] [Google Scholar]
- 16.Feinmesser M, Raiter A, Hardy B. Prevention of melanoma metastases in lungs of BAT treated and peptide immunized mice. Int J Oncol. 2006;29(4):911–7. [PubMed] [Google Scholar]
- 17.Hardy B, Morgenstern S, Raiter A, Rodionov G, Fadaeev L, Niv Y. BAT monoclonal antibody immunotherapy of human metastatic colorectal carcinoma in mice. Cancer Lett. 2005;229(2):217–22. doi: 10.1016/j.canlet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 19.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–86. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359(6):613–26. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 21.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23(33):8447–52. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 23.Davis TA, Grillo-Lopez AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18(17):3135–43. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 24.Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin’s lymphoma--a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23(6):1088–95. doi: 10.1200/JCO.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, et al. Revised Response Criteria for Malignant Lymphoma. Journal of Clinical Oncology. 2007;25(5):579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Ma W, Wang M, Wang ZQ, et al. Effect of long-term storage in TRIzol on microarray-based gene expression profiling. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2445–52. doi: 10.1158/1055-9965.EPI-10-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–65. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 28.Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–62. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- 29.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ame-Thomas P, Le Priol J, Yssel H, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26(5):1053–63. doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawal S, Chu F, Zhang M, et al. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. J Immunol. 2013;190(12):6681–93. doi: 10.4049/jimmunol.1201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CH, Curtis JD, Maggi LB, Jr, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–51. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coiffier B, Osmanov EA, Hong X, et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol. 2011;12(8):773–84. doi: 10.1016/S1470-2045(11)70150-4. [DOI] [PubMed] [Google Scholar]
- 34.Armand P, Nagler A, Weller EA, et al. Disabling Immune Tolerance by Programmed Death-1 Blockade With Pidilizumab After Autologous Hematopoietic Stem-Cell Transplantation for Diffuse Large B-Cell Lymphoma: Results of an International Phase II Trial. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–8. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27(9):1470–6. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 39.Wahlin BE, Aggarwal M, Montes-Moreno S, et al. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1--positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin Cancer Res. 2010;16(2):637–50. doi: 10.1158/1078-0432.CCR-09-2487. [DOI] [PubMed] [Google Scholar]
- 40.Richendollar BG, Pohlman B, Elson P, Hsi ED. Follicular programmed death 1-positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Hum Pathol. 2011;42(4):552–7. doi: 10.1016/j.humpath.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi H, Tomita N, Sakata S, et al. Prognostic significance of programmed cell death-1-positive cells in follicular lymphoma patients may alter in the rituximab era. Eur J Haematol. 2013;90(4):286–90. doi: 10.1111/ejh.12075. [DOI] [PubMed] [Google Scholar]
- 42.Koch K, Hoster E, Unterhalt M, et al. The composition of the microenvironment in follicular lymphoma is associated with the stage of the disease. Hum Pathol. 2012;43(12):2274–81. doi: 10.1016/j.humpath.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 45.Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(20):6446–53. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang ZZ, Grote DM, Ziesmer SC, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122(4):1271–82. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Mahony D, Morris JC, Quinn C, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13(3):958–64. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 48.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuster SJ, Neelapu SS, Gause BL, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29(20):2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113(15):3546–52. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marabelle A, Kohrt H, Sagiv-Barfi I, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123(6):2447–63. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houot R, Goldstein MJ, Kohrt HE, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114(16):3431–8. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.