Abstract
A priority of Fragile X Syndrome (FXS) research is to determine the molecular mechanisms underlying the functional, behavioral, and structural deficits in humans and in the FXS mouse model. Given that metabotropic glutamate receptor (mGluR) long-term depression (LTD) is exaggerated in FXS mice, considerable effort has focused on proteins that regulate this form of synaptic plasticity. STriatal-Enriched protein tyrosine Phosphatase (STEP) is a brain-specific phosphatase implicated as an ‘LTD protein’ because it mediates AMPA receptor internalization during mGluR LTD. STEP also promotes NMDA receptor endocytosis and inactivates ERK1/2 and Fyn, thereby opposing synaptic strengthening. We hypothesized that dysregulation of STEP may contribute to the pathophysiology of FXS. We review how STEP’s expression and activity are regulated by dendritic protein synthesis, ubiquitination, proteolysis, and phosphorylation. We also discuss implications for STEP in FXS and other disorders, including Alzheimer’s disease. As highlighted here, pharmacological interventions targeting STEP may prove successful for FXS.
11.1 Introduction
A priority of Fragile X Syndrome (FXS) research is to identify potential therapeutic targets by focusing on mRNAs regulated by Fragile X Mental Retardation Protein (FMRP) and whose translated proteins regulate the expression of synaptic plasticity. STriatal-Enriched protein tyrosine Phosphatase (STEP) is one such candidate protein. STEP is a brain-specific tyrosine phosphatase that regulates dendritic proteins involved in synaptic plasticity, including ERK1/2, Fyn, NMDA receptors (NMDARs), and AMPA receptors (AMPARs) (Nguyen et al. 2002; Paul et al. 2003; Pelkey et al. 2002; Snyder et al. 2005; Zhang et al. 2008). Dysregulation of these proteins is proposed to contribute to the pathophysiology of FXS (Eadie et al. 2010; Kim et al. 2008; Nakamoto et al. 2007). STEP reduces ERK1/2 activity by dephosphorylating one of its regulatory tyrosine residues, Tyr204 (Paul et al. 2003), and inactivates the Src family tyrosine kinase (SFK) Fyn by dephosphorylating its regulatory site (Nguyen et al. 2002). Dephosphorylation of NMDARs and AMPARs promotes internalization of these receptors (Snyder et al. 2005; Zhang et al. 2008), and STEP is thought to mediate group 1 metabotropic glutamate receptor (mGluR)-dependent long-term depression (LTD) (Gladding et al. 2009; Moult et al. 2006; Zhang et al. 2008). Consistent with the mGluR theory of FXS (Bear et al. 2004), STEP is translated in response to mGluR stimulation (Zhang et al. 2008). Over-activation of mGluRs in the mouse model for FXS [Fmr1 knock-out (KO)] is associated with a tyrosine-phosphatase-dependent reduction in ERK1/2 phosphorylation (Kim et al. 2008), suggesting upregulation of an unknown tyrosine phosphatase in FXS. STEP is an excellent candidate for being this unknown tyrosine phosphatase. In this chapter, we review the current understanding of STEP and its potential role in the physiological and behavioral deficits associated with FXS.
11.2. STEP basics
11.2.1. Isoforms, domain function, and STEP regulation
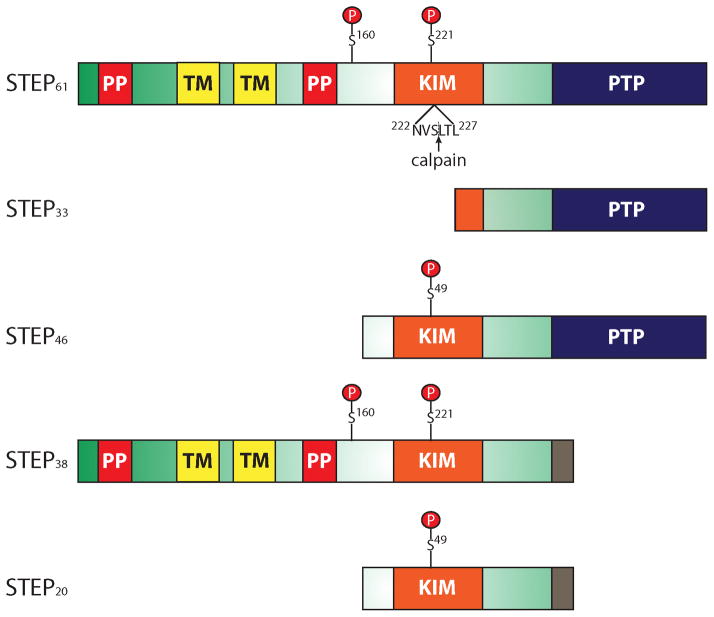
STEP was cloned and identified as a brain-specific tyrosine phosphatase twenty years ago (Lombroso et al. 1991; Lombroso et al. 1993). As its name implies, STEP is enriched in the striatum but is also found in other CNS structures including the hippocampus, cortex, amygdala, optic nerve, and spinal cord (Boulanger et al. 1995; Lorber et al. 2004). STEP is not expressed in the cerebellum (Lombroso et al. 1991). To date, four alternatively spliced variants of STEP have been identified: STEP61, STEP46, STEP38, and STEP20 (Fig. 1) (Bult et al. 1997; Bult et al. 1996; Sharma et al. 1995). The two major isoforms, STEP61 and STEP46, contain a signature consensus tyrosine phosphatase sequence, [I/V]HCxAGxxR[S/T]G, that is necessary for its catalytic activity, as well as a kinase-interacting motif (KIM) essential for substrate binding (Bult et al. 1996). STEP38 and STEP20 do not contain the consensus tyrosine phosphatase sequence and are therefore inactive variants of STEP with unknown function (Bult et al. 1997; Sharma et al. 1995).
Figure 1. Schematic of STEP.
To date, four alternatively-spliced variants of STEP (STEP61, STEP46, STEP38, and STEP20) and one calpain cleavage product (STEP33) have been identified. The kinase-interacting motif (KIM) domain is essential for substrate binding, and the consensus protein tyrosine phosphatase (PTP) sequence, [I/V]HCxAGxxR[S/T]G, is required for phosphatase activity. Since STEP61 and STEP46 are the only two that contain both the KIM and PTP sequence, they are the only active forms of STEP. STEP38 and STEP20 do not contain the PTP sequence and are inactive variants of STEP with unknown function. It is possible that these two inactive isoforms function as dominant-negative variants that compete with active STEP variants for substrate binding, or they possess other functions yet to be discovered. A unique ten amino acid sequence at the C-terminus of STEP38 and STEP20 is introduced during splicing. A calpain cleavage site resides within the KIM domain between Ser224 and Leu225 which is utilized to generate STEP33. Cleavage at this site disrupts the ability of STEP33 to interact with its substrates. STEP61 also has an additional 172 amino acids in its N-terminus which contains two transmembrane (TM) domains, two polyproline-rich (PP) regions, and an adjacent PEST sequence (not labeled). The TM regions target STEP61 to the endoplasmic reticulum, as well as the post-synaptic density. Without these TM regions, STEP46 is restricted to the cytosol. The PP regions impart substrate binding specificity. PKA phosphorylates STEP within the KIM domain (Ser221 and Ser49 on STEP61 and STEP46, respectively), as well as in the region adjacent to the PP regions (Ser160 on STEP61). Although the function of the additional phosphorylation site on STEP61 remains unclear, current investigations are aimed at determining if phosphorylation at this or other sites is a signal for calpain-mediated cleavage and/or ubiquitination.
Unlike STEP46, STEP61 contains an additional 172 amino acids at its N terminus targeting it to membranous organelles including the endoplasmic reticulum and the post-synaptic density (Fig. 1) (Boulanger et al. 1995; Bult et al. 1996; Oyama et al. 1995). Without this targeting sequence, STEP46 is restricted to the cytosol (Bult et al. 1996). The N-terminal portion of STEP61 also has two polyproline-rich regions that impart substrate specificity. For example, the first polyproline region is required for the interaction of STEP61 with Fyn (in addition to the KIM domain), and STEP61 has a ten-fold greater affinity for Fyn than STEP46 (Nguyen et al. 2002).
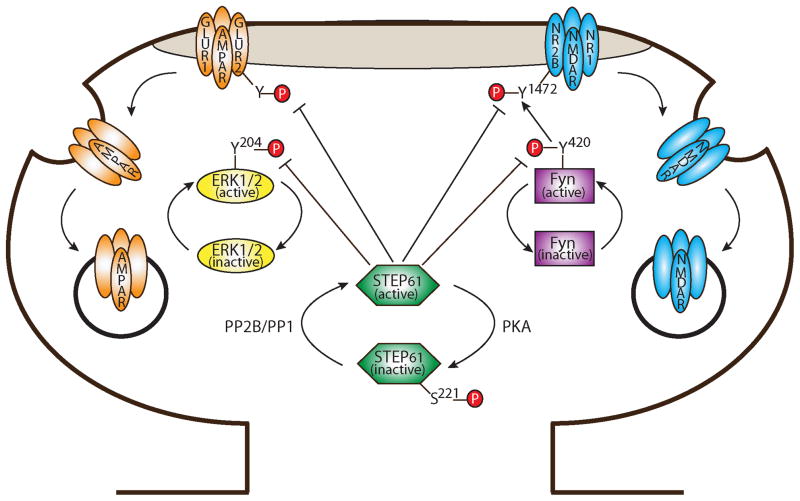
As mentioned, the KIM domain is required for binding STEP to its substrates, and this interaction is tightly regulated (Fig. 2). In all variants, dopamine-induced PKA phosphorylation of STEP in the KIM domain (Ser221 in STEP61 and Ser49 in STEP46) decreases the ability of STEP to bind to substrates due to steric interference (Paul et al. 2003). Conversely, activation of PP2B/calcineurin and PP1 (via NMDAR or α7nAChR stimulation) dephosphorylates STEP at this serine residue and increases its substrate affinity. STEP integrates dopaminergic, glutamatergic and nicotinic signaling, suggesting a potential role in psychostimulant addiction (Tashev et al. 2009; Valjent et al. 2005) and Alzheimer’s disease (Kurup et al. 2010; Snyder et al. 2005; Zhang et al. 2010). Additional evidence for its role in Alzheimer’s disease is discussed below.
Figure 2. Regulation of STEP and its substrates by phosphorylation.
In response to dopamine D1 receptor activation, PKA phosphorylation of STEP61 at Ser221 sterically prevents binding of STEP61 to its substrates. In contrast, stimulation of NMDARs initiates calcium influx and activation of PP2B (calcineurin) and PP1 to dephosphorylate and activate STEP61. When active, STEP dephosphorylates ERK1/2 and Fyn at their regulatory tyrosine residues, Tyr204 and Tyr420 (respectively), and inactivates them. STEP61 regulates the phosphorylation of NR2B-containing NMDARs by two parallel mechanisms. First, when Fyn is inactivated by STEP61, Fyn is unable to phosphorylate NR2B Tyr1472. Second, STEP61 dephosphorylates NR2B Tyr1472 directly. Dephosphorylation of Tyr1472 promotes the interaction of NR2B with clathrin adaptor proteins and leads to endocytosis of these receptors. STEP61 is also required for the internalization of GluR1/GluR2-containing AMPARs following mGluR stimulation. While the molecular mechanisms are still incompletely understood, STEP61 appears to promote the endocytosis of AMPARs in a similar manner to NMDARs.
A truncated STEP product, STEP33, is a calpain-mediated cleavage product generated during extrasynaptic NMDAR stimulation (Fig. 1) (Gurd et al. 1999; Nguyen et al. 1999; Xu et al. 2009). The calpain cleavage site resides in the KIM domain between residues Ser224 and Leu225 (Xu et al. 2009). Cleavage at this site disrupts the ability of STEP to associate with and dephosphorylate its substrates. Consequently, proteolytic cleavage of STEP after extrasynaptic NMDAR stimulation results in the activation of one of STEP’s substrates, p38, and initiates the cell death signaling cascade. Preventing STEP cleavage through use of a competitive peptide significantly attenuates cell death after either glutamate excitotoxicity or oxygen-glucose deprivation models (Xu et al. 2009). The inactivation of STEP by calpain cleavage is a possible mechanism for the observed increase in NMDAR tyrosine phosphorylation following cerebral hypoxia-ischemia (Besshoh et al. 2005; Gurd et al. 1999).
11.2.2. Developmental profile, brain region specificity, and subcellular localization
STEP61 and STEP46 are differentially expressed during rodent development (Raghunathan et al. 1996). STEP61 is abundant at birth and remains relatively unchanged into adulthood, whereas STEP46 is virtually undetectable at birth. STEP46 appears around postnatal day 6 and is enriched by 14 days (Okamura et al. 1997; Raghunathan et al. 1996). STEP46 plateaus at postnatal day 30 and remains constant throughout adulthood. Given that the first few weeks of life are associated with extensive cell migration and synaptogenesis (reviewed in Cayre et al. 2009), the onset of STEP46 expression during this time suggests a role in synaptogenesis. In middle aged (12-month old) mice, STEP61 is elevated relative to 3–6 month olds (Kurup et al. 2010), suggesting that expression of STEP61 also changes during aging.
The expression of STEP61 and STEP46 is brain-region specific (Boulanger et al. 1995; Lorber et al. 2004). The striatum, central nucleus of the amygdala, and optic nerve express both STEP isoforms. In contrast, the hippocampus, neocortex, spinal cord, and lateral amygdala express only STEP61 (Boulanger et al. 1995). Initial subcellular characterizations showed that STEP46 is enriched in cytosolic fractions, while STEP61 is enriched in light membrane fractions (which include endoplasmic reticulum, golgi, and endosomes) (Bult et al. 1996; Lombroso et al. 1993). Further investigation with electron microscopy showed that STEP is also targeted to the post-synaptic density (Oyama et al. 1995), and biochemical purification of these densities showed an enrichment of the STEP61 isoform (Gurd and Lombroso, unpublished data). Recent work demonstrates that the concentration of STEP61 is higher in extrasynaptic membranes than synaptic membranes (Goebel-Goody et al. 2009). Given that clathrin-mediated internalization of membrane proteins is thought to occur extrasynaptically (Blanpied et al. 2002; Racz et al. 2004), enrichment of STEP in extrasynaptic compartments might play a role in facilitating endocytosis of glutamate receptors in this part of the dendritic spine.
11.3. STEP substrates implicated in FXS
11.3.1. Mitogen-activated kinase ERK1/2
Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is critical for the induction and maintenance of synaptic plasticity. These kinases have been implicated in the regulation of membrane electrical properties (via the Kv4-family channels and subsequent NMDAR activation), local dendritic protein synthesis, nuclear transcriptional regulation, and the formation and stabilization of dendritic spines (reviewed in Sweatt 2004). ERK1/2 inactivation disrupts these processes. STEP inactivates ERK1/2 by dephosphorylating the regulatory tyrosine residue (Tyr204) in the activation loop (Fig. 2) (Paul et al. 2003). In wild-type synaptoneurosomal preparations, ERK1/2 phosphorylation, as detected by a dual-specificity Thr202/Tyr204 phospho-specific antibody, is rapidly increased upon mGluR stimulation via the selective group 1 mGluR agonist (RS)-3,3-dihydroxyphenylglycine (DHPG) (Kim et al. 2008). In contrast, in Fmr1 KO synaptoneurosomes, ERK1/2 phosphorylation is quickly and abnormally reduced following mGluR stimulation (Kim et al. 2008). A broad spectrum tyrosine phosphatase inhibitor (orthovanadate) prevents the DHPG-mediated decrease in ERK1/2 phosphorylation, suggesting over-activation of a tyrosine phosphatase in FXS (Kim et al. 2008). Given that STEP dephosphorylates and inactivates ERK1/2 (Paul et al. 2003), STEP is a likely candidate for the tyrosine phosphatase that is upregulated in FXS. Given that the early-phase kinetics of ERK activation is delayed in some individuals with FXS, ERK activation may be an useful biomarker of metabolic status in FXS (Weng et al. 2008).
Further support for the regulation of ERK1/2 by STEP stems from studies of STEP KO mice (Venkitaramani et al. 2009). ERK1/2 phosphorylation is significantly enhanced in the striatum, CA2 region of the hippocampus, and central/lateral amygdala in STEP KOs. Moreover, activation of ERK1/2 phosphorylation following DHPG treatment is more pronounced in STEP KOs relative to wild-type (Venkitaramani et al. 2009), corroborating earlier work implicating a tyrosine phosphatase in the regulation of ERK1/2 activity following mGluR stimulation (Kim et al. 2008).
ERK1/2 translocation to the nucleus and subsequent initiation of gene transcription is required for the formation of fear memories and the expression of synaptic plasticity in the lateral amygdala (Schafe et al. 2000). Infusion of a substrate trapping membrane-permeable fusion protein of STEP46 (TAT-STEPC-S) in the lateral amygdala of rats inhibits Pavlovian fear conditioning (Paul et al. 2007). This mutant STEP protein binds to its substrates, but cannot dephosphorylate them and does not release them, thereby disrupting their downstream signaling. Bath application of this construct also blocks the induction of LTP in the lateral amygdala and prevents ERK1/2 translocation to the nucleus during memory consolidation and synaptic plasticity (Paul et al. 2007). A recent report shows decreased LTP in the lateral amygdala of Fmr1 KO mice that is not rescued by the mGluR5-specific inverse agonist, MPEP (Suvrathan et al. 2010). Inhibitors targeted at other proteins upregulated in FXS, such as STEP, should be tested for their capacity to rescue these deficits.
11.3.2. Fyn and NMDA receptors
STEP regulates the phosphorylation and surface expression of NMDARs by two parallel pathways (Fig. 2): indirectly via dephosphorylation and inactivation of Fyn, one of the SFKs that phosphorylates NMDARs (Nguyen et al. 2002) and directly by dephosphorylation of the NMDAR subunit NR2B (Snyder et al. 2005; Kurup et al. 2010). When activated, Fyn phosphorylates the NMDAR NR2B subunit at Tyr1472 (Nakazawa et al. 2001). Full activation of Fyn is achieved by phosphorylation of Tyr420 in its catalytic domain (Smart et al. 1981). STEP dephosphorylates Fyn at this site (Nguyen et al. 2002), thereby inactivating Fyn and reducing Fyn-mediated phosphorylation of NR2B at Tyr1472 (Fig. 2). Additionally, STEP interacts with NMDARs (Pelkey et al. 2002) and dephosphorylates Tyr1472 directly (Kurup et al. 2010; Snyder et al. 2005). Tyr1472 resides within a conserved tyrosine-dependent endocytic motif (YXXΦ: X=any amino acid, Φ=bulky hydrophobic amino acid) (Roche et al. 2001). When not phosphorylated, the tyrosine residue in this motif binds to clathrin adapter proteins via strong hydrophobic interactions (reviewed in Marsh and McMahon 1999). In this way, STEP mediates endocytosis of NR2B-containing NMDARs by promoting the interaction between NMDARs and clathrin adapter proteins (Nakazawa et al. 2006).
Consistent with STEP’s role in mediating NMDAR endocytosis, the surface expression of NR1/NR2B receptor complexes is elevated in STEP KO mice and reduced in the presence of increased STEP levels (Kurup et al. 2010; Zhang et al. 2010). STEP’s modulation of surface NMDARs also affects their function. For example, application of recombinant STEP to the cytoplasmic face of neurons decreases NMDAR excitatory post-synaptic currents (EPSCs) and prevents the induction of long-term potentiation (LTP), whereas inhibiting endogenous STEP with an anti-STEP antibody enhances NMDAR EPSCs and occludes LTP (Pelkey et al. 2002). Moreover, theta-burst LTP is significantly increased in STEP KO mice relative to wild-type (Zhang et al. 2010). Taken together, STEP dephosphorylates Tyr1472, promotes internalization of surface NR1/NR2B receptors, and subsequently acts as a ‘brake’ on the induction of NMDAR-dependent LTP (Braithwaite et al. 2006; Kurup et al. 2010; Pelkey et al. 2002; Snyder et al. 2005; Zhang et al. 2010).
Some forms of NMDAR-dependent synaptic plasticity and learning are impaired in Fmr1 KO mice, lending support to the hypothesis that over-activation of STEP may contribute to hypofunction of NMDARs in FXS. NMDAR-dependent LTP and LTD are significantly attenuated in the dendate gyrus of Fmr1 KO mice, which is associated with a decrease in NMDAR EPSC amplitude (Eadie et al. 2010). Learning impairments are also observed in the ability of Fmr1 KOs to discriminate between two similar contexts and during contextual fear extinction, both of which require functional NMDARs (Eadie et al. 2010). Moreover, Desai et al. (2006) demonstrated that NMDAR-dependent spike timing-dependent plasticity potentiation is attenuated in Fmr1 KOs relative to WT. While hippocampal CA1 NMDAR-dependent LTD using a 1-Hz low-frequency stimulation protocol is unaffected in Fmr1 KOs (Huber et al. 2002), it is clear that hypofunction of NMDARs in some brain regions, perhaps via abnormal dephosphorylation by STEP, may contribute to NMDAR-dependent physiological and behavioral deficits in Fmr1 KO mice.
11.3.3. AMPA receptors
Pioneering work in the FXS field demonstrated that Fmr1 KO mice have exaggerated mGluR-dependent LTD (Huber et al. 2002). Both NMDARs and AMPARs are internalized during mGluR stimulation with the pharmacological mGluR agonist DHPG (Snyder et al. 2001), so identification of a common mechanism regulating endocytosis of these receptors would enhance our understanding of synaptic deficits in FXS. Given that STEP also regulates the internalization of GluR1/GluR2-containing AMPARs (Zhang et al. 2008), it is an excellent candidate for regulating this common mechanism.
Mounting evidence supports the hypothesis that a tyrosine phosphatase regulates the expression of mGluR-dependent LTD. Moult and colleagues (2006) reported that blocking tyrosine phosphatases with a broad spectrum tyrosine phosphatase inhibitor (phenylarsine oxide) prevents the expression of DHPG-mediated LTD. Pre-treatment of slices with the SFK inhibitor, PP2, prevents the tyrosine phosphatase-dependent block of mGluR-LTD (Moult et al. 2006), suggesting that SFKs counteract tyrosine phosphatases during this form of synaptic plasticity. Tyrosine phosphorylation of GluR2 is reduced during DHPG-mediated LTD, and this dephosphorylation is associated with internalization of AMPARs (Gladding et al. 2009; Moult et al. 2006). These findings point to a model in which a tyrosine phosphatase is activated during mGluR stimulation to dephosphorylate GluR2-containing receptors and mediate their endocytosis.
STEP appears to be the tyrosine phosphatase that mediates AMPAR internalization during mGluR stimulation (Fig. 2) (Zhang et al. 2008). Internalization of surface GluR1/GluR2 receptors is associated with an increase in STEP protein levels following DHPG treatment, and this occurs in conjunction with dephosphorylation of tyrosine residues on GluR2. DHPG-induced internalization of AMPARs is abolished in STEP KO hippocampal slices and cultures and restored with the addition of a wild-type TAT-STEP fusion protein to STEP KO neurons (Zhang et al. 2008). In this way, STEP completes the model proposed by Moult et al. (2006) and Gladding et al. (2009): it is activated by mGluR stimulation and leads to the dephosphorylation and subsequent internalization of AMPARs (Zhang et al. 2008).
While the molecular mechanisms governing AMPAR endocytosis following mGluR activation are still uncertain, tyrosine phosphorylation seems to play an important role. There are four tyrosine residues in the C-terminal tail of GluR2 (Tyr837, Tyr869, Tyr873, Tyr876) (Hayashi and Huganir 2004); yet none appear to reside within a conserved tyrosine-dependent endocytic motif (YXXΦ). A new model proposed by Scholz et al. (2010) sheds light on this paradox. These authors report that the GluR2 subunit directly interacts with the synaptic protein BRAG2, which is a synaptically-localized protein that functions as a guanine-exchange factor (GEF) for the GTPase Arf6. This interaction requires dephosphorylation of Tyr876 by an unknown tyrosine phosphatase (Scholz et al. 2010). When Arf6 is activated by BRAG2, it recruits the adaptor protein AP2 and clathrin to synaptic membranes (Krauss et al. 2003), thereby promoting endocytosis of GluR2 (Scholz et al. 2010). It is compelling to speculate that STEP is the tyrosine phosphatase that dephosphorylates GluR2 and mediates its internalization. Consistent with this hypothesis, the surface expression of GluR1/GluR2-containing AMPARs is significantly elevated in the absence of STEP (Zhang et al. 2008). Ongoing efforts are directed at confirming the site that STEP dephosphorylates on GluR2.
Given that Fmr1 KO mice have exaggerated mGluR-dependent LTD (Huber et al. 2002), one prediction is that the steady-state endocytosis of AMPARs would be upregulated in the absence of FMRP. Nakamoto et al. (2007) used siRNA of FMRP in cultured hippocampal neurons to demonstrate that DHPG induces excessive internalization of GluR1/GluR2 in FMRP-deficient neurons as compared to controls. More recently, aberrant constitutive AMPAR internalization was reported in hippocampal Fmr1 KO neurons relative to wild-type neurons (Gross et al. 2010), and surface levels of GluR1 are reduced in the amygdala of Fmr1 KO slices compared to wild-type (Suvrathan et al. 2010). Linking dysregulation of STEP in Fmr1 KOs to aberrant endocytosis of AMPARs is the subject of current investigation.
Because of its role in regulating AMPAR endocytosis following mGluR stimulation (Zhang et al. 2008), STEP was coined an ‘LTD protein’ by Luscher and Huber (2010). There are likely several ‘LTD proteins’ that are dysregulated in FXS. For example, microtubule associated protein 1b (MAP1b) is elevated early in development in Fmr1 KO mice (Lu et al. 2004) and regulates AMPAR surface expression by binding to the AMPAR scaffolding protein GRIP1 (Davidkova and Carroll 2007; Seog 2004). When bound to GRIP1, MAP1b sequesters GRIP-AMPAR bound complexes away from the synaptic surface, thereby negatively regulating AMPAR surface expression (Davidkova and Carroll 2007). Increased levels of MAP1b in Fmr1 KOs could therefore result in greater GRIP-AMPAR bound complexes and may explain increased AMPAR internalization. Even so, there is no evidence to date suggesting that strategies which decrease the expression and/or function of MAP1b improves behavioral performance in Fmr1 KOs. Another example of an ‘LTD protein’ that is dysregulated in FXS is activity-regulated cytoskeletal associated protein (Arc). Increased polysomal-associated Arc mRNA has also been reported in Fmr1 KOs (Zalfa et al. 2003); however, Arc protein levels do not appear to be significantly upregulated at baseline in Fmr1 KOs (Park et al. 2008). Arc directly associates with dynamin 2 and endophilin, which are proteins required for AMPAR endocytosis. As a consequence, Arc promotes internalization of AMPARs via interaction with these proteins (Chowdhury et al. 2006; Rial Verde et al. 2006; Shepherd et al. 2006). Arc is rapidly translated in response to DHPG stimulation in wild-type neurons, but this increase in Arc protein is absent in Fmr1 KOs (Park et al. 2008), supporting work from (Zalfa et al. 2003) which demonstrated elevated constitutive translation of Arc in Fmr1 KOs. Nonetheless, deletion of Arc in Fmr1 KO mice does not entirely prevent the exaggerated mGluR-LTD (Park et al. 2008), suggesting that additional proteins contribute to this electrophysiological deficit.
11.4. Regulation of STEP expression
11.4.1. Synaptic activity: mGluR stimulation and FMRP
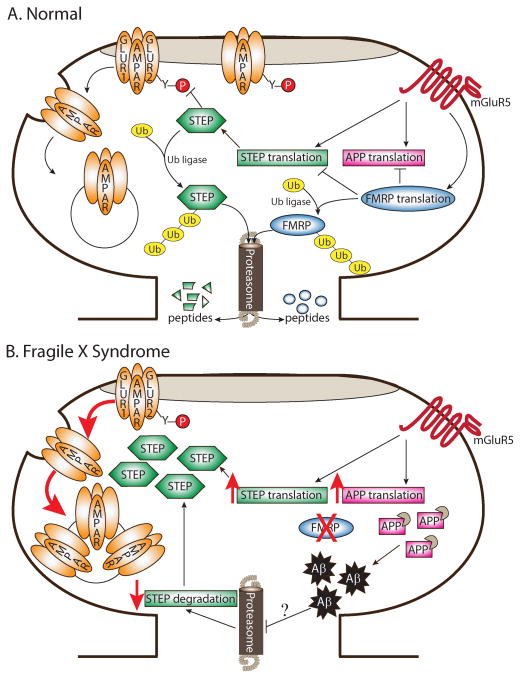
The mGluR hypothesis of FXS proposes that stimulation of mGluRs leads to local translation of synaptic proteins that are responsible for mediating mGluR-dependent LTD (Bear et al. 2004). Moreover, this theory posits that many FXS-related phenotypes originate in exaggerated signaling through mGluRs. FMRP normally suppresses translation of several mRNAs downstream of mGluR stimulation (Li et al. 2001). In FXS, FMRP is functionally absent, so many of these synaptically-localized proteins are upregulated (Gross et al. 2010 but see also Park et al. 2008). Here we review evidence that STEP is downstream of mGluR activation (Zhang et al. 2008), interacts with FMRP, and is upregulated in Fmr1 KO mice (Goebel-Goody et al. 2010). Given that STEP also regulates internalization of both NMDARs and AMPARs (Zhang et al. 2010; Zhang et al. 2008) and that mGluR-dependent LTD is enhanced in Fmr1 KOs (Huber et al. 2002), a revised model of the FXS mGluR theory emerges where exaggerated signaling through mGluRs causes dysregulation of STEP translation and a subsequent increase in the endocytosis rate of glutamate receptors (Fig. 3). These events likely lead to enhanced mGluR-LTD in Fmr1 KOs.
Figure 3. Mechanisms governing STEP protein expression and implications for Fragile X Syndrome.
(A) In normal (or wild-type) neurons, brief stimulation of mGluR5 receptors with DHPG triggers translation of STEP mRNA, as well as translation of other mRNAs including APP and FMRP. STEP dephosphorylates a tyrosine residue on GluR2 and initiates endocytosis of AMPARs following DHPG treatment. FMRP associates with both STEP and APP mRNA and likely acts as a translation suppressor to prevent excessive translation of these mRNAs. Ubiquitination and degradation by the proteasome regulate STEP and FMRP protein levels. Upon stimulation of mGluRs with DHPG, FMRP is rapidly degraded by the proteasome, presumably to permit translation of FMRP targets and allow the expression of LTD. (B) In the absence of FMRP, STEP protein expression might be inappropriately elevated by two parallel pathways. First, without the suppression of STEP mRNA translation by FMRP, the steady-state translation rate of STEP would be upregulated. Similarly, translation of APP is increased in Fmr1 KO mice. Elevated levels of APP provide more targets for β- and γ-secretase-mediated cleavage and result in exacerbated Aβ production in aged Fmr1 KO mice. Given that Aβ inhibits the ubiquitin proteasome system (UPS) in Alzheimer’s disease, it is possible that the UPS is blocked in FXS later in life. Consequently, inhibition of UPS by Aβ could lead to reduced degradation of STEP. Elevated STEP levels in FXS could therefore maintain the persistent internalization of AMPARs and exaggerated mGluR-dependent LTD. Of note, for simplicity, NR2B-containing NMDARs, ERK1/2, and Fyn were removed from this figure; however, it is possible that these proteins would also be more dephosphorylated and inactivated in the presence of elevated STEP levels.
Stimulation of mGluRs with DHPG leads to a rapid, dose-dependent increase in the translation of STEP (Fig. 3A) (Zhang et al. 2008). This increase is also time-dependent and occurs within synaptoneurosomes, suggesting that STEP is dendritically-translated by mGluR stimulation. Translation inhibitors block the DHPG-induced increase in STEP, whereas transcription inhibitors have no effect. In line with the requirement for the MAPK and phosphoinositide-3 kinase (PI3K) pathways in translation-dependent mGluR-LTD, preincubation of MEK (MAPK kinase) and PI3K inhibitors abolishes the DHPG-induced increase in STEP protein levels (Zhang et al. 2008). The use of specific group I mGluR1 and mGluR5 inhibitors verify that the DHPG-mediated increase in STEP translation occurs primarily through mGluR5 activation. As discussed previously, this DHPG-induced increase in STEP levels is required for the internalization of AMPARs following mGluR stimulation (Zhang et al. 2008).
We are beginning to uncover the molecular mechanisms underlying the DHPG-induced translation of STEP (Zhang et al. 2008). In agreement with the mGluR theory, STEP appears to associate with FMRP (Goebel-Goody et al. 2010; Darnell, unpublished results). Moreover, STEP protein levels are elevated in the brains of adult Fmr1 KO mice (Goebel-Goody et al. 2010), suggesting aberrant translation of STEP in the absence of FMRP (Fig. 3B). These findings are consistent with FMRP suppressing STEP translation under normal conditions and STEP translation being abnormally upregulated in the absence of FMRP.
Accordingly, genetically reducing STEP in Fmr1 KO mice ameliorates some FXS-associated behavioral deficits. For example, Fmr1 KO mice are well-characterized for their susceptibility to audiogenic seizures (Musumeci et al. 2000; Yan et al. 2005; Dolen et al. 2007). Given that STEP KO mice are more resistant to pilocarpine-induced seizures (Briggs et al. 2011), mice were generated that are null for both STEP and Fmr1 (Goebel-Goody et al. 2010). STEP/Fmr1 double KOs had fewer audiogenic seizures and less seizure-induced c-Fos positive neurons in the periaqueductal gray relative to Fmr1 KOs. Similarly, genetically reducing STEP in Fmr1 KOs decreases hyperactivity and spatial anxiety in an open field behavioral task (Goebel-Goody et al. 2010). Thus, inhibitors of STEP may be promising therapeutic strategies in FXS.
11.4.2. Ubiquitination via beta amyloid
In addition to regulation of STEP by mGluRs and FMRP, STEP expression is regulated by ubiquitination (Fig. 3A) (Kurup et al. 2010). The covalent attachment of ubiquitin targets proteins to the 26S proteasome for degradation (reviewed in Hegde 2010). In some neurological disorders, the ubiquitin proteasome system (UPS) is impaired. One pertinent example is Alzheimer’s disease (AD). AD is a debilitating neurodegenerative disorder associated with memory impairments. Accumulation of beta amyloid (Aβ) and the formation of amyloid plaques are characteristic features of AD, both of which are implicated in synaptic loss and cognitive decline (Hardy and Selkoe 2002; Lacor et al. 2004). In both human AD brains and mouse models of AD, the buildup of Aβ inhibits the proteasome and consequently leads to reduced degradation of proteins normally regulated by the UPS (Keller et al. 2000; Lam et al. 2000; Oh et al. 2005; Almeida et al. 2006). Recent studies demonstrate that STEP protein levels are increased in three mouse models of AD (Tg-2576, J20, 3xTg-AD) (Chin et al. 2005; Kurup et al. 2010; Zhang et al. 2010) and in the prefrontal cortex of human AD patients (Kurup et al. 2010). Elevated STEP levels in AD mouse models results in increased STEP activity, decreased phosphorylation and surface expression of NR2B-containing NMDARs, and decreased cognitive ability (Kurup et al. 2010; Zhang et al. 2010). STEP protein abundance is increased in AD due to Aβ-induced inhibition of the UPS (Kurup et al. 2010). Specifically, STEP61-ubiquitin conjugates are increased in the cortex of Tg-2576 AD mice and in wild-type cortical slices following Aβ treatment (Kurup et al. 2010). Genetically eliminating STEP in the 3xTg-AD mouse model improves cognitive performance, restores the NR1/NR2B surface expression deficit, and enhances synaptic plasticity (Zhang et al. 2010), validating STEP as a target for drug discovery for the treatment of AD.
Elucidating the molecular mechanisms underlying AD is relevant to FXS research because amyloid precursor protein (APP), the precursor protein that is cleaved by β- and γ-secretases to generate Aβ, is regulated by mGluRs and FMRP (Fig. 3A) (Westmark and Malter 2007). In particular, translation of APP is increased following mGluR stimulation, and APP mRNA associates with FMRP (Westmark and Malter 2007). As predicted by the mGluR hypothesis (Bear et al. 2004), APP translation is elevated in Fmr1 KO mice (Fig. 3B) (Westmark and Malter 2007). Greater levels of APP are also associated with increased soluble Aβ in middle-aged (11–13 months old) Fmr1 KOs (Westmark and Malter 2007). To study the role of APP and FMRP further, Westmark et al. (2008) created a novel mouse model (FRAXAD) where Tg-2576 AD mice were crossed with Fmr1 KO mice. FRAXAD mice have even greater levels of APP and Aβ than Tg-2576 mice alone (Westmark et al. 2008), suggesting an additive effect of the two mutations. Moreover, FRAXAD mice are more susceptible to seizures than either Fmr1 KO or Tg-2576 mice (Westmark et al. 2008; Westmark et al. 2009), and this deficit is abrogated when mice are pretreated with the mGluR5 antagonist MPEP (Westmark et al. 2009). Taken together, these findings reveal that some FXS-associated behaviors may be due to the accumulation of APP and Aβ in middle-aged Fmr1 KOs.
Given that Aβ inhibits the UPS, it is possible that the UPS is blocked in FXS later in life (Fig. 3B). Aβ-induced inhibition of the UPS could lead to reduced degradation of proteins normally regulated by the UPS. As a result, two possible explanations may exist for the accumulation of proteins in FXS (Fig. 3B): (1) Lack of translation suppression by FMRP and (2) Increased inhibition of the UPS by Aβ. Since STEP is regulated by both FMRP and the UPS, it is a likely candidate for a protein being upregulated by these two mechanisms in FXS. Current investigations are aimed at addressing these intriguing unanswered questions.
The UPS has already been implicated in FXS and Fragile X-associated tremor/ataxia syndrome (FXTAS) by a number of studies in recent years (Hou et al. 2006; Greco et al. 2002; Greco et al. 2006; Iwahashi et al. 2006). Inhibitors of the UPS block the expression of mGluR-LTD in wild-type mice (Fig. 3A). Specifically, FMRP is rapidly degraded by the UPS after mGluR stimulation, presumably to permit FMRP-bound mRNAs to be translated. In contrast, the enhanced mGluR-LTD observed in Fmr1 KOs is insensitive to UPS inhibitors, demonstrating that degradation of FMRP is required for regulating the expression of mGluR-LTD (Hou et al. 2006).
Ubiquitin-positive intranuclear neuronal and astroglial inclusion bodies are a feature of FXTAS, a late-onset neurodegenerative disorder affecting pre-mutation carriers of expanded CGG repeats (50–200) in the 5′ untranslated region of the Fmr1 gene (Greco et al. 2002; Greco et al. 2006; Iwahashi et al. 2006). In particular, a striking correlation exists between CGG length and the number of ubiquitin-positive inclusions (Greco et al. 2006). Given that FXTAS patients present with cognitive decline and dementia, among other symptoms (Garcia-Arocena and Hagerman 2010), it is likely that the presence of inclusion bodies in the hippocampus contribute to these cognitive deficits. While ubiquitinated proteins are only a minor component of inclusion bodies (Iwahashi et al. 2006), future studies are required which rigorously address whether the UPS is inhibited and what possible ubiquitin-conjugated proteins are upregulated in FXTAS.
11.5. Summary and conclusions
Over the last two decades, considerable evidence has mounted which supports the role of STEP in synaptic plasticity. Specifically, STEP dephosphorylates both NMDARs and AMPARs and negatively regulates their surface expression by promoting their interaction with clathrin-associated proteins. Additionally, STEP dephosphorylates and inactivates both ERK1/2 and Fyn. For these reasons, STEP acts as a ‘tonic brake’ on LTP and is one of a handful of ‘LTD proteins’ that promote the expression of LTD. STEP’s expression and activity is regulated by several mechanisms, including mGluR-stimulated translation, ubiquitination, proteolysis, and phosphorylation. Dysregulation of any of these mechanisms can flip the balance of STEP function such that it is more or less active. Either of these results could contribute to a disease state. In the case of FXS, enhanced activity of mGluRs in the absence of FMRP appears to increase the translation of STEP. Given that APP and Aβ levels are elevated in Fmr1 KOs and that Aβ inhibits the UPS in AD, it is also an intriguing possibility that Aβ blocks the UPS in FXS and leads to less degradation of STEP. Genetically reducing STEP reduces some FXS-associated behaviors in Fmr1 KOs. As highlighted here, pharmacological treatments that target STEP may be successful strategies not only for FXS, but also for other neurological disorders such as AD in which increased levels of STEP contribute to cognitive decline. While a number of unanswered questions remain, we continue to take one step at a time to advance our understanding of how STEP contributes to the pathophysiology of FXS.
Acknowledgments
We thank Dr. Marilee Ogren for critical reading of this chapter. This work was supported by the FRAXA Research Foundation and National Institute of Mental Health Grant MH052711.
11.7. References
- Almeida CG, Takahashi RH, Gouras GK. Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J Neurosci. 2006;26:4277–4288. doi: 10.1523/JNEUROSCI.5078-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, Oksenberg D, Nikolich K. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006;23:2847–2856. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- Briggs SW, Walker J, Asik K, Lombroso P, Naegele J, Aaron G. STEP regulation of seizure thresholds in the hippocampus. Epilepsia. 2011;52:497–506. doi: 10.1111/j.1528-1167.2010.02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Raghunathan A, Solimena M, Lombroso PJ. STEP: a family of brain-enriched PTPs. Alternative splicing produces transmembrane, cytosolic and truncated isoforms. Eur J Cell Biol. 1997;72:337–344. [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Sharma E, Lukacsi E, Solimena M, Naegele JR, Lombroso PJ. STEP61: a member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci. 1996;16:7821–7831. doi: 10.1523/JNEUROSCI.16-24-07821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 2006;96:1734–1745. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2010 doi: 10.1002/hipo.20890. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:83–39. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Collett VJ, Jia Z, Bashir ZI, Collingridge GL, Molnar E. Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol Cell Neurosci. 2009;40:267–279. doi: 10.1016/j.mcn.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Wallis ED, Li L, Hall DV, Royston S, Baum M, Naegele JR, Lombroso PJ. Loss of striatal-enriched protein tyrosine phosphatase (STEP) reverses deficits in a fragile x syndrome mouse model. Society for Neuroscience Annual Meeting; San Diego CA. 2010. Program No. 452.4. [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Cudley AE, Del Bigio MR, Jacquemont S, Leehey M, Haterman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd JW, Bissoon N, Nguyen TH, Lombroso PJ, Rider CC, Beesley PW, Vannucci SJ. Hypoxia-ischemia in perinatal rat brain induces the formation of a low molecular weight isoform of striatal enriched tyrosine phosphatase (STEP) J Neurochem. 1999;73:1990–1994. [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN. The ubiquitin-proteasome pathway and synaptic plasticity. Learn Mem. 2010;17:314–327. doi: 10.1101/lm.1504010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129:256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Markham JA, Weiler IJ, Greenough WT. Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. Proc Natl Acad Sci USA. 2008;105:4429–4434. doi: 10.1073/pnas.0800257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol. 2003;162:113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YA, Pickart CM, Alban A, Landon M, Jamieson C, Ramage R, Mayer RJ, Layfield R. Inhibition of the ubiquitin-proteasome system in Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:9902–9906. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci USA. 1991;88:7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B, Berry M, Hendriks W, den Hertog J, Pulido R, Logan A. Stimulated regeneration of the crushed adult rat optic nerve correlates with attenuated expression of the protein tyrosine phosphatases RPTPalpha, STEP, and LAR. Mol Cell Neurosci. 2004;27:404–416. doi: 10.1016/j.mcn.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O’Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci USA. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Paul S, Xu Y, Gurd JW, Lombroso PJ. Calcium-dependent cleavage of striatal enriched tyrosine phosphatase (STEP) J Neurochem. 1999;73:1995–2001. [PubMed] [Google Scholar]
- Oh S, Hong HS, Hwang E, Sim HJ, Lee W, Shin SJ, Mook-Jung I. Amyloid peptide attenuates the proteasome activity in neuronal cells. Mech Ageing Dev. 2005;126:1292–1299. doi: 10.1016/j.mad.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Okamura A, Goto S, Nishi T, Yamada K, Yoshikawa M, Ushio Y. Postnatal ontogeny of striatal-enriched protein tyrosine phosphatase (STEP) in rat striatum. Exp Neurol. 1997;145:228–234. doi: 10.1006/exnr.1997.6435. [DOI] [PubMed] [Google Scholar]
- Oyama T, Goto S, Nishi T, Sato K, Yamada K, Yoshikawa M, Ushio Y. Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum: a light and electron microscopic study with a complementary DNA-generated polyclonal antibody. Neuroscience. 1995;69:869–880. doi: 10.1016/0306-4522(95)00278-q. [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, Lombroso PJ. The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Raghunathan A, Matthews GA, Lombroso PJ, Naegele JR. Transient compartmental expression of a family of protein tyrosine phosphatases in the developing striatum. Brain Res Dev Brain Res. 1996;91:190–199. doi: 10.1016/0165-3806(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune LC, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R, Berberich S, Rathgeber L, Kolleker A, Kohr G, Kornau HC. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron. 2010;66:768–780. doi: 10.1016/j.neuron.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Seog DH. Glutamate receptor-interacting protein 1 protein binds to the microtubule-associated protein. Biosci Biotechnol Biochem. 2004;68:1808–1810. doi: 10.1271/bbb.68.1808. [DOI] [PubMed] [Google Scholar]
- Sharma E, Zhao F, Bult A, Lombroso PJ. Identification of two alternatively spliced transcripts of STEP: a subfamily of brain-enriched protein tyrosine phosphatases. Brain Res Mol Brain Res. 1995;32:87–93. doi: 10.1016/0169-328x(95)00066-2. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart JE, Oppermann H, Czernilofsky AP, Purchio AF, Erikson RL, Bishop JM. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src) Proc Natl Acad Sci USA. 1981;78:6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA. 2010;107:11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tashev R, Moura PJ, Venkitaramani DV, Prosperetti C, Centonze D, Paul S, Lombroso PJ. A substrate trapping mutant form of striatal-enriched protein tyrosine phosphatase prevents amphetamine-induced stereotypies and long-term potentiation in the striatum. Biol Psychiatry. 2009;65:637–645. doi: 10.1016/j.biopsych.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng N, Weiler IJ, Sumis A, Berry-Kravis E, Greenough WT. Early-phase ERK activation as a biomarker for metabolic status in fragile X syndrome. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1253–1257. doi: 10.1002/ajmg.b.30765. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Westmark PR, Beard AM, Hildebrandt SM, Malter JS. Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. Int J Clin Exp Pathol. 2008;1:157–168. [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Westmark PR, Malter JS. MPEP reduces seizure severity in Fmr-1 KO mice over expressing human Abeta. Int J Clin Exp Pathol. 2009;3:56–68. [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]