Abstract
The family of dedicator of cytokinesis (Dock), a protein family that belongs to the atypical Rho guanine nucleotide exchange factors (GEFs) for Rac and/or Cdc42 GTPases, plays pivotal roles in various processes of brain development. To date, 11 members of Docks have been identified in the mammalian system. Emerging evidence has suggested that members of the Dock family are associated with several neurodegenerative and neuropsychiatric diseases, including Alzheimer disease and autism spectrum disorders. This review summarizes recent advances on the understanding of the roles of the Dock protein family in normal and diseased processes in the nervous system. Furthermore, interacting proteins and the molecular regulation of Docks are discussed.
Keywords: actin, axon, Cdc42, dedicator of cytokinesis, dendrite, ELMO, guanine nucleotide exchange factor, neurite, Rac, Rho GTPase, synapse
Introduction of Dock protein family
The evolutionarily conserved Dock protein family is a newly characterized family of atypical Rho guanine nucleotide exchange factor (GEF) for Rac and/or Cdc42 GTPases.1,2 The typical Dbl family of Rho GEFs possesses a pleckstrin homology (PH)-Dbl homology (DH) module, of which PH domain is important for the phospholipid-binding and membrane targeting of Dbl GEFs, and DH domain is responsible for its GEF activity.3 By contrast, Dock family lacks the PH-DH module, but instead contains a Dock homology region (DHR) 1-DHR2 module. DHR1-DHR2 module plays similar roles as PH-DH module, of which DHR1 is important for the phospholipid-binding and membrane targeting of Docks, and DHR2 is responsible for their GEF activity.
To date, 11 members of Docks, namely Dock1 (Dock180) to 11, have been identified in mammalian system. Based on sequence homology, these Docks are divided into 4 subfamilies: Dock-A, which includes Dock180, Dock2, and Dock5; Dock-B, which includes Dock3 and Dock4; Dock-C (also called zizimin-related, or zir family), which includes Dock6, Dock7, and Dock8; and Dock-D (also called zizimin family), which includes Dock9, Dock10, and Dock11 (Fig. 1).1,2 In addition to the DHR1-DHR2 module, Dock-A and -B members contain an N-terminal Src homology 3 (SH3) domain and a proline-rich C-terminus, thus are more phylogenetic related to each other. On the other hand, Dock-C members lack recognizable domains outside of DHR1-DHR2 module, whereas Dock-D members contain an N-terminal PH domain. Both Dock-A and -B members preferentially activate Rac, whereas Dock-D members preferentially activate Cdc42. Dock-C members do not show unified GEF activity, i.e., Dock6 and Dock7 are capable of activating both Rac or Cdc42, whereas Dock8 preferentially activates Cdc42.4
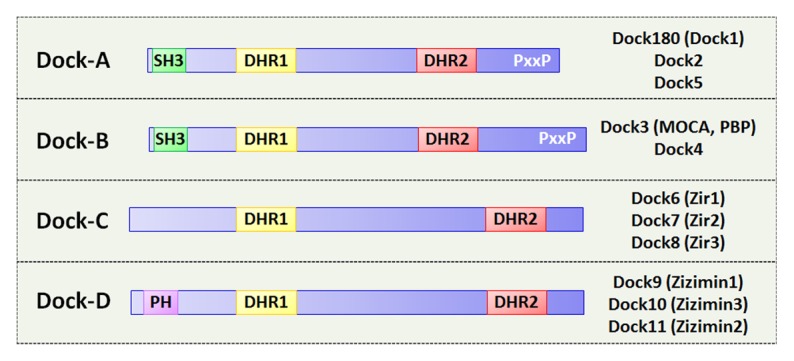
Figure 1. Schematic structure of different members of Dock protein family. Dock family proteins are divided into 4 subfamilies, Dock-A–D. Members of each subfamily and their alternate names are listed (MOCA, modifier of cell adhesion; PBP, presenilin-binding protein; Zir, zizimin-related). Structure of different domains, including SH3 (Src Homology 3), PH (pleckstrin homology), DHR (Dock homology region) 1, DHR2, and the proline-rich region (PxxP) are indicated.
Members of Dock protein family have been found to play important roles in multiple processes of brain development, including the development and functioning of neurons, microglia, and Schwann cells.5 Notably, emerging evidence has linked Docks with neuropsychiatric and neurodegenerative disorders, including autism spectrum disorders, schizophrenia, and Alzheimer and Parkinson diseases (AD and PD, respectively; Table 1). This review summarizes the current understanding of the roles of Dock protein family in nervous system during physiological and pathological conditions.
Table 1. Function of Dock proteins in nervous system and their related neurological diseases.
| Function | Related neurological diseases | Ref. | |
|---|---|---|---|
| Dock180 | Axon pathfinding, dendritic spine morphogenesis | 10–12,14 | |
| Dock2 | Neuroinflammation, microglial function | Alzheimer disease | 19, 20 |
| Dock3 | Axonal growth and regeneration, neurite outgrowth, neuroprotection | Alzheimer disease, attention deficit hyperactivity disorder | 21–32 |
| Dock4 | Neurite differentiation, dendritic spine morphogenesis | Autism, dyslexia, schizophrenia | 34–40 |
| Dock5 | Parkinson disease | 6 | |
| Dock6 | Neurite outgrowth, axon growth and regeneration | 43, 44 | |
| Dock7 | Neuronal polarization, cortical neurogenesis, Schwann cell development | 45, 46, 48, 49 | |
| Dock8 | Mental retardation, autism | 55, 56 | |
| Dock9 | Dendrite development | Bipolar disorder | 58, 59 |
| Dock10 | Neurite dynamics | Autism | 60, 61 |
Function of Dock protein family in nervous system and its related neurological diseases
Dock-A and Dock-B
Dock-A and -B members are the most studied Dock proteins in nervous and other systems. The neural functions of Dock1–4 have been revealed by multiple in vitro and in vivo studies. The neural function of Dock5, however, has remained to be explored, although evidence from genetic studies has implicated that Dock5 may be associated with PD.6 It was found that members of these 2 subfamilies form evolutionarily conserved associations with 2 groups of adaptor proteins, engulfment and cell motility (ELMO) and CT10 regulator of kinase (Crk) adaptor proteins.7 Formation of the bipartite complex of Docks and ELMO is one of the most important regulatory ways to activate the GEF activity of Dock-A and -B members toward Rac.8,9
Dock180
As the first identified member of Dock protein family, Dock180 has been found to play diverse roles in phagocytosis and cell migration. In nervous system, Dock180 is essentially involved in the regulation of axon guidance and dendritic spine morphogenesis. Dock180 binds to the netrin receptor DCC (deleted in colorectal cancer) and mediates netrin-induced Rac1 activation and axon growth.10 Such regulation is important for the commissural axon projection. Interestingly, Dock180 is also important for the axon pruning induced by ephrin-B3 reverse signaling and RhoG.11,12 Dock180 couples to ephrin-B3 through interacting with the adaptor protein Grb4/Nck2, and hence mediating ephrin-B3 signaling toward Rac1 activation and pruning of hippocampal mossy fiber axons.11,13 Moreover, the Dock180-ELMO complex participates in RhoG-mediated reduction of axonal complexity.12 The bi-faced roles of Dock180 in axon attraction or repulsion suggests that precise regulation of Dock180 at the axonal growth cones is an essential molecular control of the axon tip motility. Dock180 is also found to be involved in synapse development. The RhoG-ELMO1-Dock180 complex promotes Rac1 activation and leads to dendritic spine morphogenesis in hippocampal neurons.14
Although Dock180 plays critical roles in these neural developmental processes, it has not been reported that Dock180 itself is linked to neurological diseases. However, brain-specific angiogenesis inhibitor-1 (BAI-1), an interacting protein of Dock180-ELMO module, has been found to be linked to schizophrenia, bipolar disorder, and addiction.15,16 As BAI-1 regulates dendrite morphogenesis and synaptogenesis,16,17 it is of interest to explore whether Dock180 modulates the neural function of BAI-1 and whether such regulation is implicated in the pathogenesis of neuropsychiatric disorders.
Dock2
Dock2 is highly expressed in the immune system and regulates immune-cell functions.18 In brain, Dock2 is expressed exclusively in microglia and is implicated in neuroinflammation of AD pathology.19,20 It has been shown that the number of Dock2-expressing microglia is abnormally increased in brains of AD patients.19 The expression of Dock2 is positively regulated by prostaglandin E2 receptors, which mediate inflammatory, neurotoxic, and amyloidogenic effects induced by the increased secretion of microglial prostaglandins during AD pathogenesis.19 Importantly, Dock2 deficiency significantly reduces the area and size of β-amyloid (Aβ) plaque in cerebral cortex and hippocampus of a mouse model of AD.20 Thus, Dock2 may be a key molecule that contributes to the innate immune activation and Aβ plaque burden in AD.
Dock3
Dock3 was first identified as a presenilin-binding protein (PBP), and is found to be localized to the particulate fraction of sporadic AD brains.21 Multiple lines of evidence have suggested a complex mechanism involved in Dock3 signaling that contributes to AD pathogenesis. First, Dock3 is associated with neurofibrillary tangles and promotes the phosphorylation of tau protein.22 Second, Dock3 is shown to integrate the neuronal death signals transduced from familial AD-linked amyloid β precursor protein (APP) and presenilin (PS) mutants.23 Both of these findings point to a role of Dock3 in the neurodegenerative process in AD. On the other hand, studies from different groups have demonstrated neuroprotective roles of Dock3. First, Dock3 decreases the secretion of APP and Aβ peptide by accelerating the proteasome-dependent degradation of APP.24 Second, Dock3 ameliorates the neurotoxicity induced by N-methyl-D-aspartate receptors (NMDARs) via interacting with the C-terminus of NMDAR subunits.25,26 Third, Dock3 is important for maintaining the functional integrity of axons, as loss of Dock3 leads to axon degeneration.27 Given that Dock3 is appeared to play dual roles in neural degeneration and protection, further analysis on its precise temporal and spatial regulation is required for the understanding of Dock3′s role in AD pathology. In addition to AD, Dock3 has also been implicated to link to psychiatric disorders such as attention deficit hyperactivity disorder.28
Studies from in vivo and in vitro experiments have demonstrated that a major neural function of Dock3 is to promote neurite and axonal growth. Several molecular mechanisms have been revealed underlying Dock3-mediated neurite and axon growth. First, Dock3 associates with ELMO and RhoG to form the conventional ternary Dock-ELMO-RhoG complex, which is important for Rac1 activation during brain-derived neurotrophic factor (BDNF)-TrkB mediated neurite outgrowth.29 Moreover, Dock3 regulates actin cytoskeleton, microtubule assembly, and cell–cell adhesion by interacting with or regulating WAVE (Wiskott-Aldrich syndrome protein family verprolin-homologous), GSK-3β (glycogen synthase kinase 3β), and N-cadherin, respectively.30-32 Importantly, these Dock3-regulated molecular events all participate in BDNF-induced neurite outgrowth. Dock3 is also found as a negative regulator of Wnt/β-catenin signaling, as Dock3 inhibits the nuclear expression of β-catenin.33
Dock4
The gene encoding Dock4 has been found to be associated with several neuropsychiatric diseases, including autism, dyslexia, and schizophrenia.34-37 Of note, a rare heterozygous microdeletion found to be associated with autism and dyslexia leads to a fusion transcript that generates a shorter Dock4 protein product lacking the complete DHR2 domain and the C-terminus.34,35 Indeed, the DHR2-dependent Rac1 activation and actin organization is important for Dock4 in regulating the neurite differentiation of neuroblastoma cells.38 The SH3-dependent interaction of Dock4 with ELMO2 is important for this regulation, whereas the C-terminus of Dock4 is dispensable.38 In hippocampal neurons, Dock4 regulates the establishment of axon-dendrite polarity and dendrite arborization, which is also dependent on the SH3 domain and GEF activity of Dock4. Interestingly, the C-terminus of Dock4 is not important for polarity establishment, but may play regulatory roles in dendrite development.38,39 Furthermore, Dock4 is expressed in dendritic spines and participates in spine morphogenesis that dependent on its GEF activity and C-terminus.40 Importantly, the C-terminus of Dock4 was shown to both regulate the synaptic localization of Dock4 and mediate the interaction with the actin-binding protein cortactin.40 It is thus of interest to further explore the detailed role of the C-terminus of Dock4 during spine morphogenesis. Among all Docks, Dock4 is the only member that is also capable of activating Rap1, a Ras-related small GTPase involved in neuronal migration and spine dynamics.41,42 Whether the regulation of Rap1 is important for Dock4-dependent neural functions awaits further study.
Dock-C
Dock-C members, also called zizimin-related proteins, play regulatory roles in both central and peripheral nervous systems. In comparison with Dock-A and -B, Dock-C members do not interact with ELMO and Crk adaptors.
Dock6
Dock6 was found to promote neurite outgrowth and regulate axonal growth and regeneration of sensory neurons.43,44 Although Dock6 is capable of activating both Rac1 and Cdc42 in vitro, it preferentially activates Rac1 in dorsal root ganglion (DRG) neurons.44 Importantly, the GEF activity of Dock6 toward Rac1 is negatively regulated by Akt-dependent phosphorylation at Ser1194. During the initiation of axon growth at embryonic stages or after injury, Dock6 interacts with the protein phosphatase PP2A, which dephosphorylates Dock6 at Ser1194 and activates its GEF activity and axon growth. In later developmental stages, Dock6 switches to bind to Akt, which phosphorylates Dock6 and inhibits its GEF activity. This Akt-dependent phosphorylation of Dock6 is regulated by nerve-derived factor (NGF)-TrkA signaling and phosphoinositide 3-kinase. Re-introducing a nonphosphorylatable mutant (Ser1194A) and a phosphomimetic mutant (S1194E) in mice lacking Dock6 provides in vivo evidence that the phosphorylation status of Dock6 is a molecular determinant for axon growth.
Dock7
Dock7 is highly expressed in the developing brain and has been found to play important roles in several neuronal developmental processes.45,46 First, Dock7 regulates the neurogenesis in the neocortex by promoting the differentiation of radial glial progenitor cells (RGCs) to basal progenitors and neurons.46 Interestingly, such regulation is not dependent on the GEF activity of Dock7, but is through interaction with the microtubule- and centrosome-associating protein TACC3 (transforming acidic coiled-coil-containing protein 3). Binding to Dock7 inhibits the function of TACC3 on microtubule growth, hence promoting the interkinetic nuclear migration of RGCs and cortical neurogenesis. A second neural function of Dock7 in the central nervous system is the regulation of neuronal polarity and axon formation.45 Dock7 is preferentially expressed in the axons of developing hippocampal neurons, where it activates Rac and promotes axon formation. A microtubule destabilizing protein stathmin/Op18 is inactivated by Dock7 and Rac, which is a critical molecular event toward modulating the microtubule network during Dock7-regulated axon formation. Furthermore, Dock7 is found to form a complex with myosin VI in neuronal cells, and may thus be implicated in the regulation of myosin VI-dependent motor transport or actin cytoskeletal organization in neurons.47
In the peripheral nervous system, Dock7 is important for the development of Schwann cells, the glial cells that ensheath the axons of motor and sensory neurons.48,49 Dock7 negatively regulates the differentiation of Schwann cells and the onset of myelination in both primary Schwann cells in vitro and sciatic nerves in vivo.49 Knockdown of Dock7 leads to a downregulation of Rac1 and Cdc42, concomitant with an activation of RhoA.49 Although Dock7 is a negative regulator for Schwann cell differentiation, it promotes Schwann cell migration mediated by neuregulin.48 Dock7 directly binds to the neuregulin receptor ErbB2, which activates the GEF activity of Dock7 by phosphorylating it at Tyr1118.
Dock7 has been found as an interacting partner of tuberous sclerosis complex 1 (TSC1) and TSC2.50,51 Mutations of the genes encoding TSC1 and TSC2 are the main causes of multi-system benign tumors in tuberous sclerosis, and are also associated with neural developmental diseases, including mental retardation and epilepsy. It is of importance to investigate whether Dock7 regulates the function of TSC1/2 and whether such regulation is implicated in neural developmental diseases. Interestingly, despite Dock7 has multiple roles in neuronal development and Schwann cell myelination, a study reported that 2 mice with Dock7 mutations resulted from a chemical mutagenesis screen exhibited normal activities in several neurobehavioral studies, including tests for depressive and anxiety-like behaviors, memory, and locomotion.52 It is possible that other Docks may play redundant roles to compensate the loss-of-function of Dock7. Nonetheless, a more detailed molecular and behavioral analysis on specific neural function, such as cognition, will be required to determine the in vivo role of Dock7.
Dock8
Dock8 is highly expressed in the immune system, and Dock8 deficiency causes immune-related disorders.18 Not like other Dock-C members, Dock8 exhibits specific activity toward Cdc42 but not Rac1.4,53,54 Importantly, Dock8 is expressed in multiple regions of human brain, and mutations of the gene encoding Dock8 has been found in mental retardation and autism patients.55,56 This suggests that Dock8 may play important neural functions, which need further investigation.
Dock-D
Dock-D members, also called zizimin family proteins, exhibit specific GEF activity toward Cdc42. The biological functions of Dock-D members have been relatively less studied. Although Dock9 and Dock10 expressions are detected in brain, the neural function of them is only beginning to be understood. On the other hand, Dock11 is expressed predominantly in lymphocytes, and has not been found to play roles in brain yet.57
Dock9
Dock9 is expressed in brain at later developmental stages and is important for dendrite development.58 The GEF activity of Dock9 toward Cdc42 activation is important for dendritic outgrowth of cultured hippocampal neurons. Moreover, the PH domain and the DHR1 domain of Dock9 are important for its membrane targeting and activation of Cdc42. It is noteworthy that sequence variations have been found in the gene encoding Dock9 in bipolar disorder patients, and mutations of Dock9 gene are associated with several typical symptoms of the disease.59 This suggests that Dock9 may contribute to both risk and increased illness severity in bipolar disorder.
Dock10
Variations of Dock10 gene have been found in patients of autism spectrum disorders.60 Although the neural function of Dock10 is not clearly understood, it was found to be highly expressed in neurites of neuroblastoma cells and implicated in the extension/retraction dynamics of neurites.61
Molecular function and regulation of Dock protein family
Dock proteins act in both GEF-dependent and -independent ways
The activation of Docks toward Rac1 and/or Cdc42 is a classical function of Dock protein family in modulating actin dynamics. However, Docks can also act in a GEF-independent way during several neuronal developmental processes. For example, Docks can interact with the actin-binding proteins WAVE, WASP (Wiskott-Aldrich syndrome protein), and cortactin to regulate actin organization independent of their GEF activity.30,40,54 Docks can also regulate microtubule dynamics through binding to the microtubule-regulating proteins GSK-3β and TACC3.31,46 Moreover, Docks can regulate assembly, cellular localization, and degradation of other signaling molecules, such as Wnt signaling molecules and NMDA receptors.25,26,62 Table 2 summarizes the interacting proteins of Docks found in nervous and other systems.63-65
Table 2. A summary of interacting proteins of Dock1–8*,#.
| Interacting proteins | Domains of Docks for interaction | Regulatory roles | Function | Ref. | |
|---|---|---|---|---|---|
| Dock180 | ELMO1 | 1–161 a.a., containing SH3 | Increasing GEF activity of Dock180 toward Rac1 | Dendritic spine morphogenesis, reducing axonal complexity | 67 |
| CrkII | 1752–1865 a.a. of the C-terminus | Increasing GEF activity of Dock180 toward Rac1 | Cell migration | 9, 69 | |
| Grb4/Nck2 | 1793–1952 a.a. of the C-terminus | Mediating the recruitment of Dock180 to activated ephrin-B3 | Axon pruning | 11, 13 | |
| DCC | Increasing GEF activity of Dock180 toward Rac1 | Axon guidance | 10 | ||
| GRASP/Tamalin | SH3 | Scaffolding Dock180 to ARF-Rac signaling | Epithelial cell migration | 63 | |
| ANKN28 | SH3 | Cell migration and focal adhesion formation | 64 | ||
| SNX5 | DHR1 | Endosome-to-trans-Golgi-network transport | 65 | ||
| WAVE1–3 | DHR1 | 30 | |||
| Dock5 | 76 | ||||
| Dock2 | ELMO1 | SH3 | Increasing GEF activity of Dock2 toward Rac1 | Lymphocyte migration | 66 |
| CrkL | 1–515 a.a. and 939–1476 a.a. | Increasing GEF activity of Dock2 toward Rac1 | Cytoskeletal regulation in leukemia cells | 70 | |
| Vav | Cytoskeletal regulation in leukemia cells | 70 | |||
| WAVE1–3 | DHR1 | 30 | |||
| Dock3 | ELMO | 1–160 a.a., containing SH3 | Increasing GEF activity of Dock3 toward Rac1 | Neurite outgrowth | 29 |
| Presenilin | AD pathogenesis | 21 | |||
| β-catenin | Inhibiting nuclear β-catenin expression | Inhibiting Wnt signaling | 33 | ||
| Fyn | 1773–2028 a.a. of the C-terminus | Recruiting Dock3 to activated TrkB receptors | Axonal outgrowth and regeneration | 30 | |
| WAVE1–3 | DHR1 | Recruiting WAVE complex to activated TrkB receptors | Axonal outgrowth and regeneration | 30 | |
| GSK-3β | 1628–1777 a.a. of the C-terminus | Inhibiting GSK-3β activity by increasing its phosphorylation | Axonal outgrowth and regeneration | 31 | |
| NR2B | Increases NR2B degradation | Ameliorating NMDA-mediated neurotoxicity | 26 | ||
| NR2D | 796–1154 a.a. (the linker between DHR1 and DHR2 and part of the DHR2) | Ameliorating NMDA-mediated neurotoxicity | 25 | ||
| Dock4 | ELMO2 | 1–161 a.a., containing SH3 | Increasing GEF activity of Dock4 toward Rac1 | Neurite and dendrite development | 38, 39 |
| CrkII | C-terminus | Increasing GEF activity of Dock4 toward Rac1 | Dendrite development | 39 | |
| Cortactin | C-terminus | Synaptic localization of Dock4 | Dendritic spine morphogenesis | 40 | |
| APC | DHR2 | Stabilizing β-catenin, cell migration | 62 | ||
| Axin | C-terminus | Increasing Axin degradation | Stabilizing β-catenin, cell migration | 62 | |
| GSK-3β | C-terminus | Phosphorylation of Dock4 by GSK-3β, increasing Dock4 GEF activity toward Rac | Stabilizing β-catenin, cell migration | 62 | |
| WAVE1–3 | DHR1 | 30 | |||
| Dock5 | CrkII | 1736–1784 a.a. of the C-terminus | Intestinal epithelial cell spreading and migration | 71 | |
| CrkL | 1738–1870 a.a. of the C-terminus | Intestinal epithelial cell spreading and migration | 71 | ||
| Dock180 | 76 | ||||
| Dock6 | Akt | DHR1 | Inhibiting Dock6 GEF activity toward Rac1 by phosphorylating Dock6 at S1194 | Axon growth and regeneration | 44 |
| PP2A | DHR2 | Increasing Dock6 GEF activity toward Rac1 by dephosphorylating Dock6 at S1194 | Axon growth and regeneration | 44 | |
| Dock7 | TACC3 | 933–1164 a.a. (a region following DHR1 domain) | Antagonizing TACC3 function on microtubule growth or stabilization | Interkinetic nuclear migration of radial glial cells and cortical neurogenesis. | 46 |
| ErbB2 | 692–1431 a.a. (the whole sequence between DHR1 and DHR2) | Increasing Dock7 GEF activity toward Rac1 and Cdc42 by phosphorylating Dock7 at Y1118 | Schwann cell migration | 48 | |
| Myosin VI | 47 | ||||
| TSC1/2 | 50, 51 | ||||
| Dock8 | WASP | 754–1452 a.a. of the linker region between DHR1 and DHR2 | Mediating the localization of WASP in immune cells | Regulating F-actin organization of immune cells. | 54 |
| Talin | 1453–2099 a.a. (C-terminus including DHR2) | Mediating the localization of talin in immuno cells | Regulating integrin-mediated adhesion of immune cells. | 54 |
* As Rac and/or Cdc42 are known to bind to the DHR2 domain of all Docks, these 2 GTPases are not summarized in this table.
# It has been unknown for the interacting proteins of Dock9–11.
Abbreviations: ANKN28, ankyrin repeat domain 28; APC, adenomatous polyposis coli; Crk, CT10 regulator of kinase; CrkL, Crk-like; DCC, deleted in colorectal cancer; ELMO, engulfment and cell motility; GRASP, Golgi reassembly and stacking protein; GSK-3β, glycogen synthase kinase-3β; NR2B or NR2D, N-methyl-d-aspartic acid (NMDA) receptor 2B or 2D subunit; PP2A, protein phosphatase 2A; SNX5, sorting nexin 5; TACC3, transforming acidic coiled-coil-containing protein 3; TSC, tuberous sclerosis complex; WASP, Wiskott-Aldrich syndrome protein; WAVE, WASP family verprolin-homologous
Regulation of Dock Activity
Protein–protein interactions
The most well known regulation of the Dock family, in particular Dock-A and Dock-B members, is the interaction with ELMO.29,38,39,66,67 ELMO binds to the SH3 domain of Docks, thus leading to the release of the autoinhibitory status of Docks and exposure of their DHR2 domain for Rac activation.67 Moreover, ELMO acts as a scaffold protein to link Docks to other signaling molecules, such as RhoG.68 Crk adaptor proteins, including CrkII and CrkL, are another group of adaptors that activates the GEF activity of Dock-A and -B members through binding to their C-terminus.39,69-71 As Dock-C and Dock-D members lack the N-terminal SH3 domain and the proline-rich C-terminal region, they do not bind to ELMO and Crk adaptors. Whether common adaptor proteins bind to these 2 subfamilies of Docks to function similarly as ELMO and Crk remains to be investigated.
GEFs for Rho GTPases normally couple to membrane receptors or signaling molecules to transduce extracellular stimuli toward activation of Rho GTPases.72 Dock proteins participate in a number of signaling pathways, among which Trk receptors, Neuregulin-ErB, Eph-Ephrin, and Wnt mediated signaling pathways are important in nervous system.11,29,33,48,62,73 Docks can be directly or indirectly recruited to these signaling receptor complexes to be activated (Table 2).
Homodimerization and heterodimerization
Dock1, Dock2, and Dock9 can self-dimerize to form homodimers.74,75 It has been revealed that dimerization of Dock2 does not alter its GEF activity in vitro, but is important for its function under physiological conditions.75 This suggests that dimerization probably increases the signaling capacity of Docks. Given that the conserved DHR2 domain mediates the self-binding, dimerization through this domain may be a general mechanism for all Docks.74,75 In addition to homodimerization, heterodimerization formed between different Dock proteins (e.g., Dock1 and Dock5) is also evident.76 It is thus likely that clustering of one or more Dock proteins is one mechanism to regulate the local activity of Docks.
Phospholipid-binding and membrane targeting
Structural analysis of Dock180 has identified a common C2 domain scaffold and surface loops in the DHR1 domain of all Docks, which mediates the direct binding to phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3).77 Studies have confirmed that the DHR1-phospholipid binding is a common characteristic of Docks and such binding is important for the cellular function of Docks.58,78-81 In addition to DHR1 domain, members of Dock-D subfamily may also use the N-terminal PH domain to interact with phospholipids.82 The phospholipid binding regulates the function of Docks in 2 ways. First, this interaction releases the autoinhibitory structure of Docks and frees the DHR2 domain for GTPase activation. Second, this interaction translocates Dock proteins to the plasma membrane, where they locally activate Rho GTPases. Consistent with this notion, the phospholipid-binding regulated activation of GEF activity and membrane targeting is one of the important regulatory ways of Dock proteins in various biological processes, including cell polarization and migration.
Phosphorylation
Emerging evidence has identified that phosphorylation is important for the GEF activities of Dock proteins. For instance, tyrosine phosphorylation of Dock180 at multiple sites by Src kinase activates its GEF activity during tumorigenesis.83,84 Moreover, Dock4 is phosphorylated at the C-terminus by GSK-3β, which is important for Wnt-induced Rac activation.62 In the nervous system, 2 phosphorylation events, Ser1194 phosphorylation of Dock6 by Akt and Tyr1118 of Dock7 by ErbB2 receptors, have been found to control Dock activity during axon growth and Swchann cell migration, respectively.44,48 Interestingly, Ser1194 phosphorylation of Dock6 inhibits its activity, while Tyr1118 of Dock7 activates its activity. This suggests that phosphorylation can regulate Dock activity in both ways.
Concluding remarks
Members of Dock family play roles in diverse processes of nervous system, including the development and functioning of neurons, microglia, and Schwann cells. More importantly, many Dock proteins have been implicated in neurological diseases or associated with disease-related molecules. Nonetheless, there is still limited evidence that how deregulation of individual Dock proteins links to the system disorders in the brain. The neural function of several Docks, such as Dock5 and Dock8, has not been explored, although the genes encoding these 2 Docks have been shown to link with neurological diseases.6,55,56 Therefore, investigations on the synaptic network connectivity and neural behaviors in animals with manipulations of individual Dock genes are important to reveal the physiological roles of Docks in brain. Furthermore, this review summarizes the interacting proteins and molecular regulation of Docks. However, the detailed roles of Docks in transducing extracellular signals into actin reorganization or other cellular changes in the nervous system still remain to be fully understood.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Program for New Century Excellent Talents in University of China, the National Key Basic Research Program of China (Grant No. 2013CB530900), the National Natural Science Foundation of China for Young Scholars (Grant no. 81101015), the Research Fund for the Doctoral Program of Higher Education of China (Grant no. 20110001120103), the Fundamental Research Funds for the Central Universities of China (Grant no. 21612205), the Bureau of Science and Information Technology of Guangzhou Municipality for Young Scholars (Grant no. 2011J2200048), and the Science, Industry, Trade, and Information Technology Commission of Shenzhen Municipality for Distinguished Young Scholars (Grant no. JC201005260217A).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26839
References
- 1.Côté JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci. 2002;115:4901–13. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 2.Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci. 2005;118:4937–46. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 3.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 4.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–61. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto Y, Yamauchi J. Cellular signaling of Dock family proteins in neural function. Cell Signal. 2010;22:175–82. doi: 10.1016/j.cellsig.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Pankratz N, Dumitriu A, Hetrick KN, Sun M, Latourelle JC, Wilk JB, Halter C, Doheny KF, Gusella JF, Nichols WC, et al. PSG-PROGENI and GenePD Investigators, Coordinators and Molecular Genetic Laboratories Copy number variation in familial Parkinson disease. PLoS One. 2011;6:e20988. doi: 10.1371/journal.pone.0020988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–93. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–82. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 9.Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–97. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Gao X, Liu G, Xiong W, Wu J, Rao Y. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–76. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franke K, Otto W, Johannes S, Baumgart J, Nitsch R, Schumacher S. miR-124-regulated RhoG reduces neuronal process complexity via ELMO/Dock180/Rac1 and Cdc42 signalling. EMBO J. 2012;31:2908–21. doi: 10.1038/emboj.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu Y, Kucik DF, Wu C. Identification and kinetic analysis of the interaction between Nck-2 and DOCK180. FEBS Lett. 2001;491:193–9. doi: 10.1016/S0014-5793(01)02195-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Oh MH, Bernard LP, Macara IG, Zhang H. The RhoG/ELMO1/Dock180 signaling module is required for spine morphogenesis in hippocampal neurons. J Biol Chem. 2011;286:37615–24. doi: 10.1074/jbc.M111.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–4. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 16.Lanoue V, Usardi A, Sigoillot SM, Talleur M, Iyer K, Mariani J, Isope P, Vodjdani G, Heintz N, Selimi F. The adhesion-GPCR BAI3, a gene linked to psychiatric disorders, regulates dendrite morphogenesis in neurons. Mol Psychiatry. 2013;18:943–50. doi: 10.1038/mp.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duman JG, Tzeng CP, Tu YK, Munjal T, Schwechter B, Ho TS, Tolias KF. The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J Neurosci. 2013;33:6964–78. doi: 10.1523/JNEUROSCI.3978-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishikimi A, Kukimoto-Niino M, Yokoyama S, Fukui Y. Immune regulatory functions of DOCK family proteins in health and disease. Exp Cell Res. 2013;319:2343–9. doi: 10.1016/j.yexcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Cimino PJ, Sokal I, Leverenz J, Fukui Y, Montine TJ. DOCK2 is a microglial specific regulator of central nervous system innate immunity found in normal and Alzheimer’s disease brain. Am J Pathol. 2009;175:1622–30. doi: 10.2353/ajpath.2009.090443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cimino PJ, Yang Y, Li X, Hemingway JF, Cherne MK, Khademi SB, Fukui Y, Montine KS, Montine TJ, Keene CD. Ablation of the microglial protein DOCK2 reduces amyloid burden in a mouse model of Alzheimer’s disease. Exp Mol Pathol. 2013;94:366–71. doi: 10.1016/j.yexmp.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwa A, Yoshida H, Lee S, Paladino T, Liu Y, Chen Q, Dargusch R, Schubert D, Kimura H. Isolation and characterization of novel presenilin binding protein. J Neurochem. 2000;75:109–16. doi: 10.1046/j.1471-4159.2000.0750109.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Yoshida H, Schubert D, Maher P, Mallory M, Masliah E. Presenilin binding protein is associated with neurofibrillary alterations in Alzheimer’s disease and stimulates tau phosphorylation. Am J Pathol. 2001;159:1597–602. doi: 10.1016/S0002-9440(10)63005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tachi N, Hashimoto Y, Matsuoka M. MOCA is an integrator of the neuronal death signals that are activated by familial Alzheimer’s disease-related mutants of amyloid β precursor protein and presenilins. Biochem J. 2012;442:413–22. doi: 10.1042/BJ20100993. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Kimura H, Schubert D. A novel mechanism for the regulation of amyloid precursor protein metabolism. J Cell Biol. 2002;158:79–89. doi: 10.1083/jcb.200110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai N, Hayashi H, Aida T, Namekata K, Harada T, Mishina M, Tanaka K. Dock3 interaction with a glutamate-receptor NR2D subunit protects neurons from excitotoxicity. Mol Brain. 2013;6:22. doi: 10.1186/1756-6606-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namekata K, Kimura A, Kawamura K, Guo X, Harada C, Tanaka K, Harada T. Dock3 attenuates neural cell death due to NMDA neurotoxicity and oxidative stress in a mouse model of normal tension glaucoma. Cell Death Differ. 2013;20:1250–6. doi: 10.1038/cdd.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, Peto CA, Shelton GD, Mizisin A, Sawchenko PE, Schubert D. Loss of modifier of cell adhesion reveals a pathway leading to axonal degeneration. J Neurosci. 2009;29:118–30. doi: 10.1523/JNEUROSCI.3985-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Silva MG, Elliott K, Dahl HH, Fitzpatrick E, Wilcox S, Delatycki M, Williamson R, Efron D, Lynch M, Forrest S. Disruption of a novel member of a sodium/hydrogen exchanger family and DOCK3 is associated with an attention deficit hyperactivity disorder-like phenotype. J Med Genet. 2003;40:733–40. doi: 10.1136/jmg.40.10.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namekata K, Watanabe H, Guo X, Kittaka D, Kawamura K, Kimura A, Harada C, Harada T. Dock3 regulates BDNF-TrkB signaling for neurite outgrowth by forming a ternary complex with Elmo and RhoG. Genes Cells. 2012;17:688–97. doi: 10.1111/j.1365-2443.2012.01616.x. [DOI] [PubMed] [Google Scholar]
- 30.Namekata K, Harada C, Taya C, Guo X, Kimura H, Parada LF, Harada T. Dock3 induces axonal outgrowth by stimulating membrane recruitment of the WAVE complex. Proc Natl Acad Sci U S A. 2010;107:7586–91. doi: 10.1073/pnas.0914514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namekata K, Harada C, Guo X, Kimura A, Kittaka D, Watanabe H, Harada T. Dock3 stimulates axonal outgrowth via GSK-3β-mediated microtubule assembly. J Neurosci. 2012;32:264–74. doi: 10.1523/JNEUROSCI.4884-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Chen TJ, Letourneau PC, Costa LdaF, Schubert D. Modifier of cell adhesion regulates N-cadherin-mediated cell-cell adhesion and neurite outgrowth. J Neurosci. 2005;25:281–90. doi: 10.1523/JNEUROSCI.3692-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caspi E, Rosin-Arbesfeld R. A novel functional screen in human cells identifies MOCA as a negative regulator of Wnt signaling. Mol Biol Cell. 2008;19:4660–74. doi: 10.1091/mbc.E07-10-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagnamenta AT, Bacchelli E, de Jonge MV, Mirza G, Scerri TS, Minopoli F, Chiocchetti A, Ludwig KU, Hoffmann P, Paracchini S, et al. International Molecular Genetic Study Of Autism Consortium Characterization of a family with rare deletions in CNTNAP5 and DOCK4 suggests novel risk loci for autism and dyslexia. Biol Psychiatry. 2010;68:320–8. doi: 10.1016/j.biopsych.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maestrini E, Pagnamenta AT, Lamb JA, Bacchelli E, Sykes NH, Sousa I, Toma C, Barnby G, Butler H, Winchester L, et al. IMGSAC High-density SNP association study and copy number variation analysis of the AUTS1 and AUTS5 loci implicate the IMMP2L-DOCK4 gene region in autism susceptibility. Mol Psychiatry. 2010;15:954–68. doi: 10.1038/mp.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poelmans G, Buitelaar JK, Pauls DL, Franke B. A theoretical molecular network for dyslexia: integrating available genetic findings. Mol Psychiatry. 2011;16:365–82. doi: 10.1038/mp.2010.105. [DOI] [PubMed] [Google Scholar]
- 37.Alkelai A, Lupoli S, Greenbaum L, Kohn Y, Kanyas-Sarner K, Ben-Asher E, Lancet D, Macciardi F, Lerer B. DOCK4 and CEACAM21 as novel schizophrenia candidate genes in the Jewish population. Int J Neuropsychopharmacol. 2012;15:459–69. doi: 10.1017/S1461145711000903. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Y, Peng Y, Wan J, Tang G, Chen Y, Tang J, Ye WC, Ip NY, Shi L. The atypical guanine nucleotide exchange factor Dock4 regulates neurite differentiation through modulation of Rac1 GTPase and actin dynamics. J Biol Chem. 2013;288:20034–45. doi: 10.1074/jbc.M113.458612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueda S, Fujimoto S, Hiramoto K, Negishi M, Katoh H. Dock4 regulates dendritic development in hippocampal neurons. J Neurosci Res. 2008;86:3052–61. doi: 10.1002/jnr.21763. [DOI] [PubMed] [Google Scholar]
- 40.Ueda S, Negishi M, Katoh H. Rac GEF Dock4 interacts with cortactin to regulate dendritic spine formation. Mol Biol Cell. 2013;24:1602–13. doi: 10.1091/mbc.E12-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–97. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–18. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto Y, Yamauchi J, Sanbe A, Tanoue A. Dock6, a Dock-C subfamily guanine nucleotide exchanger, has the dual specificity for Rac1 and Cdc42 and regulates neurite outgrowth. Exp Cell Res. 2007;313:791–804. doi: 10.1016/j.yexcr.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto Y, Torii T, Yamamori N, Ogata T, Tanoue A, Yamauchi J. Akt and PP2A reciprocally regulate the guanine nucleotide exchange factor Dock6 to control axon growth of sensory neurons. Sci Signal. 2013;6:ra15. doi: 10.1126/scisignal.2003661. [DOI] [PubMed] [Google Scholar]
- 45.Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron. 2006;51:727–39. doi: 10.1016/j.neuron.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 46.Yang YT, Wang CL, Van Aelst L. DOCK7 interacts with TACC3 to regulate interkinetic nuclear migration and cortical neurogenesis. Nat Neurosci. 2012;15:1201–10. doi: 10.1038/nn.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majewski Ł, Sobczak M, Havrylov S, Jóźwiak J, Rędowicz MJ. Dock7: a GEF for Rho-family GTPases and a novel myosin VI-binding partner in neuronal PC12 cells. Biochem Cell Biol. 2012;90:565–74. doi: 10.1139/o2012-009. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi J, Miyamoto Y, Chan JR, Tanoue A. ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration. J Cell Biol. 2008;181:351–65. doi: 10.1083/jcb.200709033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamauchi J, Miyamoto Y, Hamasaki H, Sanbe A, Kusakawa S, Nakamura A, Tsumura H, Maeda M, Nemoto N, Kawahara K, et al. The atypical Guanine-nucleotide exchange factor, dock7, negatively regulates schwann cell differentiation and myelination. J Neurosci. 2011;31:12579–92. doi: 10.1523/JNEUROSCI.2738-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nellist M, Burgers PC, van den Ouweland AM, Halley DJ, Luider TM. Phosphorylation and binding partner analysis of the TSC1-TSC2 complex. Biochem Biophys Res Commun. 2005;333:818–26. doi: 10.1016/j.bbrc.2005.05.175. [DOI] [PubMed] [Google Scholar]
- 51.Guo L, Ying W, Zhang J, Yuan Y, Qian X, Wang J, Yang X, He F. Tandem affinity purification and identification of the human TSC1 protein complex. Acta Biochim Biophys Sin (Shanghai) 2010;42:266–73. doi: 10.1093/abbs/gmq014. [DOI] [PubMed] [Google Scholar]
- 52.Blasius AL, Brandl K, Crozat K, Xia Y, Khovananth K, Krebs P, Smart NG, Zampolli A, Ruggeri ZM, Beutler BA. Mice with mutations of Dock7 have generalized hypopigmentation and white-spotting but show normal neurological function. Proc Natl Acad Sci U S A. 2009;106:2706–11. doi: 10.1073/pnas.0813208106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruusala A, Aspenström P. Isolation and characterisation of DOCK8, a member of the DOCK180-related regulators of cell morphology. FEBS Lett. 2004;572:159–66. doi: 10.1016/j.febslet.2004.06.095. [DOI] [PubMed] [Google Scholar]
- 54.Ham H, Guerrier S, Kim J, Schoon RA, Anderson EL, Hamann MJ, Lou Z, Billadeau DD. Dedicator of cytokinesis 8 interacts with talin and Wiskott-Aldrich syndrome protein to regulate NK cell cytotoxicity. J Immunol. 2013;190:3661–9. doi: 10.4049/jimmunol.1202792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinci G, Chantot-Bastaraud S, El Houate B, Lortat-Jacob S, Brauner R, McElreavey K. Association of deletion 9p, 46,XY gonadal dysgenesis and autistic spectrum disorder. Mol Hum Reprod. 2007;13:685–9. doi: 10.1093/molehr/gam045. [DOI] [PubMed] [Google Scholar]
- 56.Griggs BL, Ladd S, Saul RA, DuPont BR, Srivastava AK. Dedicator of cytokinesis 8 is disrupted in two patients with mental retardation and developmental disabilities. Genomics. 2008;91:195–202. doi: 10.1016/j.ygeno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishikimi A, Meller N, Uekawa N, Isobe K, Schwartz MA, Maruyama M. Zizimin2: a novel, DOCK180-related Cdc42 guanine nucleotide exchange factor expressed predominantly in lymphocytes. FEBS Lett. 2005;579:1039–46. doi: 10.1016/j.febslet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Kuramoto K, Negishi M, Katoh H. Regulation of dendrite growth by the Cdc42 activator Zizimin1/Dock9 in hippocampal neurons. J Neurosci Res. 2009;87:1794–805. doi: 10.1002/jnr.21997. [DOI] [PubMed] [Google Scholar]
- 59.Detera-Wadleigh SD, Liu CY, Maheshwari M, Cardona I, Corona W, Akula N, Steele CJ, Badner JA, Kundu M, Kassem L, et al. NIMH Genetics Initiative for Bipolar Disorder Consortium Sequence variation in DOCK9 and heterogeneity in bipolar disorder. Psychiatr Genet. 2007;17:274–86. doi: 10.1097/YPG.0b013e328133f352. [DOI] [PubMed] [Google Scholar]
- 60.Nava C, Keren B, Mignot C, Rastetter A, Chantot-Bastaraud S, Faudet A, Fonteneau E, Amiet C, Laurent C, Jacquette A, et al. Prospective diagnostic analysis of copy number variants using SNP microarrays in individuals with autism spectrum disorders. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pertz OC, Wang Y, Yang F, Wang W, Gay LJ, Gristenko MA, Clauss TR, Anderson DJ, Liu T, Auberry KJ, et al. Spatial mapping of the neurite and soma proteomes reveals a functional Cdc42/Rac regulatory network. Proc Natl Acad Sci U S A. 2008;105:1931–6. doi: 10.1073/pnas.0706545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Upadhyay G, Goessling W, North TE, Xavier R, Zon LI, Yajnik V. Molecular association between beta-catenin degradation complex and Rac guanine exchange factor DOCK4 is essential for Wnt/beta-catenin signaling. Oncogene. 2008;27:5845–55. doi: 10.1038/onc.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Attar MA, Santy LC. The scaffolding protein GRASP/Tamalin directly binds to Dock180 as well as to cytohesins facilitating GTPase crosstalk in epithelial cell migration. BMC Cell Biol. 2013;14:9. doi: 10.1186/1471-2121-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tachibana M, Kiyokawa E, Hara S, Iemura S, Natsume T, Manabe T, Matsuda M. Ankyrin repeat domain 28 (ANKRD28), a novel binding partner of DOCK180, promotes cell migration by regulating focal adhesion formation. Exp Cell Res. 2009;315:863–76. doi: 10.1016/j.yexcr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Hara S, Kiyokawa E, Iemura S, Natsume T, Wassmer T, Cullen PJ, Hiai H, Matsuda M. The DHR1 domain of DOCK180 binds to SNX5 and regulates cation-independent mannose 6-phosphate receptor transport. Mol Biol Cell. 2008;19:3823–35. doi: 10.1091/mbc.E08-03-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanui T, Inayoshi A, Noda M, Iwata E, Stein JV, Sasazuki T, Fukui Y. DOCK2 regulates Rac activation and cytoskeletal reorganization through interaction with ELMO1. Blood. 2003;102:2948–50. doi: 10.1182/blood-2003-01-0173. [DOI] [PubMed] [Google Scholar]
- 67.Lu M, Kinchen JM, Rossman KL, Grimsley C, Hall M, Sondek J, Hengartner MO, Yajnik V, Ravichandran KS. A Steric-inhibition model for regulation of nucleotide exchange via the Dock180 family of GEFs. Curr Biol. 2005;15:371–7. doi: 10.1016/j.cub.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 68.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–4. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 69.Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–6. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishihara H, Maeda M, Oda A, Tsuda M, Sawa H, Nagashima K, Tanaka S. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100:3968–74. doi: 10.1182/blood-2001-11-0032. [DOI] [PubMed] [Google Scholar]
- 71.Sanders MA, Ampasala D, Basson MD. DOCK5 and DOCK1 regulate Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J Biol Chem. 2009;284:27–35. doi: 10.1074/jbc.M808010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 73.Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, Katoh H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol. 2010;190:461–77. doi: 10.1083/jcb.201005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meller N, Irani-Tehrani M, Ratnikov BI, Paschal BM, Schwartz MA. The novel Cdc42 guanine nucleotide exchange factor, zizimin1, dimerizes via the Cdc42-binding CZH2 domain. J Biol Chem. 2004;279:37470–6. doi: 10.1074/jbc.M404535200. [DOI] [PubMed] [Google Scholar]
- 75.Terasawa M, Uruno T, Mori S, Kukimoto-Niino M, Nishikimi A, Sanematsu F, Tanaka Y, Yokoyama S, Fukui Y. Dimerization of DOCK2 is essential for DOCK2-mediated Rac activation and lymphocyte migration. PLoS One. 2012;7:e46277. doi: 10.1371/journal.pone.0046277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel M, Pelletier A, Côté JF. Opening up on ELMO regulation: New insights into the control of Rac signaling by the DOCK180/ELMO complex. Small GTPases. 2011;2:268–75. doi: 10.4161/sgtp.2.5.17716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Premkumar L, Bobkov AA, Patel M, Jaroszewski L, Bankston LA, Stec B, Vuori K, Côté JF, Liddington RC. Structural basis of membrane targeting by the Dock180 family of Rho family guanine exchange factors (Rho-GEFs) J Biol Chem. 2010;285:13211–22. doi: 10.1074/jbc.M110.102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobayashi S, Shirai T, Kiyokawa E, Mochizuki N, Matsuda M, Fukui Y. Membrane recruitment of DOCK180 by binding to PtdIns(3,4,5)P3. Biochem J. 2001;354:73–8. doi: 10.1042/0264-6021:3540073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Côté JF, Motoyama AB, Bush JA, Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol. 2005;7:797–807. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–52. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanai A, Ihara S, Ohdaira T, Shinohara-Kanda A, Iwamatsu A, Fukui Y. Identification of DOCK4 and its splicing variant as PIP3 binding proteins. IUBMB Life. 2008;60:467–72. doi: 10.1002/iub.67. [DOI] [PubMed] [Google Scholar]
- 82.Meller N, Westbrook MJ, Shannon JD, Guda C, Schwartz MA. Function of the N-terminus of zizimin1: autoinhibition and membrane targeting. Biochem J. 2008;409:525–33. doi: 10.1042/BJ20071263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng H, Hu B, Liu KW, Li Y, Lu X, Cheng T, Yiin JJ, Lu S, Keezer S, Fenton T, et al. Activation of Rac1 by Src-dependent phosphorylation of Dock180(Y1811) mediates PDGFRα-stimulated glioma tumorigenesis in mice and humans. J Clin Invest. 2011;121:4670–84. doi: 10.1172/JCI58559. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Feng H, Hu B, Jarzynka MJ, Li Y, Keezer S, Johns TG, Tang CK, Hamilton RL, Vuori K, Nishikawa R, et al. Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine residue Y722 by Src family kinases mediates EGFRvIII-driven glioblastoma tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:3018–23. doi: 10.1073/pnas.1121457109. [DOI] [PMC free article] [PubMed] [Google Scholar]


