Abstract
DNA damage causally contributes to cancer development and tissue degeneration with aging.1 Cellular DNA damage responses (DDR) mediate cell cycle arrest to allow time for DNA repair, or induce cellular senescence and apoptosis to eliminate damaged cells.2 In contrast to cell-autonomous DNA damage responses, it remains less clear how organisms respond to genome instability in certain cell types and how distinct tissues interact when responding to tissue-specific DNA damage. C. elegans comprises an intriguing system to study the interaction between distinct tissues as germ cells evoke conserved DDR mechanisms, while somatic tissues are highly radio resistant.3,4 The recent discovery of the “germline DNA damage-induced systemic stress response” (GDISR) sheds new light on non-cell autonomous responses to genome instability.5 GDISR is mediated by ERK MAP kinase MPK-1 induced putative secreted peptides that are associated with innate immunity. The innate immune response leads to activation of the ubiquitin-proteasome-system (UPS) in somatic tissues, which confers systemic stress resistance. We discuss the role of the innate immunity in mediating systemic DNA damage responses and how UPS activity promotes endurance of somatic tissues.
Keywords: DNA damage response, Innate immunity, stress resistance, C. elegans, aging
In contrast to cell-autonomous DNA damage checkpoint mechanisms, it has remained largely unexplored how multicellular organisms respond systemically to tissue-specific DNA damage. In the metazoan model system C. elegans GDISR leads to enhanced resistance to heat and oxidative stress in somatic tissues.5 The somatic stress resistance is evoked in response to distinct types of DNA damage such as UV-induced bulky lesions, IR-induced DNA strand breaks, HU-induced replication stalling, and even meiotic double strand breaks (DSBs). In adult C. elegans, only germ cells proliferate while somatic tissues are postmitotic. DNA damage checkpoints halt the cell cycle progression in mitotically dividing germ cells resulting in a drop of progeny production.3 In animals that were exposed to genotoxic stress, the generation of offspring is shifted to later ages.5 These observations suggest that in analogy to cellular DNA damage checkpoints that allow time for DNA repair, the somatic stress resistance might function as systemic DNA damage checkpoint that preserves somatic functions when offspring generation is delayed as a consequence of germ cell DNA damage.
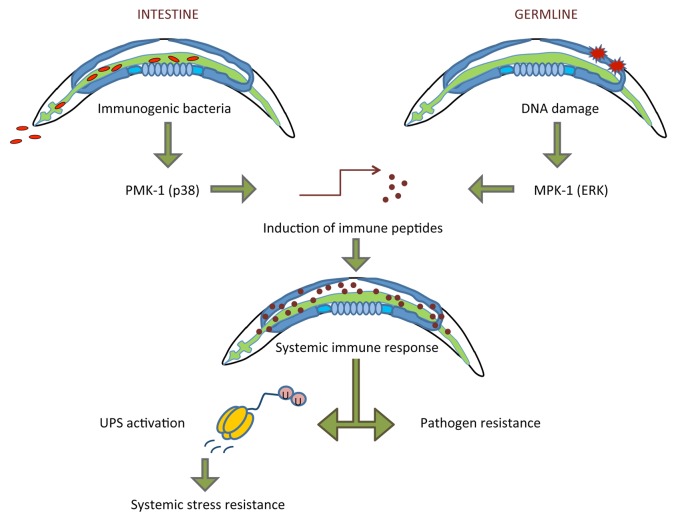
Upon DNA damage, the C. elegans ERK1/2 MAP kinase MPK-1 is hyper-phosphorylated in wild type worms but not in worms lacking the germline due to mutation in the Notch receptor glp-1. Both glp-1 and mpk-1 deficient animals also fail to develop DNA damage-induced stress resistance, altogether suggesting that MPK-1 activity in germ cells is required for evoking somatic stress resistance upon genotoxic stress (Fig. 1). MAPK signaling mediates responses to DNA damage and to pathogen infection in various species including C. elegans.6,7 Upon DNA damage, a gene expression program is induced that bears similarity to pathogen responses that are mediated through the MAPKs MPK-1/ERK and PMK-1/p38. Both mpk-1 and glp-1 mutant worms fail to upregulate the expression of innate immunity-associated genes upon genotoxic stress, indicating that MPK-1 activity in the germline is required for inducing innate immune genes. Indeed, not only DNA damage but also exposure to pathogenic bacteria leads to elevated stress resistance.5 In contrast to GDISR, the pathogen-induced stress resistance is mediated by the p38 MAPK PMK-1. The similarly induced gene sets largely comprise putative secreted peptides, suggesting that innate immune factors might function as diffusible mediators of the systemic stress response. These observations established that germ cell DNA damage triggers an effective innate immune response that results in systemic stress resistance.
Figure 1. Model for GDISR. Infection of the intestine leads to induction of the p38 MAPK PMK-1, while DNA damage in germ cells activates the ERK1/2 MAPK MPK-1. MAPK signaling triggers transcriptional induction of innate immune genes that comprise putative secreted peptides. The innate immune response mediates pathogen resistance and, through activating the UPS, confers systemic stress resistance.
Interestingly, both mpk-1 mutant worms and germline deficient glp-1 mutants exhibit elevated baseline stress resistance when compared with wild type control animals.5,8 The stress resistance of glp-1 mutants is thought to reflect the general stress resistance that can be caused by a complete ablation of the reproductive germline. The “germline pathway” of stress resistance is known to be dependent on the activity of the FOXO transcription factor DAF-16 in somatic tissues.8 Intriguingly, GDISR functions entirely independently of the DAF-16 pathway. The increased base line immune gene expression in the mpk-1 mutant strain is likely to be mediated via a compensatory MAPK signaling.5 Indeed combining glp-1 mutation with lack of DAF-16 and mpk-1 mutation with pmk-1/p38 RNAi knock down reduced the baseline stress resistance of the 2 strains without having any effect on the lack of DNA damage-induced heat stress tolerance.5
Innate immune responses to DNA damage from worms to humans
C. elegans possesses an ancestral innate immune system that is comprised of a large number of closely related CUB (complement C1r/C1s, Uegf, Bmp1) domain peptides, C-type lectins, and other antimicrobial peptides.7,9,10 The functioning of this ancestral immune system in pathogen defense is yet poorly understood. C-type lectins recognize sugar moieties on pathogens, while in humans, CUB domains are found in complement factors and secreted growth factors.10 The closely related putative secreted peptides that are induced upon DNA damage and upon pathogen infection are likely to act in a highly redundant fashion since inactivation of individual factors does not reduce pathogen resistance.11 Immune reactions to DNA damage also occur in higher species. Some bacterial infections themselves can induce DNA damage such as E. coli and Helicobacter pylori infection in HeLa cells12 and host cells,13 respectively. Additionally, replication fork stalling and genotoxic stress leads to induction of ligands for the NKG2D receptor that is expressed on natural killer cells.14 The human innate immune system can recognize microbial and host DNA by the toll-like receptor TLR-9, the cGAMP synthase cGAS, and the inflammasome15-17 and it will be highly interesting to determine whether any of these DNA sensing mechanisms might also play a role in the response to nuclear DNA damage.
Physiological consequences of cell non-autonomous immune responses to DNA damage are particularly apparent in the human skin where UV irradiation results in highly complex immune reactions ranging from local inflammation to systemic immune suppression.18 Human cells that enter senescence as a result of high irradiation doses secrete cytokines and growth factors that may have both tumor-promoting and -suppressive consequences.19 In mice, DNA damage-induced IL-6 secretion by thymic epithelial cells establishes a chemo-protective niche for lymphoma cells,20 while p53-induced cytokine release may induce both innate and adaptive immune responses leading to tumor cell clearance.21,22 It is thought that DNA damage accumulation with aging causes chronic inflammation that contributes to tissue dysfunction and immune aging.23 Cell non-autonomous responses to DNA damage are thus likely to play a major role not only in tumor suppression, but also in the physiological adjustments of the organism with aging. However, both the mechanisms and the consequences of systemic DNA damage responses remained poorly understood. GDISR confers systemic protection of somatic tissues against multiple stress factors and suggests that innate immunity plays an important role in adjusting somatic endurance to reproduction.
The role of the ubiquitin-proteasome system (UPS) in systemic stress resistance
GDISR is established in the soma through increased UPS activity in somatic tissues.5 Activation of the UPS likely leads to enhanced protein homeostasis that elevates resistance to environmental stress. It has been shown that increased UPS activity can replace the requirement for heat shock proteins24 and forced expression of the proteasome subunit RPN-6 enhances resistance to proteotoxic conditions such as heat and oxidative stress.25 Also life span extension in response to dietary restriction or lack of insulin/insulin-like growth factor-1-signaling is dependent on the activity of proteasomal E3 ubiquitin ligase complexes.26,27 Efficient protein turnover might ensure that damaged proteins in somatic cells are eliminated. As the recognition of DNA damage is highly sensitive with a low threshold for triggering DNA damage responses in dividing cells, activation of the UPS might ameliorate protein turnover already at low damage levels before massive protein misfolding ensues in a toxic environment. Intriguingly, somatic UPS is activated upon induction of an innate immune response. The enhanced proteostasis in somatic tissues might thus confer elevated somatic endurance also under conditions of pathogen infection. In addition, it is conceivable that UPS activity facilitates the successful switch of the cellular proteome to the production of large amounts of immune peptides. The unfolded protein response (UPR) in the endoplasmic reticulum is required for survival during immune activation28; thus, UPS might facilitate the successful establishing of the innate immune response by alleviating pressure from the protein-folding machinery as well as by increasing the pool of free amino acids through enhanced protein degradation. Consistent with this hypothesis, a transient immune response triggered by DNA damage infliction or short-term exposure with immunogenic B. subtilis leads to efficient activation of protein turnover, while sustained feeding on B. subtilis evokes a strong protein accumulation indicative of chronic overloading of the protein degradation machinery.
Outlook
It will be highly interesting to address whether innate immune responses induced by DNA damage in humans might systemically enhance tissue maintenance before chronic inflammation is manifested. Conceptually consistent with this possibility, components of the mammalian innate immune system have recently been implicated in the maintenance of tissue homeostasis and regeneration.29,30 We propose that GDISR comprises an ancestral somatic stress resistance program that protects the organism from pathogen infection and prolongs somatic preservation when genomically compromised germ cells require extended somatic endurance to ensure offspring generation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Ermolaeva M received the EMBO long-term fellowship, Schumacher B acknowledges funding from the DFG (CECAD, SFB 829, and KFO 286), the ERC (Starting grant 260383), Marie Curie (FP7 ITN CodeAge 316354, aDDRess 316390, MARRIAGE 316964, and ERG 239330), the German-Israeli Foundation (GIF, 2213–1935.13/2008 and 1104–68.11/2010), the Deutsche Krebshilfe (109453), and the BMBF (SyBaCol).
References
- 1.Schumacher B, Garinis GA, Hoeijmakers JH. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–45. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage--induced apoptosis and cell cycle arrest in C. elegans. Mol Cell. 2000;5:435–43. doi: 10.1016/S1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- 4.Hartman PS, Simpson VJ, Johnson T, Mitchell D. Radiation sensitivity and DNA repair in Caenorhabditis elegans strains with different mean life spans. Mutat Res. 1988;208:77–82. doi: 10.1016/S0165-7992(98)90003-3. [DOI] [PubMed] [Google Scholar]
- 5.Ermolaeva MA, Segref A, Dakhovnik A, Ou HL, Schneider JI, Utermöhlen O, Hoppe T, Schumacher B. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature. 2013;501:416–20. doi: 10.1038/nature12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutkowski R, Dickinson R, Stewart G, Craig A, Schimpl M, Keyse SM, Gartner A. Regulation of Caenorhabditis elegans p53/CEP-1-dependent germ cell apoptosis by Ras/MAPK signaling. PLoS Genet. 2011;7:e1002238. doi: 10.1371/journal.pgen.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irazoqui JE, Urbach JM, Ausubel FM. Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol. 2010;10:47–58. doi: 10.1038/nri2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–5. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 9.Thomas JH. Concerted evolution of two novel protein families in Caenorhabditis species. Genetics. 2006;172:2269–81. doi: 10.1534/genetics.105.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivers RP, Youngman MJ, Kim DH. Transcriptional responses to pathogens in Caenorhabditis elegans. Curr Opin Microbiol. 2008;11:251–6. doi: 10.1016/j.mib.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–51. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 13.Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M, Kalali B, Gerhard M, Sartori AA, Lopes M, et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A. 2011;108:14944–9. doi: 10.1073/pnas.1100959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–7. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz T, Schwarz A. Molecular mechanisms of ultraviolet radiation-induced immunosuppression. Eur J Cell Biol. 2011;90:560–4. doi: 10.1016/j.ejcb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–66. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 23.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012;24:488–93. doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friant S, Meier KD, Riezman H. Increased ubiquitin-dependent degradation can replace the essential requirement for heat shock protein induction. EMBO J. 2003;22:3783–91. doi: 10.1093/emboj/cdg375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilchez D, Morantte I, Liu Z, Douglas PM, Merkwirth C, Rodrigues AP, Manning G, Dillin A. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489:263–8. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 26.Ghazi A, Henis-Korenblit S, Kenyon C. Regulation of Caenorhabditis elegans lifespan by a proteasomal E3 ligase complex. Proc Natl Acad Sci U S A. 2007;104:5947–52. doi: 10.1073/pnas.0700638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrano AC, Liu Z, Dillin A, Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature. 2009;460:396–9. doi: 10.1038/nature08130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson CE, Kooistra T, Kim DH. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature. 2010;463:1092–5. doi: 10.1038/nature08762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. 2011;17:2161–71. doi: 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–30. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]