Abstract
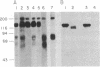
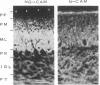
Neuron-glia cell adhesion molecule (Ng-CAM) has previously been shown to be present exclusively on neurons and to mediate adhesion between neuronal membranes and glial cells. In the present study, its chain structure, binding functions, and relation to N-CAM (the other known CAM on neurons) were investigated further. Three polypeptide components of chicken Ng-CAM (Mr 200,000, 135,000, and 80,000) have been isolated. By using specific antisera against each component, the Mr 135,000 and Mr 80,000 components were found to cross-react antigenically with the Mr 200,000 component but not with each other. The conclusion that the Mr 135,000 and 80,000 components are structurally related to different regions of the Mr 200,000 component was further supported by the finding that 32P could be incorporated in vitro into the Mr 200,000 and 80,000 components but not into the Mr 135,000 component. Ng-CAM appears to be involved in both neuron-glia adhesion and neuron-neuron adhesion by distinguishable mechanisms that appear to involve different sites or conformations of the molecule. Polyclonal antibodies and a monoclonal antibody against Ng-CAM both inhibited adhesion between glia and neurons derived from brain, cerebellum, and retina. In contrast, antibodies against N-CAM (which inhibit neuron-neuron adhesion) did not inhibit neuron-glia adhesion. These findings confirm the proposed function of Ng-CAM in neuron-glia adhesion. In addition, however, Ng-CAM was found to be involved directly or indirectly in neuron-neuron adhesion. Non-cross-reactive polyclonal anti-Ng-CAM and anti-N-CAM antibodies each inhibited the aggregation of neurons from whole brain and cerebellum and the inhibition was greater when both antibodies were present together. In contrast, monoclonal anti-Ng-CAM antibodies were found that inhibited neuron-glia adhesion but did not inhibit neuronal cell aggregation. The amount of Ng-CAM expressed on neurons was not directly predictive of the effect of anti-Ng-CAM antibodies on their homotypic aggregation. Although Ng-CAM and N-CAM can be expressed simultaneously on individual neurons, the ratio of N-CAM to Ng-CAM ranged from 1.5 for cerebellar cells to 10.0 for retinal cells. While, as expected, retinal cell aggregation was inhibitable only by anti-N-CAM, cerebellar cells, which expressed at least as much Ng-CAM as brain cells, showed significantly less inhibition by anti-Ng-CAM antibodies. These findings raise the possibility that Ng-CAM may actually interact with N-CAM to yield non-linear effects. That Ng-CAM and N-CAM may function differently in vivo was suggested by their distribution in sections of brain regions. Within the cerebellum, for example, immunofluorescent anti-N-CAM staining was relatively uniform in all layers; in contrast anti-Ng-CAM staining was absent on dividing external granule cells and was present in greatest abundance on processes of post-mitotic migratory cells in the molecular layer. These observations are consistent with the hypothesis that Ng-CAM mediates neuron-glia adhesion and is thereby also involved in neuronal migration along radial glial cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Brackenbury R., Rutishauser U., Edelman G. M. Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):387–391. doi: 10.1073/pnas.78.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gallin W. J., Delouvée A., Cunningham B. A., Thiery J. P. Early epochal maps of two different cell adhesion molecules. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4384–4388. doi: 10.1073/pnas.80.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Modulation of cell adhesion during induction, histogenesis, and perinatal development of the nervous system. Annu Rev Neurosci. 1984;7:339–377. doi: 10.1146/annurev.ne.07.030184.002011. [DOI] [PubMed] [Google Scholar]
- Goridis C., Deagostini-Bazin H., Hirn M., Hirsch M. R., Rougon G., Sadoul R., Langley O. K., Gombos G., Finne J. Neural surface antigens during nervous system development. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):527–537. doi: 10.1101/sqb.1983.048.01.057. [DOI] [PubMed] [Google Scholar]
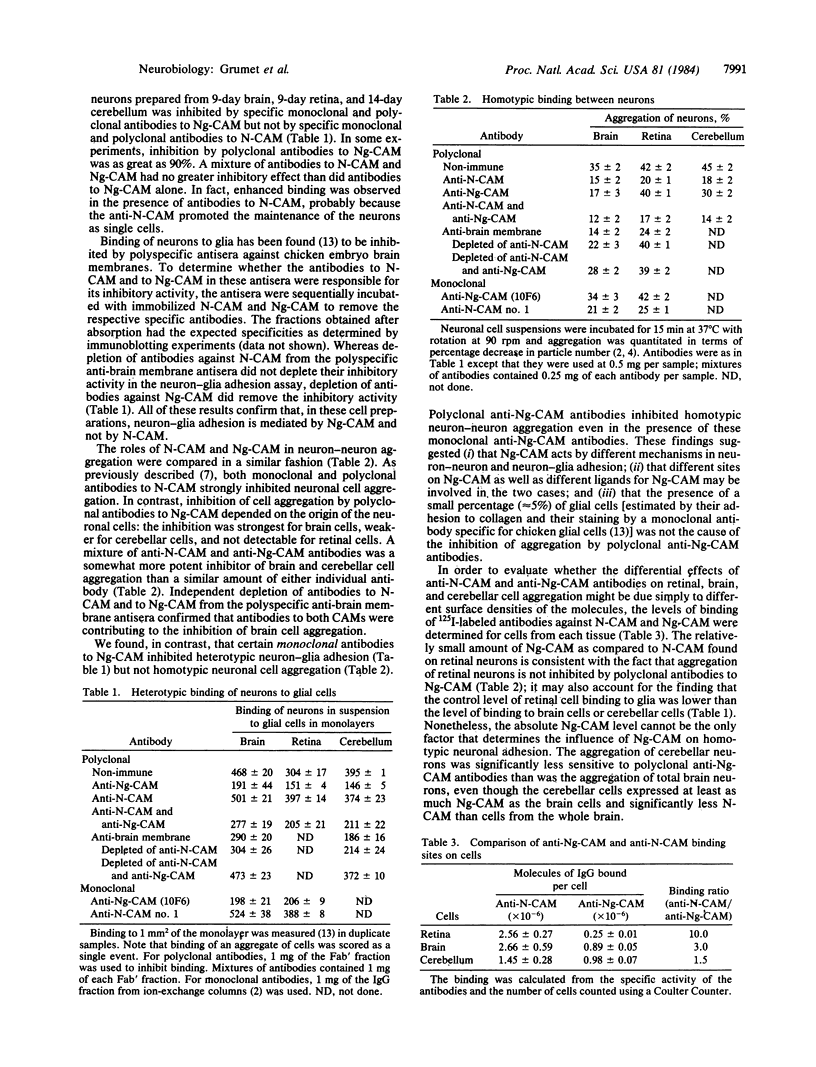
- Grumet M., Edelman G. M. Heterotypic binding between neuronal membrane vesicles and glial cells is mediated by a specific cell adhesion molecule. J Cell Biol. 1984 May;98(5):1746–1756. doi: 10.1083/jcb.98.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Edelman G. M. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984 Jan;81(1):267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Rutishauser U., Edelman G. M. Neuron-glia adhesion is inhibited by antibodies to neural determinants. Science. 1983 Oct 7;222(4619):60–62. doi: 10.1126/science.6194561. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Chuong C. M., Edelman G. M. Evolutionary conservation of key structures and binding functions of neural cell adhesion molecules. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6881–6885. doi: 10.1073/pnas.81.21.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Edelman G. M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971 Mar;141(3):283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Schachner M., Faissner A., Kruse J., Lindner J., Meier D. H., Rathjen F. G., Wernecke H. Cell-type specificity and developmental expression of neural cell-surface components involved in cell interactions and of structurally related molecules. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):557–568. doi: 10.1101/sqb.1983.048.01.060. [DOI] [PubMed] [Google Scholar]
- Sorkin B. C., Hoffman S., Edelman G. M., Cunningham B. A. Sulfation and phosphorylation of the neural cell adhesion molecule, N-CAM. Science. 1984 Sep 28;225(4669):1476–1478. doi: 10.1126/science.6474186. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Duband J. L., Rutishauser U., Edelman G. M. Cell adhesion molecules in early chicken embryogenesis. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6737–6741. doi: 10.1073/pnas.79.21.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold F. In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]