Abstract
Background
To determine if expenditures for dentistry (DENT) correlate with severity of chronic kidney disease (CKD).
Methods
A total of 10,457 subjects were enrolled from January 2008 to December 2010, divided into three groups: healthy control (HC) group (n = 1,438), high risk (HR) group (n = 3,392), and CKD group (n = 5,627). Five stages were further categorized for the CKD group. OPD utilization and expenditures for western medicine (WM), DENT, and TCM (traditional Chinese medicine) were analyzed retrospectively (2000–2008) using Taiwan's National Health Insurance Research Database. Three major areas were analyzed among groups CKD, HR and HC in this study: 1) demographic data and medical history; 2) utilization (visits/person/year) and expenditures (9-year cumulative expenditure, expenditure/person/year) for OPD services in WM, DENT, and TCM; and 3) utilization and expenditures for dental OPD services, particularly in dental filling, root canal and periodontal therapy.
Results
OPD utilization and expenditures of WM increased significantly for the CKD group compared with the HR and HC groups, and increased steadily along with the severity of CKD stages. However, overall DENT and TCM utilization and expenditures did not increase for the CKD group. In comparison among different CKD stages, the average expenditures and utilization for DENT including restorative filling and periodontal therapy, but not root canal therapy, showed significant decreases according to severity of CKD stage, indicating less DENT OPD utilization with progression of CKD.
Conclusions
Patients with advanced CKD used DENT OPD service less frequently. However, the connection between CKD and DENT service utilization requires further study.
Introduction
Chronic kidney disease (CKD) affects an increasing number of people around the world, and the prevalence of CKD appears to have increased over the past decade [1]. Evidence from the United States Renal Data System 2011 suggests that from the year 2000, Taiwan has had the highest incidence and prevalence of end-stage renal disease (ESRD) among all of the countries examined, with approximately 400 per million of the population affected [2], and ESRD is one of the leading causes of death in Taiwan [3]. In response, the government of Taiwan has launched a project of multidisciplinary care for CKD patients since 2004. It has been demonstrated that CKD is linked to many morbidities, creating a heavy burden on the medical insurance system [4]. Expenditures for CKD create significant economic burdens on patients as well and have become a major challenge for medical care systems [5]. Nevertheless, in light of the health-related expenditures, CKD treatment has been shown to be cost effective as it slows disease progression and prevents the development of comorbidities [5], [6].
CKD, a complex comorbid condition with multiple manifestations, is closely linked with cardiovascular disease, hypertension, anemia, diabetes, malnutrition, dyslipidemia, bone and mineral disorders, all of which increase the chances of morbidity, mortality, and healthcare costs [2], [7], [8]. In recent years, numerous studies have demonstrated higher rates of oral pathology in CKD patients with one or more oral symptoms; thus, a variety of changes occur in the oral cavity are strongly correlated with CKD itself or with CKD therapy [9], [10], [11]. In addition, poor oral health status is closely associated with markers of malnutrition, inflammation and increased risk of death for patients undergoing hemodialysis [12], [13]. Although the exact causality between diseases is intricate [11], [14], studies have demonstrated that poor oral health conditions and its severe consequences are closely associated with the incidence or progression of CKD [15], [16], [17]. Accordingly, it is widely accepted that CKD can have a critical impact on oral health; likewise, poor oral health has been linked to CKD [11].
Treatment of CKD through multidisciplinary approaches may improve patient outcomes and be cost-effective [6], [18], [19], [20]. On the basis of these findings, it should be emphasized that monitoring and maintaining the oral health status of CKD patients, as well as in patients who are considered for renal dialysis or as transplant candidates is essential. This would justify an increased attention to and better awareness of dental care in CKD patients. Furthermore, it might be possible to achieve better clinical and economic outcomes for CKD patients if patients are comprehensively evaluated and referred to the relevant specialty early, including dental services. However, to date, there is no retrospective epidemiologic study from a general population performed by analyzing a nationwide hospital-based database to investigate the relationship between the utilization and expenditures of dental services and CKD progression.
Recent publications focusing on medical care expenditures in CKD have concentrated mainly on Western Medicine (WM), including hospitalization [21], [22], pharmacy services [23], [24] and individual co-morbidity costs [4], [25], [26], [27], [28], [29]. Despite the emerging studies that have investigated possible associations between oral health and CKD [11], the correlation between Dentistry (DENT) and Traditional Chinese Medicine (TCM) outpatient (OPD) utilization and expenditures and the progression of kidney disease in the CKD population is largely unknown.
To the best of our knowledge, there are no large, hospital-based studies which outline the relationship between DENT and TCM utilization and expenditures for CKD patients. The objective of this study was designed to use a nationwide case control cohort to investigate DENT OPD utilization and associated expenditures in patients at various stages of CKD.
Methods
Study design and populations
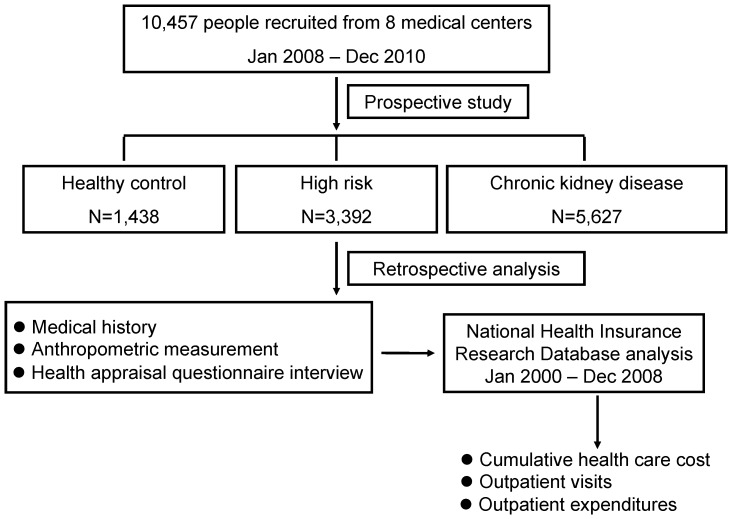
A case-control study was conducted over 3 years. Three study groups, including healthy control (HC), high risk (HR) and chronic kidney disease (CKD), were collected throughout the period January 1, 2008, to December 31, 2010. A total of 10,457 Taiwan people all covered by the National Health Insurance Program (NHIP) from 8 medical centers located in different regions of Taiwan were the subjects of this study. Participants recruited for this study were randomly selected from the participating medical centers. The design for this study is a cluster randomized without age- or gender-match. This kind of design is vulnerable to lack of comparability; however, this design makes it easy to increase sample size and calculate expenditure more accurately. A detailed medical history, anthropometric measurements, laboratory analyses, and a health appraisal questionnaire eliciting demographic, socioeconomic and behavioral risk factors were conducted through face-to-face interviews with each participant by well-trained investigators at the initial visit. Written informed consent was obtained from all study participants. At the end of the three-year study, all participants' claims data were analyzed, and their OPD utilization and expenditure, particularly in WM, DENT, and TCM, were analyzed retrospectively from the National Health Insurance Research Database (NHIRD). A flow chart summarizing the selection process of the study participants is given in Figure 1. This study protocol involving human subjects was reviewed and approved by the Institutional Review Board of Tri-Service General Hospital, National Defense Medical Center and other participating medical centers.
Figure 1. Flow chart of the selection process of the study participants.
Definition of participants
All eligible participants were categorized into 3 mutually exclusive categories: “HC”, “HR”, and “CKD,” based on estimated glomerular filtration rate (eGFR) and medical history. The level of eGFR was calculated using the Modification of Diet in Renal Disease study equation [30].
Individuals in the HC group, eGFR ≥60 (mL/min/1.73 m2) without renal abnormalities or family history of renal diseases, were recruited from health examination in the communities or participating hospital-affiliated health evaluation units.
Patients in the HR group were eGFR ≥60 (mL/min/1.73 m2) and had to meet one of the following criteria: (1) diagnosed diabetes mellitus (DM), hypertension, cardiovascular disease; (2) family member diagnosed with CKD or receiving dialysis treatment.
The CKD stages were defined according to clinical practice guidelines developed under the Kidney Disease: Improving Global Outcomes (KDIGO) classification system established by the National Kidney Foundation [31], with further classification of stage 3 disease into stage3a (eGFR <60 and ≥45 mL/min/1.73 m2) and stage3b (eGFR <45 and ≥30 mL/min/1.73 m2) [32].
Basic data collection
A face-to-face interview was conducted to obtain participants' information regarding socioeconomic status (gender, age, residence district, occupation, household income, marital status and education level) and oral health behavior (alcohol consumption, betel nut chewing and cigarette smoking habits). The geographic locations of residency were grouped into three categories of northern, central, and southern Taiwan.
Anthropometric evaluation included measurements of wrist circumference, body weight and height to calculate body mass index (BMI). According to the Bureau of Health Promotion, Department of Health, Taiwan, BMI less than 18.5 was defined as underweight, 18.5–24 as normal, between 24 and 27 as overweight, and higher than 27 as obese [33].
Retrospective analysis of past 9 years OPD utilization and expenditure of WM, DENT and TCM
Data source and validation
This hospital-based study recruited individuals from the NHIRD provided by the Bureau of National Health Insurance (BNHI), and released by National Health Research Institutes (NHRI), Miaoli, Taiwan (http://www.nhri.org.tw/nhird/). Taiwan initiated the National Health Insurance (NHI) program in March 1995 to offer affordable medical care for all residents. In addition, Taiwan has the highest incidence and prevalence of end-stage renal disease globally [2]. In response, the government of Taiwan launched a project of multidisciplinary care for CKD patients in 2004. This service is available throughout Taiwan and is covered by the NHI program. Furthermore, dental care is widely available and covered by the NHI program in Taiwan.
A distinctive characteristic of the NHIRD is its comprehensive coverage of 99% of the population, for whom the NHI program has provided universal medical coverage, comprehensive benefits, and unrestricted access to any medical institution of the patient's choice [34]. Moreover, regular justifications and claims of the medical charts are performed by the BNHI of Taiwan to ensure the fidelity of the coding system in the database. The dataset after merging from Taiwan's NHIRD was transcribed for further statistical analysis. Thus, NHIRD provides a good statistical representation for analyzing epidemiological profiles of the entire population of Taiwan. Several high-quality international peer-reviewed studies have been published based on the NHIRD data, supporting its validity for medical research [35], [36], [37], [38], [39].
Analysis of WM, DENT, and TCM utilization and expenditure
The OPD prescription and therapeutic coding system for WM (01–15, 22, 23, 81, 82, 83, 84) DENT (40–49), and TCM (60–69) of each participant were retrieved and transcribed from the NHIRD. Utilization and expenditures for WM, DENT and TCM, including cumulative medical care expenditures, annual OPD visits, and OPD expenditure per person from January 2000 to December 2008 were further analyzed.
Analysis of DENT expenditure and utilization
OPD expenditures and utilization of DENT were defined according to diagnostic code and NHI therapeutic procedure codes. The coding system by the NHI in Taiwan is performed according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Diagnostic and therapeutic procedure codes were used to define expenditure and utilization of the three most common DENT procedures: restorative therapy (ICD-9-CM code: 5210–5219; therapeutic code: 89001–89012), root canal therapy (ICD-9-CM code: 5220–5229; therapeutic code: 90001–90020), and periodontal therapy (ICD-9-CM code: 5230–5239; therapeutic code: 91001–91014). These patients' first ambulatory care visits for DENT treatment between January 1, 2000, and December 31, 2008, were assigned as the index date use of medical care.
Statistical analyses
All statistical analyses were carried out using the SAS 9.13 system (SAS system for windows, version 8.2. SAS Institute Inc. Cary. NC) and SPSS 18.0 software package (SPSS Inc., Chicago, Illinois). Mean expenditures and frequency of medical care visits where appropriate were used to describe the characteristics of the study groups. Statistical differences in categorical variables and in continuous variables between the three groups were determined using the chi-square test and one-way analysis of variance (ANOVA), respectively. Differences between each group/stage were assessed by Scheffe post hoc tests. Level of statistical significance was set at P<0.05.
Results
Demographic differences among subjects
Among the 10,457 eligible participants, clinical diagnosis was made and three mutually exclusive patient groups were categorized. Data were collected for 1,438 patients in the HC group, 3,392 patients in the HR group, and 5,627 patients in the CKD group. The number of subjects for each CKD stage (stage 1 to stage 5) was 917 (stage 1), 1108 (stage 2), 763 (stage 3a), 780 (stage 3b), 1036 (stage 4), and 1023 (stage 5). Of all the eligible individuals, significant differences existed in demographic characteristics and socioeconomic status among groups (all p<0.001) (Table 1). The majority of the patients in the CKD group were older, with a mean age 61.04±15.21 years compared with 57.59±14.30 yrs and 46.62±15.15 yrs in the HR and HC groups, respectively. Of the analyzed socioeconomic variables, patients in the CKD group were more likely to be unemployed (56.7%), have a household income ≤40,000 NT$ (71.8%), and lower education achievement<college level (84.3%) when compared with other groups (Table 1).
Table 1. Demographic characteristics and socioeconomic status of eligible subjects.
| HC (n = 1,438) | HR (n = 3,392) | CKD (n = 5,627) | |||||
| Variables | n | % | n | % | n | % | P a |
| Gender | <0.001 | ||||||
| Male | 477 | 33.2 | 1,554 | 45.8 | 3,247 | 57.7 | |
| Female | 961 | 66.8 | 1,838 | 54.2 | 2,380 | 42.3 | |
| Age (years) | <0.001 | ||||||
| mean ±SD | 46.62±15.15 | 57.59±14.30 | 61.04±15.21 | <0.001 | |||
| <45 | 680 | 47.3 | 616 | 18.2 | 796 | 14.1 | |
| 45–64 | 551 | 38.3 | 1,589 | 46.8 | 2,285 | 40.6 | |
| 65–74 | 138 | 9.6 | 794 | 23.4 | 1,386 | 24.6 | |
| >75 | 69 | 4.8 | 393 | 11.6 | 1,160 | 20.6 | |
| Living district (area of Taiwan) | <0.001 | ||||||
| Northern | 619 | 43.0 | 1,206 | 35.6 | 2,419 | 43.0 | |
| Central | 413 | 28.7 | 1,127 | 33.2 | 1,373 | 24.4 | |
| Southern | 406 | 28.3 | 1,059 | 31.2 | 1,835 | 32.6 | |
| Marital status | <0.001 | ||||||
| Married (%) | 1,017 | 70.7 | 2,754 | 81.2 | 4,496 | 79.9 | |
| Single (%) | 334 | 23.2 | 326 | 9.6 | 546 | 9.7 | |
| Other (%) | 88 | 6.1 | 312 | 9.2 | 585 | 10.4 | |
| Occupation | <0.001 | ||||||
| None | 362 | 25.2 | 1,638 | 48.3 | 3,191 | 56.7 | |
| Government | 104 | 7.2 | 149 | 4.4 | 242 | 4.3 | |
| Agriculture | 11 | 0.8 | 64 | 1.9 | 135 | 2.4 | |
| Business | 135 | 9.4 | 319 | 9.4 | 445 | 7.9 | |
| Labor | 121 | 8.4 | 282 | 8.3 | 405 | 7.2 | |
| Others | 705 | 49 | 940 | 27.7 | 1,210 | 21.5 | |
| Household income (NT$) | <0.001 | ||||||
| None (%) | 224 | 15.6 | 1,238 | 36.5 | 2,481 | 44.1 | |
| <40,000 (%) | 387 | 26.9 | 987 | 29.1 | 1,559 | 27.7 | |
| 4–90,000 (%) | 520 | 36.2 | 814 | 24.0 | 1,092 | 19.4 | |
| >90,000 (%) | 306 | 21.3 | 353 | 10.4 | 495 | 8.8 | |
| Education level | <0.001 | ||||||
| <Junior high (%) | 267 | 18.6 | 1,442 | 42.5 | 2,864 | 50.9 | |
| Senior high (%) | 598 | 41.6 | 1,323 | 39.0 | 1,879 | 33.4 | |
| >College (%) | 572 | 39.8 | 628 | 18.5 | 883 | 15.7 | |
Unless otherwise indicated, values are number (percentage). The eligible subjects were recruited patient from 2008 to 2010. N = 10,457.
Abbreviations: CKD, chronic kidney disease; HC, healthy control; HR, high risk; NT$, new Taiwan dollars.
Chi-square test. P<0.05 was considered statistically significant.
Family history, anthropometric measurements and oral health habits of participants
Among the eligible individuals, significant differences existed in family history among groups, with a higher prevalence of diabetes mellitus, heart diseases, and cerebrovascular diseases (CVDs) observed in CKD and HR patients than in HC patients (all p<0.001) (Table 2).
Table 2. Medical history, anthropometric measurements and oral habits of eligible patients.
| HC (n = 1,438) | HR (n = 3,392) | CKD (n = 5,627) | |||||
| Variables | n | % | n | % | n | % | P b |
| Medical history | |||||||
| Family history | |||||||
| Diabetes mellitus | 309 | 21.5 | 984 | 29.0 | 1,412 | 25.1 | <0.001 |
| Heart diseases | 127 | 8.8 | 271 | 8.0 | 304 | 5.4 | <0.001 |
| CVDs | 112 | 7.8 | 265 | 7.8 | 304 | 5.4 | <0.001 |
| Physical status | |||||||
| BMI (Kg/m2) | <0.001 | ||||||
| Underweighta | 97 | 6.8 | 75 | 2.2 | 214 | 3.8 | |
| Normal weighta | 679 | 47.2 | 1,275 | 37.6 | 2,223 | 39.5 | |
| Overweighta | 292 | 20.3 | 1,041 | 30.7 | 1,626 | 28.9 | |
| Obesitya | 370 | 25.7 | 1,001 | 29.5 | 1,564 | 27.8 | |
| Waist (cm) | <0.001 | ||||||
| <70 | 293 | 20.4 | 268 | 7.9 | 394 | 7 | |
| 71–80 | 585 | 40.7 | 862 | 25.4 | 1,311 | 23.3 | |
| 81–90 | 293 | 20.4 | 1,262 | 37.2 | 1,930 | 34.3 | |
| >91 | 266 | 18.5 | 1,001 | 29.5 | 1,992 | 35.4 | |
| Oral habit | |||||||
| Alcohol | 121 | 8.4 | 461 | 13.6 | 838 | 14.9 | <0.001 |
| Betel nuts | 19 | 1.3 | 122 | 3.6 | 248 | 4.4 | <0.001 |
| Cigarette | 141 | 9.8 | 638 | 18.8 | 1,283 | 22.8 | <0.001 |
The eligible subjects were recruited patient from 2008 to 2010. N = 10,457.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CVDs, cerebrovascular diseases; HC, healthy control; HR, high risk.
Underweight: BMI<18.5; Normal weight: BMI = 18.5–24; Overweight: BMI = 24–27; Obesity: BMI>27.
Chi-square test. P<0.05 was considered statistically significant.
The anthropometric evaluations of body mass index (BMI) and waist circumference showed significant differences among groups (p<0.001) (Table 2). Only a minority of eligible individuals were considered obese, with a BMI>27 (28.1%) and waist circumference >91 cm (31.2%) (Table 2).
Subjects' oral health habits, including alcohol and betel nut use, and cigarette smoking, all considered to have negative effects on oral health, were summarized (Table 2). The most frequent habits among all participants were cigarette smoking (19.7%), alcohol use (13.6%), and betel nut use (3.7%). Participants in the CKD and HR groups were more likely to have these oral habits than were those in the HC group (all p<0.001) (Table 2).
OPD utilization and expenditure in WM, DENT and TCM
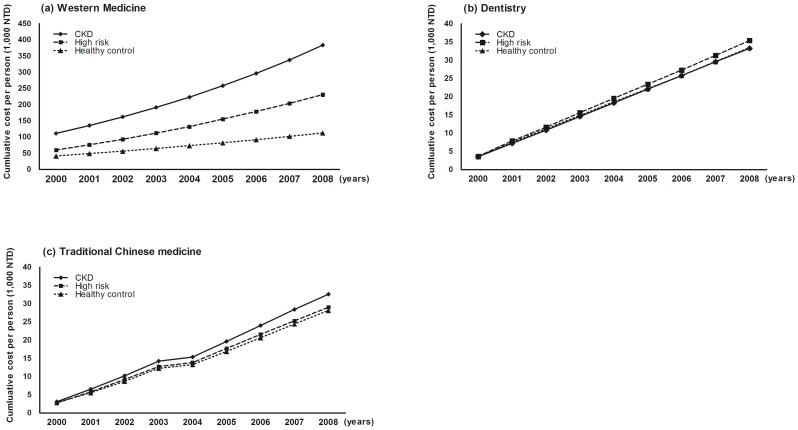
Figure 2 shows the cumulative OPD expenditures per person in WM, DENT and TCM from 2000 to 2008. In general, patients with CKD had greater overall expenditures in WM than for DENT and TCM. Interestingly, the cumulative expenditures for WM for the CKD group exhibited remarkable annual increase when compared with the HR and HC groups, whereas this tendency was not observed for DENT (Figure 2B) and TCM (Figure 2C) expenditures.
Figure 2. Cumulative OPD expenditures per person in WM, DENT, and TCM from 2000 to 2008.
The eligible subjects were recruited from 2008 to 2010. N = 10,457. Abbreviations: CKD, chronic kidney disease; DENT, Dentistry; HC, healthy control; HR, high risk; OPD, outpatient; NT$, new Taiwan dollars; TCM, Traditional Chinese Medicine; WM, Western Medicine.
The annual number of OPD visits per person, and expenditures per person (NT$) for WM and DENT exhibited significant differences among the groups (Table 3). For WM, the CKD group had higher expenditures and OPD visits than the HC or HR groups; however, these trends were not observed for DENT (Table 3). These parameters steadily increased along with the severity of CKD stages (stage 1–5) in WM (p<0.01) (Table 3). Interestingly, only the OPD visits for DENT services showed significant differences in different CKD stages (p = 0.034) although significant differences for DENT expenditures were not found (p = 0.166) (Table 3), suggesting that DENT expenditures did not increase as the patient's kidney disease became worse.
Table 3. The average annual number of OPD visits and expenditures per person in WM and DENT among eligible patients and different CKD stages from 2000 to 2008.
| CKD | |||||||||||
| Parameters | HC (n = 1,438) | HR (n = 3,392) | CKD (n = 5,627) | p b | Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 | p b |
| Western Medicine | |||||||||||
| OPD visits (mean ± SD) | 20.4±10.7 | 30.6±15.7 | 42.3±28.7 | <0.01c | 29.1±16.7 | 35.0±20.0 | 40.0±21.0 | 45.0±27.4 | 49.8±29.0 | 50.9±40.8 | <0.01e |
| OPD expendituresa (NT$, mean) | 12,986 | 26,427 | 42,213 | <0.01c | 244,703 | 340,972 | 404,935 | 452,443 | 505,490 | 532,729 | <0.01e |
| Dentistry | |||||||||||
| OPD visits (mean ± SD) | 4.1±9.2 | 4.9±2.7 | 4.7±2.0 | 0.019d | 4.3±2.3 | 4.7±2.2 | 4.4±1.7 | 4.8±1.8 | 5.5±2.4 | 4.4±1.5 | 0.034 |
| OPD expendituresa (NT$, mean) | 38,384 | 43,537 | 40,130 | 0.049 | 37,140 | 41,024 | 38,799 | 40,911 | 45,290 | 37,394 | 0.166 |
The eligible subjects were recruited patient from 2008 to 2010. N = 10,457.
Abbreviations: CKD, chronic kidney disease; DENT, Dentistry; HC, healthy control; HR, high risk NT$, new Taiwan dollars; OPD, outpatient; WM, Western Medicine.
Expenditures were rounded to the nearest whole dollar.
ANOVA. P<0.05 was considered statistically significant.
Scheffe's test : CKD>HR>HC.
Scheffe's test : HR>HC.
Scheffe's test :Stage5>Stage4>Stage3b>Stage3a>Stage2>Stage1.
Utilization and expenditure of DENT therapeutic procedures
Annual OPD visits and expenditures per person for restorative and periodontal therapy exhibited significant differences among groups; however, for root canal therapy, only OPD visits presented considerable difference among all groups (p = 0.0063) (Table 4). At different CKD stages, the average expenditures and OPD visits for restorative filling and periodontal therapy (all p<0.0001), but not root canal therapy, showed significant decreases according to severity of CKD stages (Table 4), indicating less DENT utilization with progression of CKD.
Table 4. The average annual number of OPD visits, and expenditures per person of different dental procedures among eligible patients and different CKD stages from 2000 to 2008.
| CKD | |||||||||||
| Parameters | HC (n = 1,438) | HR (n = 3,392) | CKD (n = 5,627) | p b | Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 | p b |
| Restorative therapy | |||||||||||
| OPD visits (mean ± SD) | 0.81±0.66 | 0.83±0.72 | 0.78±0.69 | 0.0027c | 0.83±0.74 | 0.82±0.69 | 0.8±0.67 | 0.81±0.7 | 0.77±0.74 | 0.64±0.59 | <0.0001h |
| OPD expendituresa (NT$, mean) | 10,040 | 9,808 | 8,812 | <0.0001d | 9,786 | 9,457 | 9,025 | 8,962 | 8,462 | 7,174 | <0.0001h |
| Root canal therapy | |||||||||||
| OPD visits (mean ± SD) | 0.38±0.34 | 0.43±0.42 | 0.42±0.39 | 0.0063e | 0.43±0.36 | 0.43±0.42 | 0.41±0.35 | 0.45±0.41 | 0.43±0.42 | 0.41±0.34 | 0.436 |
| OPD expendituresa (NT$, mean) | 6,179 | 6,522 | 6,140 | 0.0705 | 6,217 | 6,256 | 6,023 | 6,626 | 5,970 | 5,803 | 0.3 |
| Periodontal therapy | |||||||||||
| OPD visits (mean ± SD) | 0.76±0.68 | 0.87±0.86 | 0.78±0.76 | <0.0001f | 0.76±0.71 | 0.84±0.78 | 0.83±0.73 | 0.86±0.76 | 0.76±0.84 | 0.67±0.66 | <0.0001i |
| OPD expendituresa (NT$, mean) | 6,377 | 7,020 | 6,149 | <0.0001g | 6,153 | 6,640 | 6,488 | 6,697 | 5,835 | 5,262 | <0.0001i |
The eligible subjects were recruited patient from 2008 to 2010. N = 10,457.
Abbreviations: CKD, chronic kidney disease; DENT, Dentistry; HC, healthy control; HR, high risk NT$, new Taiwan dollars; OPD, outpatient; WM, Western Medicine.
Expenditures were rounded to the nearest whole dollar.
ANOVA. P<0.05 was considered statistically significant.
Scheffe's test : HR>CKD.
Scheffe's test : HC>HR>CKD.
Scheffe's test : HR>CKD>HC.
Scheffe's test : HR>CKD>HC.
Scheffe's test : HR>HC>CKD.
Scheffe's test : Stage1>Stage2>Stage3a>Stage3b>Stage4>Stage5.
Scheffe's test : Stage3b>Stage2>Stage3a>Stage4>Stage5.
Discussion
To the best of our knowledge, this is the first attempt to use a long-term, nationwide hospital-based cohort to investigate the relationship between DENT utilization and expenditures for CKD patients according to the progression of CKD stages. Our major findings were as follows: 1) group CKD demonstrated significant differences in terms of demographic data and socioeconomic performance when compared to groups HC and HR; 2) participants in group CKD had poor oral health habits compared to group HC; 3) the medical care utilization and expenditures for WM services for patients with CKD were higher when compared to groups HC and HR, but DENT and TCM services showed no significant difference among the three groups; and 4) as for DENT services, the OPD visits and expenditures of the patients receiving restorative and periodontal therapy showed a significant decrease in group CKD, but not in groups HC and HR. Moreover, the OPD visits and expenditures for group CKD decreased significantly according to the progression of CKD. All these findings provide a new understanding of the relationship between CKD and DENT services, particularly in the treatment of restorative and periodontal therapy.
First, in our study we investigated socioeconomic and demographic data, finding that group CKD was more likely to be male, unemployed or earning a low income, and more than 50% likely to have less than a junior high diploma. A US study had similar findings, in that people with CKD and limited education or low income have more risk of disability because of socioeconomic disparities [40]. Moreover, patients in our CKD group were more likely to have bad oral habits than were other groups (Table 2). A cross-sectional study regarding the oral health status of adults in Taiwan found that demographic factors (i.e., gender, marital status, and income levels) are all significantly associated with general health [41]. Thus, our findings highlight the need for more attention to DENT needs for CKD patients.
As for oral health habits, we found that group CKD had the worst habits, including alcohol use, betel nut use and smoking. It has been shown that oral health-related factors (i.e., oral hygiene, oral health status, dental care utilization, disease history, and lifestyle factors such as cigarette smoking, alcohol use, and betel nut chewing) are significantly associated with general and oral health [41]. A higher rate of concurrent usage of oral substances, particularly in the CKD group, indicates certain lifestyle patterns, which may confer a higher health risk [41]. However, previous studies demonstrated an inverse association between alcohol consumption and renal dysfunction [42], [43] because beneficial oxidative activity on endothelial function has a protective property for kidneys [42], [44]. Additionally, in Taiwan, CKD prevalence among betel-nut users is higher than in the non-users in all age groups [43]. The habit of betel-nut chewing may be associated with CKD, especially in males [43], [45]. It has been reported that relative risk for oral cancer among those who chew betel-nut in the Taiwanese population is 58.4 (95% confidence interval 7.6 to 447.6) [46]. Generally, oral health status is significantly related to socio-economic status and strongly correlated with oral health behaviors and even general health. We strongly recommend widespread public health care education targeting all three risky behaviors at the same time.
As for dental care, we found there was no difference in utilization and expenditure for dental care at different CKD stages, but the utilization of western medicine increased with the progression of CKD. Furthermore, the utilization and expenditure of periodontal therapy and restorative therapy both decreased with the progression of CKD stage (Table 4). But how can we explain this outcome? Recently, a survey was conducted from a representative database to examine self-reported dental status, dental care utilization, and dental insurance, by race/ethnicity [47], among community-dwelling older adults. The author found that Non-Hispanic White respondents reported better dental health, higher dental care utilization, and higher satisfaction with dental care compared to all other racial/ethnic groups. On the contrary, Chinese immigrants were more likely to report poor dental health, were less likely to report dental care utilization and dental insurance, and were less satisfied with their dental care compared to all other racial/ethnic groups [47]. Some factors including cost, physical disabilities, language barriers, dental fear and socio-psychological concerns may affect dental care service utilization by a specific population [41], [47], [48], [49]. It has been shown that those with CKD had a 25% lower likelihood of having a dental visit [HR = 0.75, 95% CI (0.64–0.88)] than those without CKD after adjustment for confounders [50]. In addition, the uremic patients demonstrated more dental problems than healthy controls and seem to develop their problems before they progressed to dialysis [51]. Treatments for CKD and dental care are widely available and inexpensive in Taiwan. Take CKD care, for example. According to Lin et al [52], the medical expenditures per subject per year in years 1997, 1998 and 1999 were US$ 129.7, 432.8 and 725.6 for CKD late stages, stages 3, 4 and 5. For dental care, from 1998 to 2005, the number of dentists at national level increased 30.5% from 8,020 to 10,465 and the population-dentist ratio decreased 22.0% (2,588 people per dentist in 1998 and 2,115 people per dentist in 2005). The percentage of insured population receiving dental service increased from 36.1% in 1998 to 40.8% in 2005. The dentist-to-population ratio (defined as the number of dentists per 10,000 people) was 5.0 in 2010 [53]. Thus, dental care for each participants is widely available and inexpensive in Taiwan.
It is essential to address the factors affecting the usage of dental care in CKD patients, as these may contribute to the progression of CKD stages. Greater attention to dental problems may be warranted during the progression of CKD to prevent deterioration of kidney function [51]. Furthermore, it is plausible that restorative and periodontal expenditure and utilization may provide contributory information on the deterioration of kidney function in patients with CKD [51]. Further studies to ascertain the nature of the association between oral health and CKD progression are needed.
This study has a few limitations that should be addressed. First, claims data were identified from the NHIRD under the principal payment code for DENT service and complete dental examination was not performed during face-to-face interview; however, to date, the decision criteria for subjects leading to dental treatment, including restorative or filling, endodontic and periodontal therapy is still judged by clinicians according to an imprecise coding system. Second, claims data may have minor inaccuracies even through these inaccuracies are rare. The accuracy of claims data of NHIRD is improved by a cross-checking system with full review by specialists. Thus, these inaccuracies would be unlikely to have significantly affected the results, considering the substantial sample size. Third, the study evaluated only the direct OPD expenditures, including WM, DENT and TCM expenditures. Information to determine the indirect economic burdens of CKD, such as work productivity loss and reduced quality of life, was not available. Furthermore, the current study may also suffer from detection bias. Indeed, it was not possible to capture the entire continuum of care of patients, as the NHIRD does not include information regarding the proportion of self-payment dental therapies such as denture fabrication, orthodontic treatment, dental implant placement and medical cosmetics treatment. Moreover, findings were based on a single integrated health system and may not be generalizable to larger populations because of hospital-based study design. A community- or population-based study will be needed to delineate the intricate relationship between CKD and oral health.
Since patients at advanced CKD stages in our study used DENT services less frequently, it would be very likely that individuals with more advanced CKD are older and less educated, and have lower income. Further multivariate regression analysis may have interesting findings regarding whether this association is dependent or not on some confounders such as demographics, socio-economic status and oral habits. In this study, from our collected data, we can offer some evidence regarding the factors on demographics, and socioeconomic status to support our conclusion. As for gender, our result showed a gender difference consistent with several previous studies in Taiwan [54], [55], [56]. It should be emphasized that 99% of Taiwan's population is covered by NHIP [34]. Thus, in terms of gender, there is no difference between the healthcare utilization for kidney disease [52], [57]. As for age, there is no obvious finding that individuals with more advanced CKD are older. For the later stages, such as CKD Stage 4 and Stage 5, the majority of CKD patients in our study were age 45–64 (32.8% and 39.4%, respectively), greater than for other age groups, including age >75 (28.6% for CKD Stage 4 and 24.20% for CKD Stage 5) (data not shown). Therefore, “Age” may not correlate to healthcare utilization and expenditure. As for demographic characteristics, such as residential district, a similar distribution pattern was found in our investigated groups (Table 1). The residential district may not have a significant impact on healthcare utilization for recruited patients because of the universal coverage of NHIP in Taiwan [34]. As for socioeconomic status, such as household income, more subjects in CKD Stage 2 (24%) indicate low or no income than those in Stage 5 (22.10%) or Stage 1 (19.7%) (data not shown). Lower socioeconomic status is a risk factor for CKD and progression to end-stage renal disease; however, consistent with another study [58], GFR decline was similar across income groups and patients with advanced CKD may not necessarily have lower income than those in other stages. For the “education level,” CKD patients at Stage 4 and Stage 5 have a higher likelihood of lower educational achievement (<Junior high) (Table 1). However, subjects aged 45–64 (40.9%) had less education than those 65–74 (34.0%), and those >75 (21.8%) (data not shown). Thus, in fact, we found individuals with more advanced CKD may not necessarily be less educated or have less income. Consequently, the demographic and socioeconomic factors may have only a limited influence on the analysis procedure and result of this study. Nevertheless, we should be cautions about the interpretation of the results; the interacting effects of these covariates on the correlation between CKD stages and healthcare utilization and expenditure still require further investigation.
Despite these limitations, this study has several strengths, including the important advantage of relying on real-world population-based data, a relatively substantial sample size, face-to-face questionnaire interview for each participant, and the availability of laboratory results to ascertain CKD stage.
Conclusions
In conclusion, from the horizon of dental utilization and expenditures, this hospital-based research is the first to assess dental OPD utilization and expenditures in a population with CKD. Patients at advanced CKD stages used DENT services, including periodontal therapy and restorative filling, less frequently. However, a large and prospective study is warranted to clarify the connection between CKD stages and DENT utilization in CKD subjects.
Acknowledgments
The authors acknowledge Dr. Fu-Gong Lin, and Ms. Jing-Shu Huang (School of Public Health, National Defense Medical Center (N.D.M.C)) and Ms. Hui-Chih Liu (Graduate Institute of Life Sciences, N.D.M.C) for assistance with statistical analysis. The authors also appreciate Professor Mary Goodwin's help in manuscript editing.
Funding Statement
This study is based in part on data from the NHIRD provided by the Bureau of NHI, Department of Health, and managed by National Health Research Institutes in Taiwan and supported by the National Science Council of Taiwan under grant DOH97-HP-1101, DOH-98-1110, DOH99-HP-1106, DOH100-HP-1102, DOH101-HP-1103, and DOH102-HP-1103. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kerr M, Bray B, Medcalf J, O'Donoghue DJ, Matthews B (2012) Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 27 Suppl 3: iii73–iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liang CH, Yang CY, Lu KC, Chu P, Chen CH, et al. (2011) Factors affecting peritoneal dialysis selection in Taiwanese patients with chronic kidney disease. Int Nurs Rev 58: 463–469. [DOI] [PubMed] [Google Scholar]
- 3. Chang JM, Hwang SJ, Tsukamoto Y, Chen HC (2012) Chronic kidney disease prevention–a challenge for Asian countries: report of the Third Asian Forum of Chronic Kidney Disease Initiatives. Clin Exp Nephrol 16: 187–194. [DOI] [PubMed] [Google Scholar]
- 4. Frankenfield DL, Weinhandl ED, Powers CA, Howell BL, Herzog CA, et al. (2012) Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare Part D. Am J Kidney Dis 59: 670–681. [DOI] [PubMed] [Google Scholar]
- 5. Vekeman F, Yameogo ND, Lefebvre P, Bailey RA, McKenzie RS, et al. (2010) Healthcare costs associated with nephrology care in pre-dialysis chronic kidney disease patients. J Med Econ 13: 673–680. [DOI] [PubMed] [Google Scholar]
- 6. Trivedi H (2010) Cost implications of caring for chronic kidney disease: are interventions cost-effective? Adv Chronic Kidney Dis 17: 265–270. [DOI] [PubMed] [Google Scholar]
- 7. Yang M, Fox CH, Vassalotti J, Choi M (2011) Complications of progression of CKD. Adv Chronic Kidney Dis 18: 400–405. [DOI] [PubMed] [Google Scholar]
- 8. Grima DT, Bernard LM, Dunn ES, McFarlane PA, Mendelssohn DC (2012) Cost-effectiveness analysis of therapies for chronic kidney disease patients on dialysis: a case for excluding dialysis costs. Pharmacoeconomics 30: 981–989. [DOI] [PubMed] [Google Scholar]
- 9. Summers SA, Tilakaratne WM, Fortune F, Ashman N (2007) Renal disease and the mouth. Am J Med 120: 568–573. [DOI] [PubMed] [Google Scholar]
- 10. Vesterinen M, Ruokonen H, Leivo T, Honkanen AM, Honkanen E, et al. (2007) Oral health and dental treatment of patients with renal disease. Quintessence Int 38: 211–219. [PubMed] [Google Scholar]
- 11. Akar H, Akar GC, Carrero JJ, Stenvinkel P, Lindholm B (2011) Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol 6: 218–226. [DOI] [PubMed] [Google Scholar]
- 12. Chen LP, Chiang CK, Chan CP, Hung KY, Huang CS (2006) Does periodontitis reflect inflammation and malnutrition status in hemodialysis patients? Am J Kidney Dis 47: 815–822. [DOI] [PubMed] [Google Scholar]
- 13. Chen LP, Chiang CK, Peng YS, Hsu SP, Lin CY, et al. (2011) Relationship between periodontal disease and mortality in patients treated with maintenance hemodialysis. Am J Kidney Dis 57: 276–282. [DOI] [PubMed] [Google Scholar]
- 14. Proctor R, Kumar N, Stein A, Moles D, Porter S (2005) Oral and dental aspects of chronic renal failure. J Dent Res 84: 199–208. [DOI] [PubMed] [Google Scholar]
- 15. Fisher MA, Taylor GW, Papapanou PN, Rahman M, Debanne SM (2008) Clinical and serologic markers of periodontal infection and chronic kidney disease. J Periodontol 79: 1670–1678. [DOI] [PubMed] [Google Scholar]
- 16. Fisher MA, Taylor GW, Shelton BJ, Jamerson KA, Rahman M, et al. (2008) Periodontal disease and other nontraditional risk factors for CKD. Am J Kidney Dis 51: 45–52. [DOI] [PubMed] [Google Scholar]
- 17. Grubbs V, Plantinga LC, Crews DC, Bibbins-Domingo K, Saran R, et al. (2011) Vulnerable populations and the association between periodontal and chronic kidney disease. Clin J Am Soc Nephrol 6: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foundation NK (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266. [PubMed] [Google Scholar]
- 19. Khan S, Amedia CA Jr (2008) Economic burden of chronic kidney disease. J Eval Clin Pract 14: 422–434. [DOI] [PubMed] [Google Scholar]
- 20. Luciano Ede P, Luconi PS, Sesso RC, Melaragno CS, Abreu PF, et al. (2012) [Prospective study of 2151 patients with chronic kidney disease under conservative treatment with multidisciplinary care in the Vale do Paraiba, SP]. J Bras Nefrol 34: 226–234. [DOI] [PubMed] [Google Scholar]
- 21. Wiebe N, Klarenbach SW, Chui B, Ayyalasomayajula B, Hemmelgarn BR, et al. (2012) Adding specialized clinics for remote-dwellers with chronic kidney disease: a cost-utility analysis. Clin J Am Soc Nephrol 7: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bessette RW, Carter RL (2012) Predicting hospital cost in CKD patients through blood chemistry values. BMC Nephrol 12: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hassan Y, Al-Ramahi RJ, Aziz NA, Ghazali R (2009) Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother 43: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 24. Wish JB, Coyne DW (2007) Use of erythropoiesis-stimulating agents in patients with anemia of chronic kidney disease: overcoming the pharmacological and pharmacoeconomic limitations of existing therapies. Mayo Clin Proc 82: 1371–1380. [DOI] [PubMed] [Google Scholar]
- 25. White CA, Jaffey J, Magner P (2007) Cost of applying the K/DOQI guidelines for bone metabolism and disease to a cohort of chronic hemodialysis patients. Kidney Int 71: 312–317. [DOI] [PubMed] [Google Scholar]
- 26. Schiller B, Doss S, E DEC, Del Aguila MA, Nissenson AR (2008) Costs of managing anemia with erythropoiesis-stimulating agents during hemodialysis: a time and motion study. Hemodial Int 12: 441–449. [DOI] [PubMed] [Google Scholar]
- 27. Higashiyama A, Okamura T, Watanabe M, Murakami Y, Otsuki H, et al. (2009) Effect of chronic kidney disease on individual and population medical expenditures in the Japanese population. Hypertens Res 32: 450–454. [DOI] [PubMed] [Google Scholar]
- 28. Levin A, Chaudhry MR, Djurdjev O, Beaulieu M, Komenda P (2009) Diabetes, kidney disease and cardiovascular disease patients. Assessing care of complex patients using outpatient testing and visits: additional metrics by which to evaluate health care system functioning. Nephrol Dial Transplant 24: 2714–2720. [DOI] [PubMed] [Google Scholar]
- 29. Wish J, Schulman K, Law A, Nassar G (2009) Healthcare expenditure and resource utilization in patients with anaemia and chronic kidney disease: a retrospective claims database analysis. Kidney Blood Press Res 32: 110–118. [DOI] [PubMed] [Google Scholar]
- 30. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, et al. (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 31. Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, et al. (2010) KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 55: 773–799. [DOI] [PubMed] [Google Scholar]
- 32. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 33. Yeh CJ, Chang HY, Pan WH (2011) Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac J Clin Nutr 20: 292–300. [PubMed] [Google Scholar]
- 34. Wu JC, Liu L, Chen YC, Huang WC, Chen TJ, et al. (2011) Ossification of the posterior longitudinal ligament in the cervical spine: an 11-year comprehensive national epidemiology study. Neurosurg Focus 30: E5. [DOI] [PubMed] [Google Scholar]
- 35. Yuh DY, Cheng GL, Chien WC, Chung CH, Lin FG, et al. (2013) Factors affecting treatment decisions and outcomes of root-resected molars: a nationwide study. J Periodontol 84: 1528–1535. [DOI] [PubMed] [Google Scholar]
- 36. Kang JH, Lin HC (2012) Increased risk of multiple sclerosis after traumatic brain injury: a nationwide population-based study. Journal of Neurotrauma 29: 90–95. [DOI] [PubMed] [Google Scholar]
- 37. Keller JJ, Chung SD, Lin HC (2012) A nationwide population-based study on the association between chronic periodontitis and erectile dysfunction. Journal of Clinical Periodontology 39: 507–512. [DOI] [PubMed] [Google Scholar]
- 38. Kuo CF, Luo SF, Yu KH, Chou IJ, Tseng WY, et al. (2012) Cancer risk among patients with systemic sclerosis: a nationwide population study in Taiwan. Scand J Rheumatol 41: 44–49. [DOI] [PubMed] [Google Scholar]
- 39. Wu MY, Hsu YH, Su CL, Lin YF, Lin HW (2012) Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am J Kidney Dis 60: 548–552. [DOI] [PubMed] [Google Scholar]
- 40. Plantinga LC, Johansen KL, Schillinger D, Powe NR (2012) Lower socioeconomic status and disability among US adults with chronic kidney disease, 1999–2008. Prev Chronic Dis 9: E12. [PMC free article] [PubMed] [Google Scholar]
- 41. Wang TF, Chou C, Shu Y (2012) Assessing the effects of oral health-related variables on quality of life in Taiwanese adults. Qual Life Res 22: 811–825. [DOI] [PubMed] [Google Scholar]
- 42. Schaeffner ES, Kurth T, de Jong PE, Glynn RJ, Buring JE, et al. (2005) Alcohol consumption and the risk of renal dysfunction in apparently healthy men. Arch Intern Med 165: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 43. Hsu YH, Liu WH, Chen W, Kuo YC, Hsiao CY, et al. (2011) Association of betel nut chewing with chronic kidney disease: a retrospective 7-year study in Taiwan. Nephrology (Carlton) 16: 751–757. [DOI] [PubMed] [Google Scholar]
- 44. Presti RL, Carollo C, Caimi G (2007) Wine consumption and renal diseases: new perspectives. Nutrition 23: 598–602. [DOI] [PubMed] [Google Scholar]
- 45. Chou CY, Cheng SY, Liu JH, Cheng WC, Kang IM, et al. (2009) Association between betel-nut chewing and chronic kidney disease in men. Public Health Nutr 12: 723–727. [DOI] [PubMed] [Google Scholar]
- 46. Lu CT, Yen YY, Ho CS, Ko YC, Tsai CC, et al. (1996) A case-control study of oral cancer in Changhua County, Taiwan. J Oral Pathol Med 25: 245–248. [DOI] [PubMed] [Google Scholar]
- 47. Shelley D, Russell S, Parikh NS, Fahs M (2011) Ethnic disparities in self-reported oral health status and access to care among older adults in NYC. J Urban Health 88: 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yuen HK, Wolf BJ, Bandyopadhyay D, Magruder KM, Selassie AW, et al. (2010) Factors that limit access to dental care for adults with spinal cord injury. Spec Care Dentist 30: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rohn EJ, Sankar A, Hoelscher DC, Luborsky M, Parise MH (2006) How do social-psychological concerns impede the delivery of care to people with HIV? Issues for dental education. J Dent Educ 70: 1038–1042. [PMC free article] [PubMed] [Google Scholar]
- 50. Grubbs V, Plantinga LC, Tuot DS, Powe NR (2012) Chronic kidney disease and use of dental services in a United States public healthcare system: a retrospective cohort study. BMC Nephrol 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thorman R, Neovius M, Hylander B (2009) Clinical findings in oral health during progression of chronic kidney disease to end-stage renal disease in a Swedish population. Scand J Urol Nephrol 43: 154–159. [DOI] [PubMed] [Google Scholar]
- 52. Lin MY, Hwang SJ, Mau LW, Chen HC, Hwang SC, et al. (2010) Impact of late-stage CKD and aging on medical utilization in the elderly population: a closed-cohort study in Taiwan. Nephrol Dial Transplant 25: 3230–3235. [DOI] [PubMed] [Google Scholar]
- 53. Huang CS, Cher TL, Lin CP, Wu KM (2013) Projection of the dental workforce from 2011 to 2020, based on the actual workload of 6762 dentists in 2010 in Taiwan. J Formos Med Assoc 112: 527–536. [DOI] [PubMed] [Google Scholar]
- 54. Kuo HW, Tsai SS, Tiao MM, Yang CY (2007) Epidemiological features of CKD in Taiwan. Am J Kidney Dis 49: 46–55. [DOI] [PubMed] [Google Scholar]
- 55. Chiang HH, Livneh H, Yen ML, Li TC, Tsai TY (2013) Prevalence and correlates of depression among chronic kidney disease patients in Taiwan. BMC Nephrol 14: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lin C, Hsu HT, Lin YS, Weng SF (2013) Increased risk of getting sudden sensorineural hearing loss in patients with chronic kidney disease: a population-based cohort study. Laryngoscope 123: 767–773. [DOI] [PubMed] [Google Scholar]
- 57. Chang RE, Hsieh CJ, Myrtle RC (2011) The effect of outpatient dialysis global budget cap on healthcare utilization by end-stage renal disease patients. Soc Sci Med 73: 153–159. [DOI] [PubMed] [Google Scholar]
- 58. Hidalgo G, Ng DK, Moxey-Mims M, Minnick ML, Blydt-Hansen T, et al. (2013) Association of Income Level With Kidney Disease Severity and Progression Among Children and Adolescents With CKD: A Report From the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis 62: 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]