Abstract
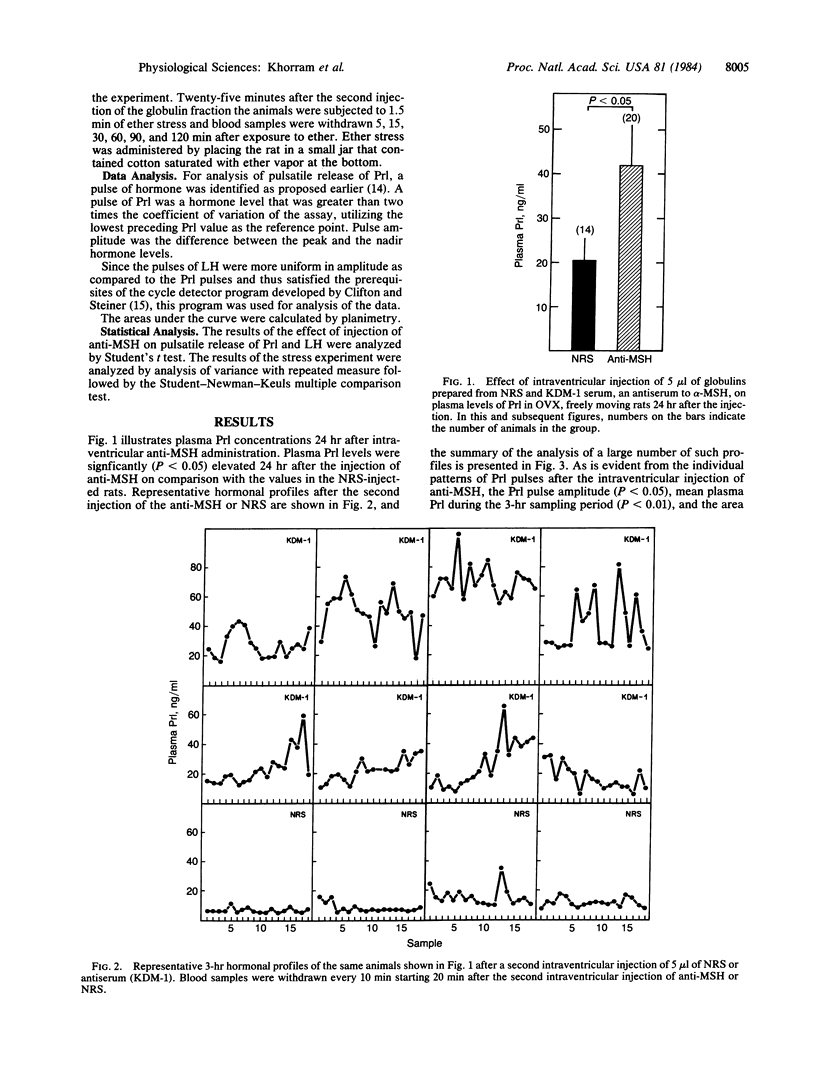
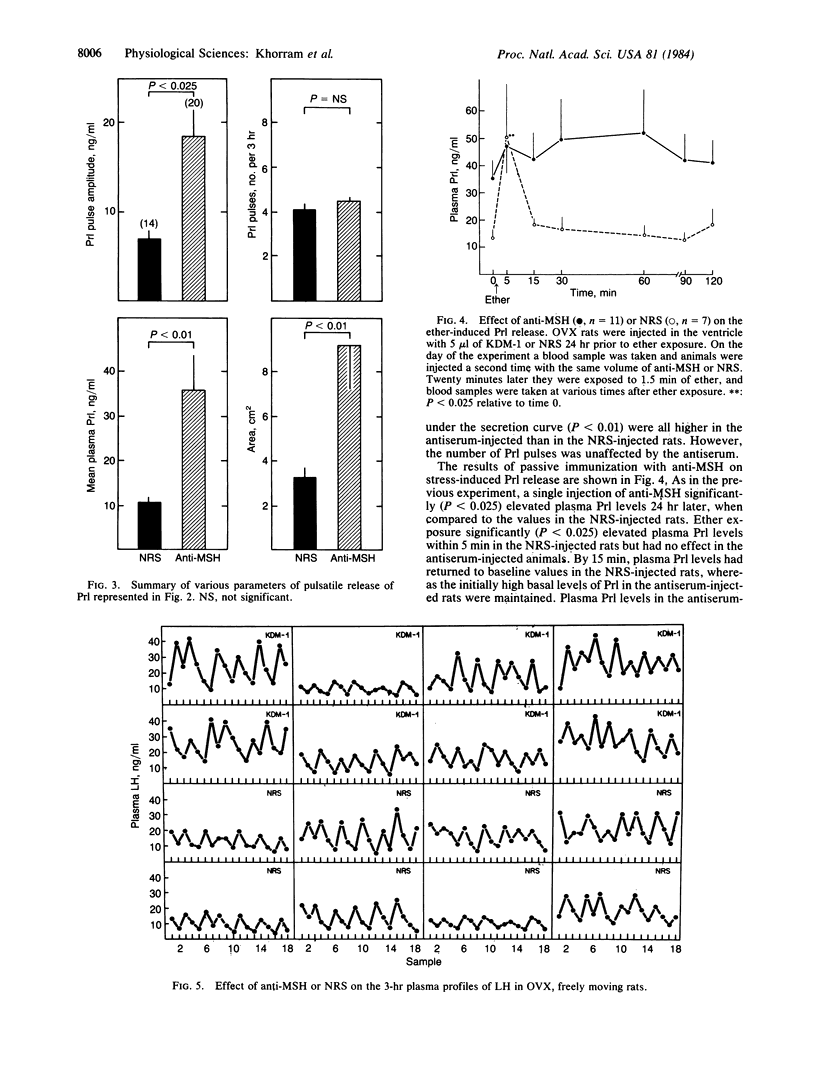
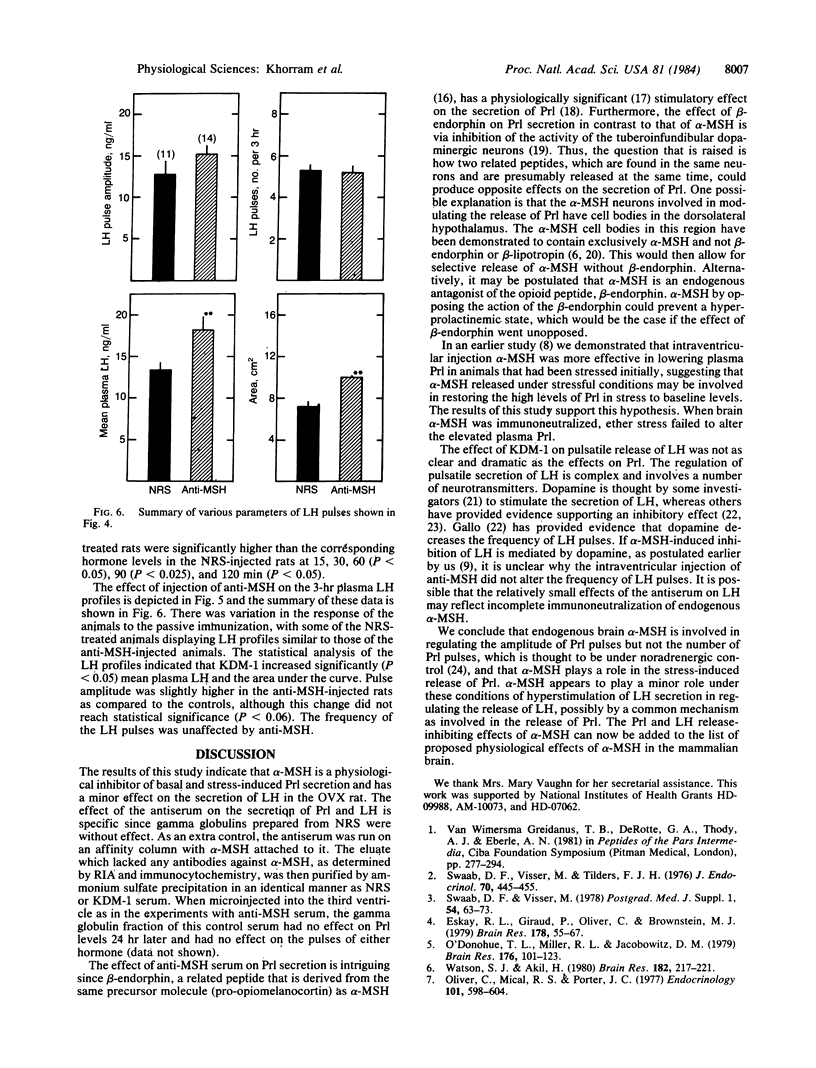
Long-term ovariectomized (OVX) rats were injected in the third cerebral ventricle with 5 microliter of the globulin fraction of an antiserum raised against alpha-melanocyte-stimulating hormone (alpha-MSH) or an equal volume of the globulin fraction of normal rabbit serum (NRS). Immunoneutralization of brain alpha-MSH produced an increase in the area under the secretion curve of prolactin (Prl), the amplitude of Prl pulses, and mean plasma Prl (P less than 0.01). In animals that had received two injections of NRS or anti-MSH and were subjected to a 2-min ether stress, Prl levels significantly increased within 5 minutes in the NRS-injected rats, whereas Prl levels in the antiserum-injected rats did not increase any further from the initially high baseline levels. The administration of antibodies against alpha-MSH produced a small increase (P less than 0.05) in the area under the secretion of luteinizing hormone (LH) and mean plasma LH; however, the number of LH pulses was unaffected. We conclude that endogenous alpha-MSH of central origin is a physiological neuromodulator of release of Prl and LH in the OVX rat and is involved in the stress-induced release of Prl.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. W., Ojeda S. R. A detailed analysis of the serum luteinizing hormone secretory profile in conscious, free-moving female rats during the time of puberty. Endocrinology. 1981 Dec;109(6):2032–2039. doi: 10.1210/endo-109-6-2032. [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., McCann S. M. Water, sodium chloride, and food intake induced by injections of cholinergic and adrenergic drugs into the third ventricle of the rat brain. Proc Soc Exp Biol Med. 1970 Apr;133(4):1464–1470. doi: 10.3181/00379727-133-34713. [DOI] [PubMed] [Google Scholar]
- Clifton D. K., Steiner R. A. Cycle detection: a technique for estimating the frequency and amplitude of episodic fluctuations in blood hormone and substrate concentrations. Endocrinology. 1983 Mar;112(3):1057–1064. doi: 10.1210/endo-112-3-1057. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Eskay R. L., Giraud P., Oliver C., Brown-Stein M. J. Distribution of alpha-melanocyte-stimulating hormone in the rat brain: evidence that alpha-MSH-containing cells in the arcuate region send projections to extrahypothalamic areas. Brain Res. 1979 Dec 7;178(1):55–67. doi: 10.1016/0006-8993(79)90087-8. [DOI] [PubMed] [Google Scholar]
- Gallo R. V. Further studies on dopamine-induced suppression of pulsatile LH release in ovariectomized rats. Neuroendocrinology. 1981 Mar;32(3):187–192. doi: 10.1159/000123154. [DOI] [PubMed] [Google Scholar]
- Gallo R. V. Neuroendocrine regulation of pulsatile luteinizing hormone release in the rat. Neuroendocrinology. 1980;30(2):122–131. doi: 10.1159/000122986. [DOI] [PubMed] [Google Scholar]
- Harms P. G., Ojeda S. R. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol. 1974 Mar;36(3):391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- Jegou S., Tonon M. C., Guy J., Vaudry H., Pelletier G. Biological and immunological characterization of alpha-melanocyte-stimulating hormone (alpha-MSH) in two neuronal systems of the rat brain. Brain Res. 1983 Jan 31;260(1):91–98. doi: 10.1016/0006-8993(83)90766-7. [DOI] [PubMed] [Google Scholar]
- Khorram O., DePalatis L. R., McCann S. M. Changes in hypothalamic and pituitary content of immunoreactive alpha-melanocyte-stimulating hormone during the gestational and postpartum periods in the rat. Proc Soc Exp Biol Med. 1984 Nov;177(2):318–326. doi: 10.3181/00379727-177-41950. [DOI] [PubMed] [Google Scholar]
- Khorram O., DePalatis L. R., McCann S. M. The effect and possible mode of action of alpha-melanocyte-stimulating hormone on gonadotropin release in the ovariectomized rat: an in vivo and in vitro analysis. Endocrinology. 1984 Jan;114(1):227–233. doi: 10.1210/endo-114-1-227. [DOI] [PubMed] [Google Scholar]
- Khorram O., Mizunuma H., McCann S. M. Effect of alpha-melanocyte-stimulating hormone on basal and stimulated release of prolactin: evidence for dopaminergic mediation. Neuroendocrinology. 1982 Jun;34(6):433–437. doi: 10.1159/000123341. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A., Ojeda S. R., Advis J. P., McCann S. M. Evidence for noradrenergic involvement in episodic prolactin and growth hormone release in ovariectomized rats. Endocrinology. 1979 Jul;105(1):86–91. doi: 10.1210/endo-105-1-86. [DOI] [PubMed] [Google Scholar]
- O'Donohue T. L., Miller R. L., Jacobowitz D. M. Identification, characterization and stereotaxic mapping of intraneuronal alpha-melanocyte stimulating hormone-like immunoreactive peptides in discrete regions of the rat brain. Brain Res. 1979 Oct 26;176(1):101–123. doi: 10.1016/0006-8993(79)90873-4. [DOI] [PubMed] [Google Scholar]
- Oliver C., Mical R. S., Porter J. C. Hypothalamic-pituitary vasculature: evidence for retrograde blood flow in the pituitary stalk. Endocrinology. 1977 Aug;101(2):598–604. doi: 10.1210/endo-101-2-598. [DOI] [PubMed] [Google Scholar]
- Ragavan V. V., Frantz A. G. Opioid regulation of prolactin secretion: evidence for a specific role of beta-endorphin. Endocrinology. 1981 Nov;109(5):1769–1771. doi: 10.1210/endo-109-5-1769. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Boer G. J., Visser M. The fetal brain and intrauterine growth. Postgrad Med J. 1978;54 (Suppl 1):63–73. [PubMed] [Google Scholar]
- Swaab D. F., Visser M., Tilders F. J. Stimulation of intra-uterine growth in rat by alpha-melanocyte-stimulating hormone. J Endocrinol. 1976 Sep;70(3):445–455. doi: 10.1677/joe.0.0700445. [DOI] [PubMed] [Google Scholar]
- Van Vugt D. A., Meites J. Influence of endogenous opiates on anterior pituitary function. Fed Proc. 1980 Jun;39(8):2533–2538. [PubMed] [Google Scholar]
- Vijayan E., McCann S. M. Re-evaluation of the role of catecholamines in control of gonadotropin and prolactin release. Neuroendocrinology. 1978;25(3):150–165. doi: 10.1159/000122737. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Akil H. alpha-MSH in rat brain: occurrence within and outside of beta-endorphin neurons. Brain Res. 1980 Jan 20;182(1):217–223. doi: 10.1016/0006-8993(80)90849-5. [DOI] [PubMed] [Google Scholar]
- van Wimersma Greidanus T. B., de Rotte G. A., Thody A. J., Eberle A. N. Melanocyte-stimulating hormone and adaptive behaviour. Ciba Found Symp. 1981;81:277–294. doi: 10.1002/9780470720646.ch16. [DOI] [PubMed] [Google Scholar]



