Abstract
In the face of changing behavioral situations, plasticity of sensory systems can be a valuable mechanism to facilitate appropriate behavioral responses. In the auditory system, the neurotransmitter serotonin is an important messenger for context-dependent regulation because it is sensitive to both external events and internal state, and it modulates neural activity. In male mice, serotonin increases in the auditory midbrain region, the inferior colliculus (IC), in response to changes in behavioral context such as restriction stress and social contact. Female mice have not been measured in similar contexts, although the serotonergic system is sexually dimorphic in many ways. In the present study, we investigated the effects of sex, experience and estrous state on the fluctuation of serotonin in the IC across contexts, as well as potential relationships between behavior and serotonin. Contrary to our expectation, there were no sex differences in increases of serotonin in response to a restriction stimulus. Both sexes had larger increases in second exposures, suggesting experience plays a role in serotonergic release in the IC. In females, serotonin increased during both restriction and interactions with males; however, the increase was more rapid during restriction. There was no effect of female estrous phase on the serotonergic change for either context, but serotonin was related to behavioral activity in females interacting with males. These results show that changes in behavioral context induce increases in serotonin in the IC by a mechanism that appears to be uninfluenced by sex or estrous state, but may depend on experience and behavioral activity.
KEY WORDS: Auditory system, Context, Estrous, Inferior colliculus, Sensory processing, Neuromodulation
INTRODUCTION
Sensory systems must be sensitive to rapid changes in behavioral context in order to enhance focus on important information or de-emphasize extraneous information as behavioral situations change. This plays out in the auditory system, where plasticity can occur in response to social stimulation or stressful situations such as predator detection. For example, in canaries and zebra finches, neurons in the caudomedial nidopallium, a secondary auditory region, can be tuned to the vocalizations of bird species composing a social group (Terleph et al., 2008). In mice, cues indicating the presence of a predator decrease auditory event-related potentials, which may influence how mice respond to the threat of predators (Halene et al., 2009).
Intrinsic information can also influence how receivers process sound signals. Intrinsic characteristics such as estrous phase, reproductive state and sex, which influence physiological processes, also influence auditory processing. For example, female mice that have given birth show more competent retrieval responses to isolated pups, as well as an improved entrainment to pup isolation calls in the auditory cortex (Ehret et al., 1987; Liu et al., 2006; Ehret and Schmid, 2009). Similar plasticity in auditory processing is found in frogs, in which the auditory neurons of unmated, receptive female frogs respond more vigorously to male calls than those of recently mated frogs (Miranda and Wilczynski, 2009b), mirroring the more vigorous behavioral responses of unmated versus mated females to the same calls. Sexually dimorphic signal reception can be found in parallel with sexually dimorphic signals, such as those often seen in courtship displays. For example, in the green tree frog, the auditory midbrains of females and males respond differently to natural vocalizations (Miranda and Wilczynski, 2009a). In addition, experience with a context may change how animals respond to repeated exposures to a stimulus, such as restriction stress (Zavala et al., 2011). Despite the abundant evidence that internal state and external context influence sensory processing, neural mechanisms that transmit these features have been less well explored. Here, we propose a common mechanism that may integrate information about physiological state, sex and specific context in the response of the auditory midbrain to changes in behavioral situation.
The serotonergic system is a centralized neurochemical network that integrates information on behavioral context and intrinsic state. In some brain regions, serotonin fluctuates with changes in behavioral context like imposition of a stressor and changes in social group composition (De Souza and Van Loon, 1986; Kawahara et al., 1993; Adell et al., 1997; Price et al., 1998; Chaouloff et al., 1999; Hall et al., 2011; Hall et al., 2012). Brain serotonin is also sensitive to internal influences such as reproductive state and sex (Maswood et al., 1999; Jitsuki et al., 2009). Estrogen levels, which fluctuate across female reproductive state and differ between the sexes, influence serotonergic function in sensory brain regions (Rubinow et al., 1998; Abizaid et al., 2005; Matragrano et al., 2012). Membrane-bound serotonin receptors decrease and binding affinity of serotonin to receptors decreases with increased estrogen levels, and during proestrus and estrus in rats (Biegon et al., 1980; Mize and Alper, 2000). These findings suggest that the serotonergic system is able to draw on information about both internal state and behavioral context in order to influence auditory processing on a time scale matched to behavior. Given that the serotonergic system is known to modulate auditory processing, it is an excellent system in which to measure the neurochemical mediation of context on sensory processing (Ebert and Ostwald, 1992; Hurley et al., 2002; Hurley and Pollak, 2005; Ji and Suga, 2007).
Fluctuations of serotonin have recently been found in the inferior colliculus (IC) of male mice during restriction stress and social interaction, but fluctuation of serotonin has not been measured in the IC of female mice during these contexts (Hall et al., 2011; Hall et al., 2012). Our goal here was to use these two paradigms to investigate the influence of multiple behavioral contexts and features of internal state in the same class of individuals, female mice. We used the paradigm of restriction as an example of a mildly stressful situation, to which we knew males would have a strong response. In this paradigm we explored sex differences by comparing the fluctuation of serotonin in males and females. To further investigate the response of female mice, we compared serotonergic fluctuation in two behavioral contexts: restriction and interaction with a male partner. We explored effects of internal state by exposing females of different estrous phases to restriction and social interaction. By measuring each individual twice, the role of repeated exposures to both restriction and interaction was examined. Furthermore, we tested whether serotonergic fluctuation was related to behaviors exhibited during restriction and social interaction, including ultrasonic vocalizations. We found that the size and timing of the serotonergic signal was distinctly responsive to some of these features, but not to others.
RESULTS
In order to investigate the influence of sex, estrus state, experience and specific context on the fluctuation of serotonin in the IC, we imposed two types of contextual stimuli on mice: restriction and social interaction. For restriction, male and female mice were placed in a restricted area, a mild stressor that prevents lateral movement. For social interaction, females were allowed to interact with familiar males.
Experiment 1: restriction stressor
Serotonin increased with restriction, but without sex or estrous phase differences
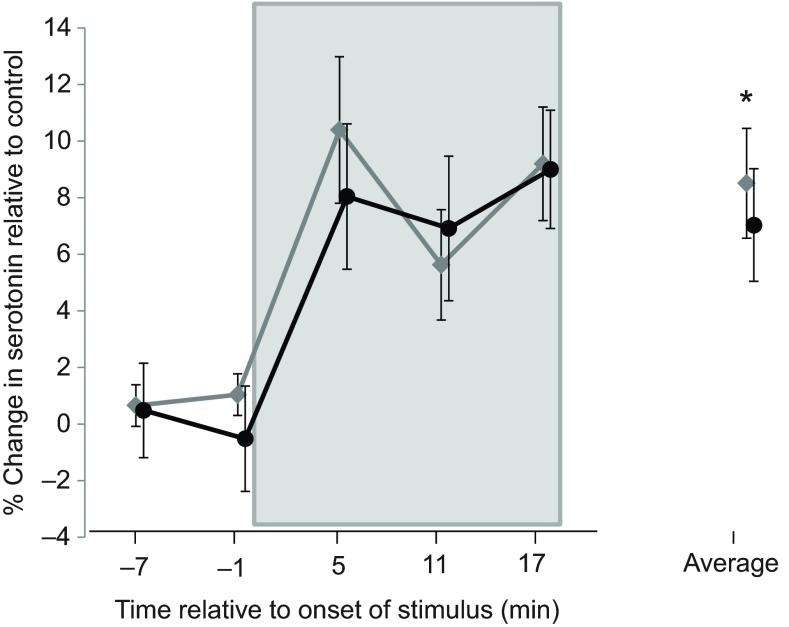
After being placed in a restricted arena, serotonin in the IC of both males (6 males in 11 trials) and females (14 females in 17 trials) had increased over that of control animals (9 males in 11 trials and 7 females in 10 trials) by 5 min, and remained elevated above controls through 17 min of restriction. Because of this, the percentage change of serotonin for all measurements taken during restriction was averaged. Serotonin in the IC of restricted animals significantly increased from baseline compared with control (mixed model F=5.382, P=0.027); however, there was no difference between males and females (mixed model F=0.338, P=0.566; Fig. 1). There was no difference in the increase of serotonin between the left and right colliculi (mixed model F=2.252, P=0.152).
Fig. 1.
Percentage change in serotonin in response to restriction. Data are for females (circles) relative to female controls and males (diamonds) relative to male controls. The box represents the time during which restriction was imposed. The average of measurements taken at 5, 11 and 17 min is shown on the right. Both males and females showed an increase in serotonin during restriction stress: the average of the three traces taken during restriction was significantly different from controls (*mixed model F=5.382, P=0.027). Males and females were not significantly different from each other. Plots are offset to display bars representing s.e.m.
Within experimental animals, although there was a qualitative increase in serotonin in the proestrus/estrus group (7 trials) over the diestrus group (10 trials) after 17 min of restriction, the difference was not significant (mixed model F=12.073, P=0.122; supplementary material Fig. S1). Three females were represented once in both groups. Testing for the effect of phase was balanced for experience, including five data points from both first and second trials in diestrus females, and four data points from first trials and three data points from second trials in proestrus/estrus females.
Experience influenced the increase of serotonin
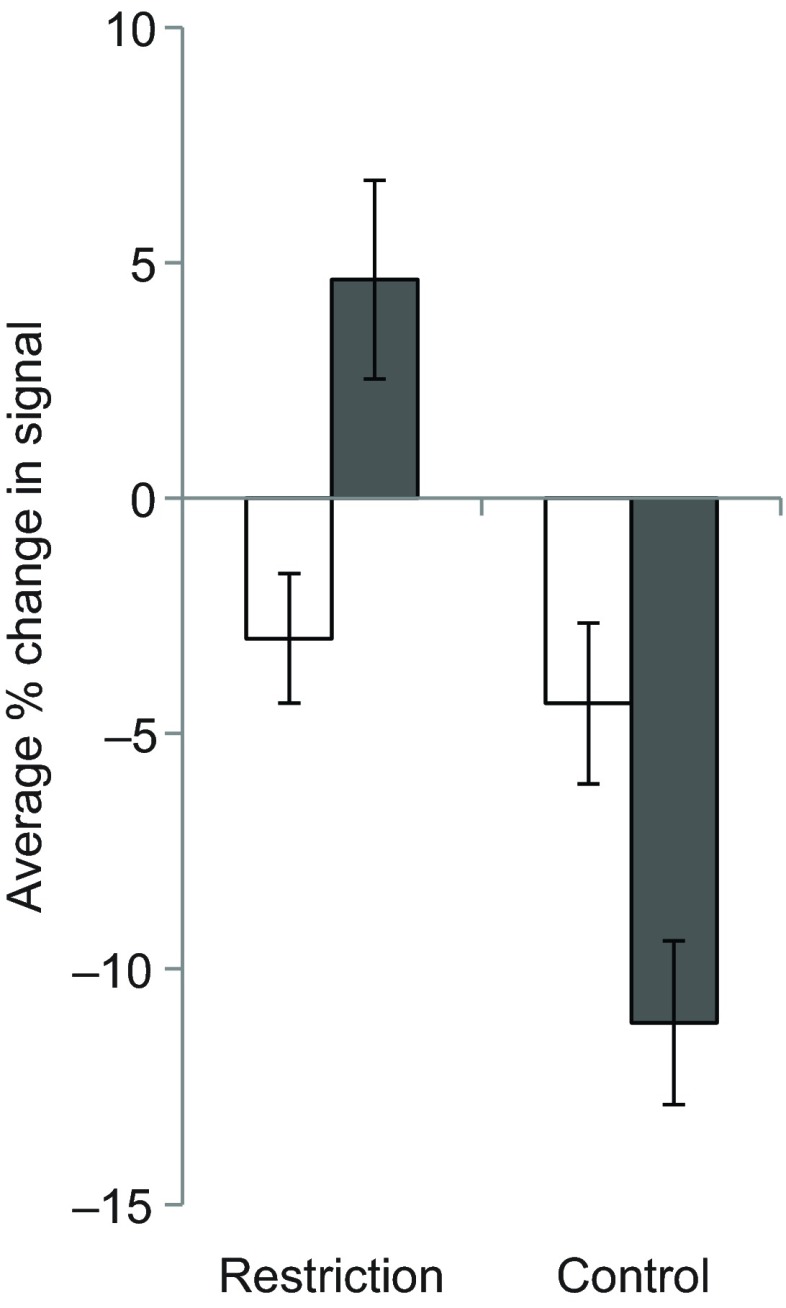
We measured serotonin levels in each animal twice, which allowed us to compare serotonergic fluctuation in first and second exposures to restriction. Increases of serotonin were larger relative to control in the second exposure than in the first (mixed model F=14.841, P=0.001). Interestingly, this appears to be caused by both an increase in the restricted animals and a decrease in the controls (Fig. 2). This is why we have focused on differences between control and experimental groups rather than changes from baseline where possible. Because in our mixed model many animals were only represented with one trial, and therefore individual variation may have influenced the results, we performed another test on only animals with two successful experimental trials (N=8: 5 males and 3 females). The increase in second trials was still larger than the increase in first trials (paired t-test P=0.018).
Fig. 2.
Restriction and control experiments shown by first (open bars) and second (filled bars) trials. Second trials for restriction experiments showed increased signal compared with first trials of restriction. Second trials for controls showed decreased signal compared to first trial controls. The effect of trial number was significant (mixed model F=14.841, P=0.001). Note that an apparent decrease in the serotonin signal may not represent an actual decrease in serotonin, but may be due to fiber electrodes becoming less sensitive over time, an effect often referred to as electrode fouling (Singh et al., 2011). Bars represent s.e.m.
Serotonin and individual variation
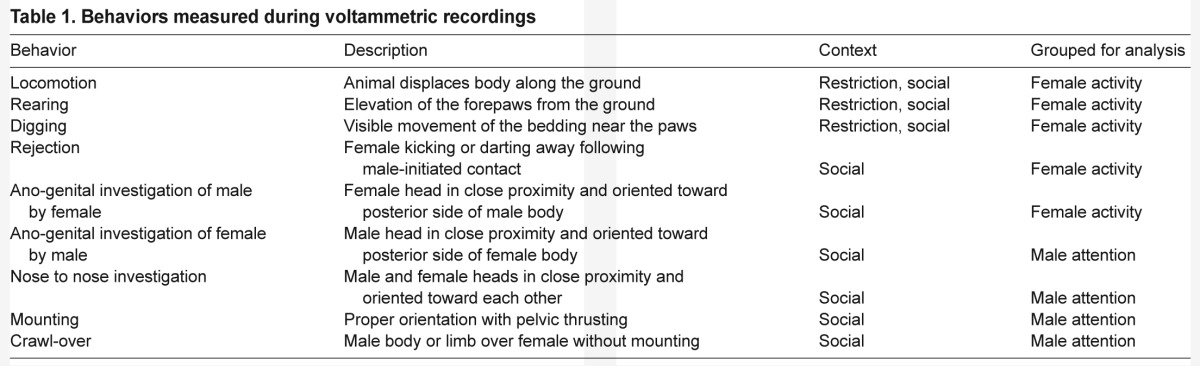
The total duration each individual spent performing locomotion, digging and rearing (Table 1) over the course of the 18 min restriction stimulus was measured. No behaviors correlated with the change of serotonin during restriction. To assess whether variation in mass and age influenced behavior or serotonin, both were measured. Mass and age were both higher for males, but were not related to each other, to any behavioral measures or to a change of serotonin. Within males and within females there were also no correlations between serotonin and the duration mice spent performing any behaviors we measured.
Table 1.
Behaviors measured during voltammetric recordings
Experiment 2: social interaction
Social interaction increased IC serotonin, but estrous phase did not influence magnitude
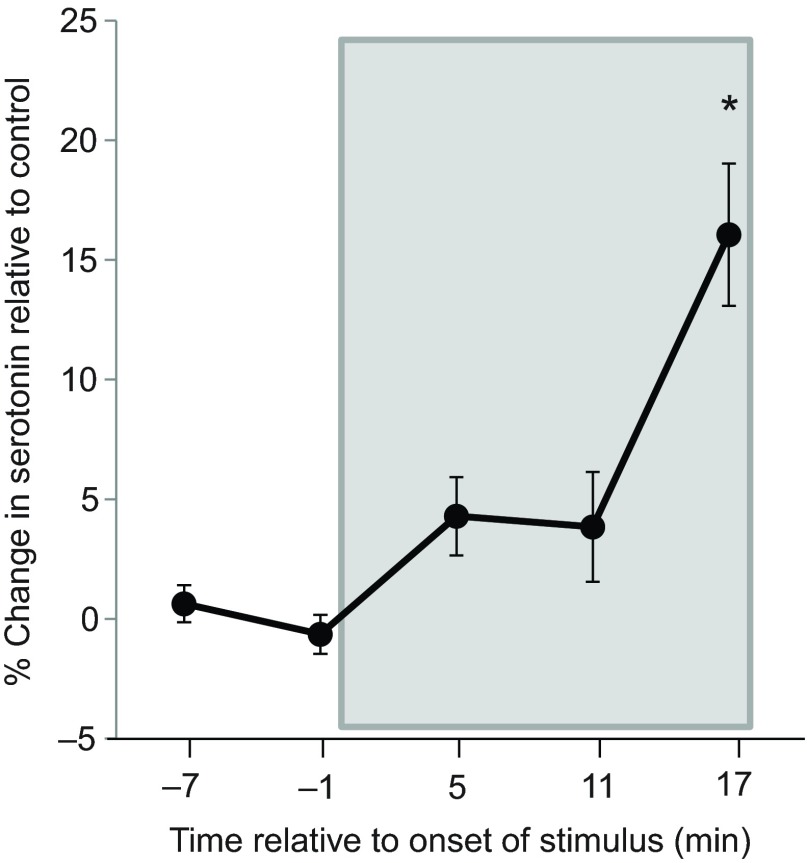
Previously, our lab has shown that serotonin increases in the IC of males in response to a male intruder (Hall et al., 2011). We sought to verify that social exposure induces increases in IC serotonin in females. During interaction with familiar males, serotonin in the IC of females increased gradually over 17 min, reaching significance from controls after 17 min (N=17 experimental and 11 control at 17 min; mixed model F=10.968, P=0.003; Fig. 3). There was no effect of whether measurements were made on the right or left side (mixed model F=0.058, P=0.813).
Fig. 3.
Percentage change in serotonin in female mice in response to interaction with a male social partner. Data are shown with controls subtracted. The box represents the time during which social interaction occurred. Females interacting with male social partners had increased serotonin over controls (*mixed model, F=10.968, P=0.003). Bars represent s.e.m.
Both the proestrus plus estrus group (8 trials) and the diestrus group (11 trials) showed gradual increases through the 17 min of social interaction (supplementary material Fig. S2). By the measurement at 17 min there was a qualitatively larger increase in the proestrus/estrus group; however, it was not significant (mixed model F=2.524, P=0.130). Two females were represented twice within the diestrus group. One female was represented twice within the proestrus/estrus group. Two females were represented once in each group. There were also no differences in behaviors exhibited between the two estrus phase groups, including mounting and female rejection of males (mixed models F=1.648, P=0.219; F=0.072, P=0.793).
Serotonergic fluctuation with respect to experience and behavioral activity
Serotonin differed between first and second social interaction trials at one time point; however, overall levels did not differ. After 11 min of social interaction, serotonin had increased more in second trials than in first trials (mixed model; 12 first trials, 7 second trials; 5 females represented twice; F=5.865, P=0.036; supplementary material Fig. S3). This difference was not present at the 5 min measurement, and had disappeared by the 17 min measurement. Because individual variation of animals only represented once in the data may have influenced the apparent differences in the mixed model, we compared data from the first and second trials of the 5 females represented twice. With this test, even at the 11 min time point, serotonin in the second trial was not significantly higher than that in the first (t-test P=0.230).
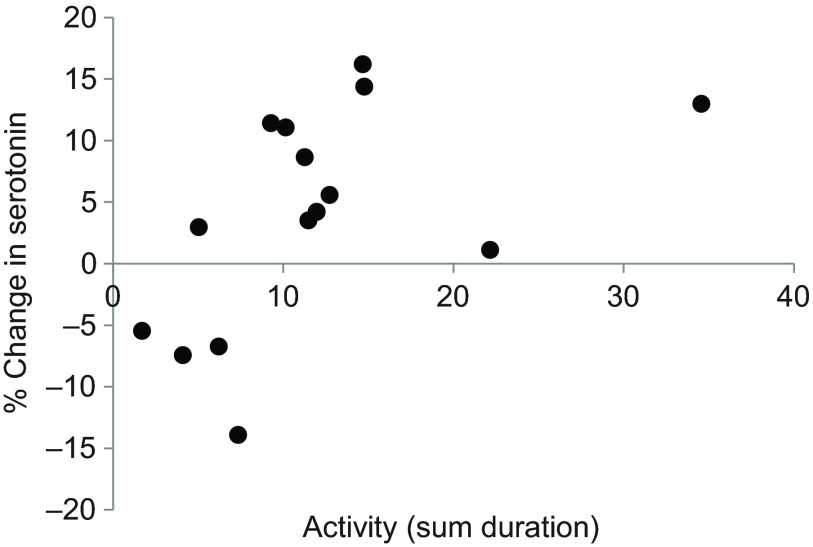
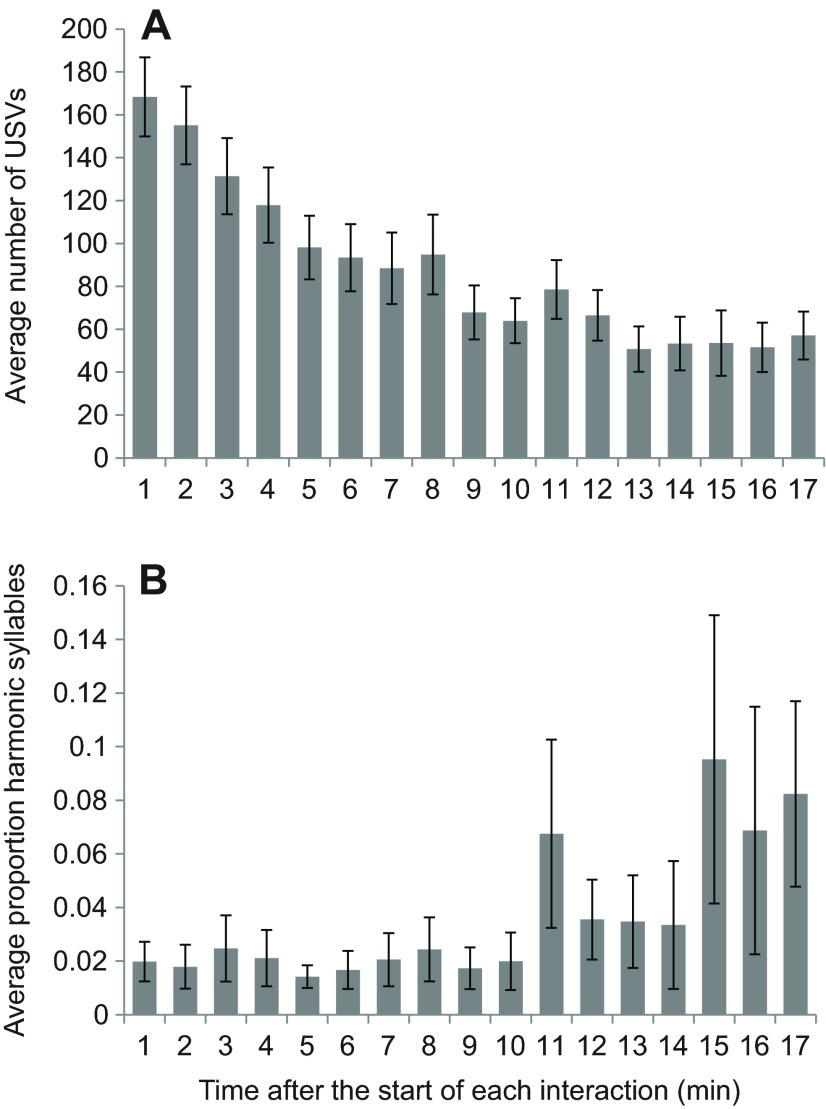
In order to determine whether differences in behavior helped to explain individual variation, we looked for correlations between the change of serotonin and female activity (Table 1). Measurements from females represented twice were averaged before assessing the strength of correlations. Females exhibited a positive correlation between the change of serotonin and activity, meaning females displaying more active behaviors had larger increases of serotonin (Spearman's ρ=0.646, P=0.009; Fig. 4). We also quantified ultrasonic vocalizations (USVs), which are produced by both males and females during social interactions or in response to novel stimuli (Moles et al., 2007; Portfors, 2007; Chabout et al., 2012). USVs occurred throughout the 17 min of interaction, gradually decreasing over time (Fig. 5A). There was no relationship between the number of USVs produced and the fluctuation of serotonin in females. Harmonic syllables, previously found in association with mounting behavior, were observed in 11 of the 15 interactions. For these trials the proportion of syllables containing harmonics increased across the 17 min (Fig. 5B). While there was no relationship between age, mass and serotonergic change, male attention and female age were negatively correlated (Spearman's ρ=−0.748, P=0.001; supplementary material Fig. S4).
Fig. 4.
Percent change in serotonin with respect to individual behavioral activity in female mice exposed to social interaction with a male. Activity was positively related to the increase in serotonin (Spearman's ρ=0.646, P=0.009, N=15). Activity was defined as the sum duration of all behaviors: locomotion, digging, rearing, rejection and ano-genital investigation (Table 1). Here, the percentage change in serotonin signal is relative to baseline, not control traces. Values may appear low due to electrode ‘fouling’.
Fig. 5.
Ultrasonic syllables recorded during 17 min of social interaction between male–female pairs. (A) The average number of ultrasonic vocalizations (USVs) produced in each minute of interaction (N=15). (B) The average proportion of syllables that contained harmonics in each minute of interaction (N=15). Bars represent s.e.m.
DISCUSSION
Here, we tested the idea that the social neuromodulator serotonin encodes different features of behavioral context and intrinsic state in a mammalian midbrain auditory nucleus, the IC. We did this by measuring serotonin in a single class of receivers, adult female mice, placed in different behavioral situations and measured in different stages of the estrous cycle. Although both a mild stressor and the presentation of a social partner caused serotonin to increase in females, the increases in these two different situations were distinguished by different time courses. Somewhat surprisingly, the serotonergic signal did not differ across estrous state in females, or between males and females placed in an identical stressful situation. Furthermore, differences between repeated measurements of serotonin in individual mice suggest that previous experience in a given context influences the serotonergic response to an immediate situation. These results contribute to our understanding of the mechanisms behind how sensory systems can be tuned precisely to the context an animal is experiencing. Below, we discuss the responsiveness of the serotonergic system to different extrinsic and intrinsic conditions in light of findings in other brain regions, and the potential function of auditory serotonin in vocal communication.
Context-dependent serotonergic fluctuation in the IC is a robust response that does not vary with sex or female estrous state
We initially predicted there would be an effect of sex on serotonergic fluctuation in the IC because serotonin measured by microdialysis in other brain areas has been found to have sex-specific extracellular levels (Jitsuki et al., 2009). Instead, during restriction stress, increases in IC serotonin occurred similarly in males and females, following the same time course and achieving similar percentage increases. In addition, females interacting with males achieved similar serotonin levels on a similar time course to that previously found in males interacting with males (Hall et al., 2012). Our results suggest that fluctuation of serotonin in the IC may be an example of a physiological process that is generalized between sexes, acting as a signal of the onset of specific contexts such as social interaction and restriction stress.
Female estrous state also did not significantly influence the change of serotonin in either the restriction or male–female experiments, which provides additional support that fluctuation over the course of several minutes in the IC is robust to the multiple hormones that vary over the estrous cycle. This is in contrast to basal levels of serotonin in the mediobasal hypothalamus in rats, which do fluctuate across the estrous cycle (Maswood et al., 1999). Direct manipulation of estrous hormones also influences the serotonergic system in multiple animal models. In seasonal breeders like female white-throated sparrows, inducing breeding condition with estradiol causes upregulation of the serotonergic system in different auditory areas, increasing the density of serotonergic fibers and increasing 5-HIAA levels in response to playback of species-specific songs (Matragrano et al., 2012).
Although we found that sex and estrous state did not influence the serotonergic response to our experimental behavioral situations, we recognize that other types of measurements that capture different features of the serotonergic system may show sex or estrous phase variation. Our voltammetric measurements were made on a time scale of minutes, which we have previously found to match the time scale of behavioral responses relatively well (Hall et al., 2011; Hall et al., 2012). Likewise, because our electrodes were relatively large, they measured large-scale fluctuation, a scale potentially relevant to ‘volume transmission’ of serotonin. Our finding of sex and estrous phase similarities in the IC does not eliminate the possibility that the fluctuation of serotonin could be dependent on these factors in other auditory areas, as serotonergic activation by a single auditory stimulus can have different effects across auditory regions (Cransac et al., 1998). Sex and estrous phase may also influence auditory processing through mechanisms other than release. For example, the density and binding affinity of membrane-bound serotonin receptors are influenced by reproductive hormones (Biegon et al., 1980; Mize and Alper, 2000). Our contribution is that, on a time scale relevant to behavior, fluctuations of serotonin in the IC appear to be robust to sex and estrous state, representing a general mechanism for responding to changes in external influences on behavioral context.
Serotonergic fluctuation occurs on different time scales in different behavioral contexts and corresponds to behavior
We exposed female mice to two different behavioral situations, restriction and interaction with males, and found a difference in time course for the change in serotonin between the two contexts. In females exposed to restriction stress, serotonin increased rapidly after onset and stayed relatively stable above control levels through each sampled time point. In females exposed to social interaction, a significant increase was not observed until after 17 min of stimulation. Serotonin has been shown to fluctuate on different time courses with respect to context in other brain regions (Adell et al., 1988). Differences in the timing of the serotonergic response between restriction and social interaction groups were previously found in the IC of male mice (Hall et al., 2011; Hall et al., 2012), which supports the idea that the context-dependent increase of serotonin in the IC is generalized between the sexes. One way to explain the cause of these context-dependent differences in time course is that the fluctuation of serotonin we observed in the IC is an indication of behavioral arousal (Jacobs and Azmitia, 1992; Jacobs and Fornal, 1999; Hall et al., 2010; Hall et al., 2011), where in potentially threatening situations serotonin increases faster, whereas over the duration of a social interaction the increase is more gradual. Although we used a controlled stress paradigm for this work, it is possible that more behaviorally relevant stress-inducing contexts also influence serotonin in the IC. For example, after detection of a predator it may be important to focus on sound stimuli determining predator type and location. This type of modulation is known to occur in the cortex of mice, where changes in auditory processing can occur with exposure to predator scents (Halene et al., 2009).
In conjunction with this hypothesis, the increase of serotonin previously measured during same-sex interaction in males occurred in parallel with the increased display of behaviors (Hall et al., 2011). In addition, serotonin is a known social modulator, with established ties to behaviors related to aggression, dominance and coping style (Daruna and Kent, 1976; Raleigh et al., 1991; Bell et al., 2007; Caramaschi et al., 2007; Koolhaas et al., 2007). Because of these relationships between serotonin and behavior, we predicted that serotonin levels in the IC of female mice would be correlated with behaviors exhibited during restriction stress and social interactions. Our findings show that during social interactions, the increase of serotonin in females was positively correlated with their activity (described in Table 1, Fig. 4). The presence of a general mechanism that increases IC serotonin in multiple behavioral contexts in accordance with behavioral arousal means that auditory modulation would occur during the presence of sounds at any time of high behavioral activity. The end result of a link between a behavioral response and the serotonergic signal, via general mechanisms of arousal, is that the resulting modulation of auditory processing by serotonin would be tied to behavioral needs.
Is the fluctuation of serotonin sensitive to prior experience?
In the present study, we observed the effects of imposing two different behavioral contexts on the fluctuation of serotonin in the IC. For both of these paradigms, mice were used in two trials, to fully make use of each subject. This design allowed us to make some interesting observations about the effects of previous experience on the serotonergic signal.
In the restriction experiment, there was a significant effect of first versus second exposure to stress on the increase of serotonin during restriction, such that in animals exposed twice, the second exposure elevated serotonin more than the first. We observed a different effect of recording order when the second trials were control trials. Because our experimental design was balanced, with most animals receiving one control and one non-control trial, each of our second-trial control trials was taken from an animal that experienced something other than control in their first trial. In these instances, the serotonergic response decreased significantly relative to first-trial controls. It is possible that the animals were primed for a stressful stimulus to occur after waking up in the voltammetric apparatus. Instead, in the absence of such a stimulus, serotonin decreased, dropping even lower than in control first trials.
Females interacting with males displayed a potential difference in the time course of increase in serotonin; however, in contrast to the restriction experiments, overall increases of serotonin were not different between first and second exposures. An important methodological difference between this experiment and the restriction experiment was that females had multiple experiences with males prior to implantation of the voltammetric hubs, and were familiar not only with the experience of interacting with males in general but also with the individual males presented in all trials. Thus, each female had about the same amount of experience going into first and second trials. A similar experiment was previously conducted on male mice, and an overall effect of recording order was found where second trials of social stimulation elevated serotonin more than first trials (Hall et al., 2011). However, because the males in the Hall et al. study were not given social experience prior to the measurement of serotonin, the interaction was novel in the first recording and not in the second, much as with our females exposed to restriction. Therefore, we hypothesize that when an animal is exposed to a new behavioral context for the first time, increases of serotonin in the IC are smaller than when the animal is exposed to the same context again, while repeated exposures to familiar contexts do not cause different amounts of serotonin to be released into the IC.
Our results suggest that experience influences serotonergic fluctuation in the IC. Whether that experience depends on behavioral context alone, or other aspects of a voltammetry experiment, is unclear. In addition to the explicit restriction stressor or social experience, all animals experienced the stress of anesthesia and of the electrode implantation procedure during the first voltammetric trial, as well as during the surgery to implant the voltammetric hubs. Although mice performed a wide range of behaviors in these trials, including eating, grooming, vocalizing and sleeping, being tethered to the voltammetric equipment may also have been stressful. It is also possible that potential damage to one IC during electrode implantation and removal, and subsequent compensation for those damages may also have influenced the response of the serotonergic system in the other IC. Increased serotonin metabolism may occur in response to brain lesions, which may reflect higher tonic release or more elevated phasic increases (Tsuiki et al., 1995). Increased serotonin after brain damage may be related to its function in vasoconstriction of the blood vessels near the damage (Lin, 1997). Our findings that second trials evoked serotonergic increases for restriction but decreases for control argue against the possibility that damage always drives increases of serotonin, however. Many of the issues raised in this section could be clarified through experiments designed explicitly to test the roles of different aspects of experience in altering serotonergic signaling.
Fluctuation of serotonin: the consequences for auditory processing and behavior
Our data suggest that serotonin is in a position to influence auditory processing of social vocalizations by IC neurons on a behaviorally relevant time scale. This hypothesis is supported by several lines of evidence, including (a) the correspondence of timing in serotonergic increases to the development of courtship and sexual behaviors in intersexual interactions in mice, and (b) the progression of vocalization production by male mice throughout a sexual encounter. In mice, an entire cycle of sexual behavior, from onset to ejaculation, may be fairly prolonged. Mounting and even intromission may occur relatively early in a courtship sequence, but often continue for substantial periods of time prior to actual ejaculation. Ejaculation latencies vary with mouse strain, male dominance status and female behavior, but ejaculation is typically delayed relative to the onset of male vocalization (McGill, 1962; Nyby, 1983; Johansen et al., 2008). A relatively recent review of rodent sexual behavior compares the phases of courtship and copulatory behavior in mice with those of two other rodent species, rats and hamsters (Hull and Dominguez, 2007). The authors of this review note substantial differences in ejaculation latencies among different strains of mice, ranging from a minimum of 9.9 min to as much as 115.7 min. Thus, events of great significance for reproduction may occur later in a mouse courtship sequence, consistent with the time scale of serotonergic increases we have shown.
Vocalizations produced by males in a courtship sequence may also change over the course of a courtship interaction. Male mice begin vocalizing soon after presentation with a female, and even in response to the odor of female secretions or urine (Nyby et al., 1979; Byatt and Nyby, 1986). Female mice also prefer to be near speakers broadcasting male vocalizations relative to silent speakers in the first few minutes after playback, a preference not shown for other types of sounds (Hammerschmidt et al., 2009; Shepard and Liu, 2011). These studies demonstrate that females preferentially investigate adult conspecific vocalizations soon after hearing them, at a time when our measurements show no change in serotonin within the IC. Vocalizations by males in the presence of females may also change qualitatively over the course of the interaction, however. White et al. (White et al., 1998) found that calls classified as 70 kHz calls were produced more robustly at the onset of a courtship interaction and subsequently declined, while vocalizations classed as 40 kHz calls usually appeared only after the first intromission. White et al. used these findings to suggest that the 70 kHz calls serve an attractive function, while the 40 kHz calls serve to coordinate copulatory behavior. Although the calls produced by males in our study declined after the initial few minutes of an interaction, they were still strongly produced throughout the full 17 min. More strikingly, the proportion of a type of call with a pronounced harmonic in the 30–40 kHz region (‘harmonic’ calls) (Portfors, 2007) is reversed relative to this trend and actually increases in later portions of the interaction. Although it is not clear whether ‘40 kHz calls’ are the same as our ‘harmonic calls’, this earlier report agrees with our finding that particular types of calls may become more prominent in the later portions of a courtship interaction. Mounting behavior itself is reduced in our voltammetry experiments, likely due to the placement of voltammetric hubs, but similar to White et al. (White et al., 1998), we have previously reported that harmonic calls are associated with mounting of females by males (Hanson and Hurley, 2012). Thus, a type of call that we know to be associated with mounting behavior is enriched in later portions of our 17 min interaction. Combined with the literature on mouse sexual and vocalization behavior, our findings therefore suggest that although serotonin would not have a strong influence on the processing of male courtship vocalizations early in an interaction, it may be in a position to influence auditory processing at a crucial point of the courtship interaction, closer to ejaculation.
This model of correspondence between the progression of social interactions and the serotonergic signal in the IC is consistent with our knowledge of the relationship between the serotonergic and auditory systems. The dorsal raphe nucleus, which supplies the majority of serotonergic input to the IC (Klepper and Herbert, 1991), is not itself auditory, and receives inputs from many different brain regions. It is thus in a position to import non-auditory social information into the IC. In our previous work, we found that the size of a serotonergic signal during social interaction does not simply indicate the presence of a social partner but also correlates with the level of behavioral response to a social partner (Hall et al., 2011). Serotonergic signals in the IC may therefore track some behaviorally relevant features of social interaction.
A final link in the model is a series of studies showing that exogenous serotonin, when applied locally to IC neurons, alters the responses to a range of different types of auditory stimuli, including species-specific vocalizations. IC neurons often have more selective responses to sound stimuli when serotonin is applied locally (Hurley and Pollak, 2001; Hurley et al., 2002). For example, in bats, serotonin causes a decrease in the number of social vocalizations to which most neurons respond (Hurley and Pollak, 2005). Because of this filtering function, endogenous increases in serotonin in the IC may facilitate more selective responses to sound, including species-specific vocalizations (Hurley and Pollak, 2001; Hurley and Pollak, 2005).
These events in the IC are of great significance to signal perception. This is because the IC collects information on stimuli from most auditory brainstem nuclei, transforms it through excitatory–inhibitory convergence, and then projects this information to higher auditory levels (Pollak et al., 2011; Ito and Oliver, 2012; Pollak, 2013). Events in some regions of the IC correspond to behavioral responses to stimuli such as courtship calls (Mangiamele and Burmeister, 2011), but signals processed within the IC are likely to be interpreted by a network of brain regions involved in social decision making, which include the anterior and ventromedial hypothalamus, bed nucleus of the stria terminalis, medial amygdala, periaqueductal gray, lateral septum and preoptic area (Newman, 1999; Goodson and Kabelik, 2009; O'Connell and Hofmann, 2011; Hoke and Pitts, 2012). These regions directly influence multiple types of social behaviors including reproductive, aggression and parental behaviors. Many, if not all, of these regions are also influenced by serotonin (Mas et al., 1995; Cologer-Clifford et al., 1997; Cheeta et al., 2000; Summers et al., 2005; Dias and Crews, 2006; Yu and Yamaguchi, 2009; Aubert et al., 2013). Motor or premotor regions controlling the production of social vocalizations are also sensitive to serotonin in a range of vertebrate model communication systems (Yu and Yamaguchi, 2009; Wood et al., 2011; Wood et al., 2013). The centralized serotonergic system would thus exemplify motivational or modulatory neural machinery impinging on the social decision network (Goodson and Kabelik, 2009; O'Connell and Hofmann, 2011; Hoke and Pitts, 2012). Our results demonstrate that, in the context of this model of social behavior, motivational machinery like the serotonergic system is important at every level, from signal processing to behavioral decision making. The global influence of serotonin further suggests that such mechanisms are important in coordinating neural activity in different regions influenced by the input of species-specific signals, to ultimately produce appropriate behavioral outputs.
Serotonin as a modulator of sensory processing across species
While we have focused on serotonergic modulation in the auditory system, serotonin is present across sensory systems, where it may also be involved in context-dependent modulation. Serotonin also modulates insect olfaction in the antennae (Kloppenburg and Mercer, 2008; Dacks et al., 2009). Serotonin is present in the olfactory and gustatory systems of mammals (Huang et al., 2005; Petzold et al., 2009). Serotonin can also alter responses to visual stimuli in the visual cortex (Waterhouse et al., 1990) and taste cell sensitivity through voltage-dependent calcium currents (Delay et al., 1997), perhaps as a result of paracrine serotonergic signaling (Kaya et al., 2004). The serotonin released throughout the brain originates in the raphe nuclei, a system that could easily modulate many sensory regions in a coordinated manner. Our finding of a fluctuation of serotonin on the time scale of minutes raises the question of whether this context-dependent fluctuation occurs in other modalities. In particular, the effect of experience on the magnitude of release of serotonin and the effect of specific context on the time course of release of serotonin may be important to how serotonin functions in other sensory systems. Together, plasticity of multiple sensory systems could be a benefit to animals exposed to cues that change across different behavioral contexts. The diffuse nature of the serotonergic release may even help sensory systems work in concert to equip animals to deal with changes that occur with threats such as predators, or those that occur over the course of a social interaction.
Summary
Our data show that increases of serotonin in the IC over a time course of minutes are dependent on the type of behavioral situation that is imposed, and the degree of experience an individual has in that situation, but not on internal influences such as sex and estrus phase. These results lead us to a model of fluctuation of serotonin in the IC as selectively context sensitive, but generalized across some intrinsic factors. The behaviorally relevant timing of serotonergic increases during interactions between male and female mice suggests a role for serotonergic modulation of courtship vocalizations.
MATERIALS AND METHODS
Animals
Adult CBA/J mice from The Jackson Laboratory were housed individually on a 14 h:10 h light:dark cycle. Food was provided ad libitum. All procedures were approved by the Bloomington Institutional Animal Care and Use Committee (Indiana University).
Estrous staging
For female mice, estrous phase was determined each day by sampling vaginal cells with a saline lavage. The liquid was smeared onto a slide and dried. Methanol was used to fix the cells, and Giemsa stain was used to help visualize the cells. Phases were determined by the relative number of cell types present. The presence of only cornified epithelial cells indicated estrus, the presence of both cornified and nucleated epithelial cells indicated proestrus, and the presence of leukocytes indicated diestrus (Goldman et al., 2007). Mice were analyzed for estrous phase every day because most did not follow a regular 4–5 day cycle. Daily staging allowed a better understanding of each mouse's phase history and standardized handling across individuals in the study. Diestrus rodent females are not considered sexually receptive, whereas females have been deemed behaviorally receptive in proestrus and estrus, although expression of receptivity is complex and variable (Blaustein, 2008). Because estrus and proestrus stage females were less common, we combined them into a single group and compared them with diestrus stage females. As the staging procedure required brief handling of the females, males measured for serotonin were also briefly restrained by hand daily to control for any effect of this handling.
Surgery
Aseptic cannulation surgeries were performed to permanently affix a headstage to the skull, allowing direct access to the IC. Mice were briefly exposed to isoflurane in order to inject a proactive dose of metacam analgesic (1 mg kg−1), and a dose of anesthetic (either 5 mg kg−1 xylazine and 120 mg kg−1 ketamine, or 5 mg kg−1 xylazine, 100 mg kg−1 ketamine and 2 mg kg−1 acepromazine). Once mice were unresponsive, the hair from the top of the mouse's head was removed, the skin was disinfected with alternating applications of Betadine and 70% ethanol, and an incision was made to deflect the skin from the area of the skull over the IC. Two holes were drilled in the skull centered at 1.1 mm posterior and 1.6 mm lateral from lambda and ~1.5 mm in diameter to expose the IC. Custom-made Teflon hubs were affixed to the skull with two bone screws (one rostral and one caudal to the hubs) and dental acrylic. The skin was replaced over the dental cement and Vetbond (3M) adhesive was used to close the skin around the hubs. Lidocaine gel and triple antibiotic ointment were applied to the surgical site. Two Teflon screws were placed into the hubs to close the surgical site while the animal was allowed to recover. Mice were allowed at least 5 days to recover before a voltammetry recording took place.
Males that participated in the male–female interaction experiments were vasectomized to prevent the production of pups. After the males were anesthetized as described above, fur was removed from the abdomen and the skin was disinfected. An incision was made on the ventral surface through the body wall and a section of both vas deferentia was removed. Free ends were ligated and the muscle and skin layers were sutured closed.
Electrode construction and voltammetric measurements
Carbon fiber microelectrodes were constructed for voltammetric recordings. A single barrel glass capillary tube was pulled to produce a narrow tip, which was broken to 11 μm inner diameter, allowing for protrusion of a carbon fiber through the tip (Cytek Industries Inc., Woodland Park, NJ, USA). The fibers were affixed to the glass tube with epoxy resin and soldered to a tinned copper wire with a low temperature melting bismuth alloy solder. The glass tube was cut to size to fit in custom-built Teflon microdrives. Electrodes were electrically pre-treated to increase sensitivity to serotonin by applying current in a solution of phosphate–citrate buffer against a Ag/AgCl reference electrode and a calomel auxiliary electrode with a bipotentiostat (EI-400; Cypress Systems, Chelmsford, MA, USA) and e-corder (EDAQ 821, Denistone East, NSW, Australia). The following protocol was applied: 70 Hz, 0–3 V triangle waveform for 30 s, followed by a 1.5 V potential for 10 s, a −0.5 V potential for 5 s, and a 1.5 V potential for 8 s. This treatment separated the oxidizing voltage of serotonin from that of other chemicals (such as catecholamines and ascorbic acid). Electrodes were chemically treated with 5% Nafion ion exchange resin (Sigma-Aldrich, St Louis, MO, USA) to help exclude 5HIAA and uric acid, which oxidize at the same voltage as serotonin even after electrical pre-treatment, but are more negatively charged. Each electrode tip was dipped 2–10 (usually 5) times into Nafion and allowed to dry between dips.
Before use in vivo, the electrodes were tested in a solution containing 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin and ascorbic acid against a Ag/AgCl reference electrode. A cyclic staircase protocol with a range of −300 to 600 mV was applied in steps of 10 mV at 30 mV s−1, completing in 1 min. Signal of serotonin was determined by measuring the height of the peak corresponding to serotonin oxidation (~300 to 450 mV) in the first derivative of the current for each trace. If the peak for serotonin was larger than those indicating DOPAC and ascorbic acid, the electrode was used in vivo (Hall et al., 2010). Measurements of serotonin were made at 6 min intervals, which in vivo represents a good tradeoff between the time course of behavioral responses to different contexts and use-dependent decreases in electrode sensitivity due to fouling, which are characteristic of carbon fiber electrodes (Singh et al., 2011).
Experiment 1: restriction stressor
Mice between 8 and 36 weeks of age were used (15 males, 19 females). On the day of a recording all subjects were weighed, given a dose of metacam analgesic, and anesthetized with 60% of the surgical dose of ketamine/xylazine. The Teflon screws were removed from the hubs and the holes were cleared of any material blocking access to the IC. A carbon fiber electrode was lowered into the hub on one side, left or right randomized per animal, so that the tip of the electrode penetrated the IC. A reference electrode (Ag/AgCl) was lowered into the hub on the opposite side to make contact with the cerebrospinal fluid. The electrodes were connected to the rig, and the mouse was allowed to recover from anesthetic in its home cage. Only data that were collected more than 1 h after recovery from anesthetic, as demonstrated by locomotion around the cage, were used. For the experimental treatment, after two baseline measurements were collected, a tube that was 8 cm in diameter, 15 cm tall and open on top was placed around the mouse in its home cage. The mouse was restricted in the tube for 18 min, allowing for three voltammetric measurements, occurring after 5, 11 and 17 min of restriction. After the final trace was completed, the tube was removed. Behavior in the tube was filmed from above with a CCD video camera (30 frames s−1), Q-See 4 channel DVR PCI video capture card, and SuperDVR software (Q-See, Digital Peripheral Solutions Inc., Anaheim, CA, USA). In control trials, an identical procedure was performed, except the animal was not subjected to restriction in a tube. Each animal was tested twice with at least 5 days of recovery in between. At the end of the second recording, a current (0.3 mA) was applied through the carbon fiber electrode using a lesion maker (Grass Instruments, Quincy, MA, USA), and mice were kllled 1 day after by perfusion. Brains were sliced to verify the location of the recording site in the IC.
Experiment 2: social interaction
Measurements of serotonin were taken from females 10–37 weeks old. Twelve stimulus males were vasectomized and used at ages 10–38 weeks, up to four times each. Females were given 5–10 iterations of 20 min social interactions with stimulus males over at least 1 week before being prepared for voltammetric recordings. Females were not used in experiments until after they had been mounted at least once.
On the day of a recording, mice were weighed, anesthetized and connected to the voltammetric equipment as in experiment 1. They were allowed to recover from anesthetic, and experimental data were not recorded for at least 1 h after the female began to locomote about the cage. After two baseline measurements were taken, either a familiar male was added for 18 min while three additional voltammetric measurements were taken at 6 min intervals (after 5, 11 and 17 min of interaction), or no male was added for a control treatment of the same duration. Each animal underwent two trials, with measurements taken once from each IC. Each animal had at least 5 days to recover in between trials, and animals were killed as described above. Females were exposed to males for both trials, given one trial of interaction with a male and one control trial, or given two control trials. All interactions were recorded with a CCD video camera (as in experiment 1). During 15 male–female interactions, vocalizations were recorded with a condenser microphone (CM16/CMPA, Avisoft Bioacoustics, Berlin, Germany) and sound card (UltraSoundGate 116Hb, Avisoft Bioacustics) with a 250 kHz sample rate (11 females; 4 recorded twice, 7 recorded once).
Behavioral analysis
Behaviors were scored by an observer blind to sex and estrous phase with ODLog software (Macropod Software, Eden Prairie, MN, USA). Behaviors measured during restriction and social interaction are described in Table 1. The duration of time spent performing each behavior was calculated for the duration of the stimulus. To reduce the number of variables used in statistical tests for the social interaction context, behaviors were collapsed into two categories: female activity and male attention (Table 1). Durations of behaviors were summed per category even though some behaviors may have occurred simultaneously, such as rejection and locomotion, so the sums do not represent absolute time, but a weighted representation of the behaviors included. Vocalizations were analyzed by hand using spectrographs (Hanson and Hurley, 2012; Avisoft SASLab Pro, Avisoft Bioacoustics). We quantified the number of USV syllables produced by male–female pairs. In addition, the number of USV syllables containing at least one harmonic were differentiated from those containing no harmonics. The total number of USVs and harmonic-type syllables were counted for the duration of the social interaction in the 15 trials that were recorded by microphone.
Data analysis
Three measurements of serotonin at 6 min intervals were taken during each experimental treatment or equivalent time point in each control treatment. The two measurements taken immediately prior to the experimental or control treatment were used as baseline. However, not all measurements were usable because of the low signal to noise ratio during vigorous movement, or the malfunction or breakage of delicate carbon fibers. Only trials with at least one usable baseline measurement and two usable experimental/control measurements were included in this study. The percentage change of serotonin was calculated from the average of two baseline measurements when possible.
Because of the unbalanced nature of the data dictated by which data were usable, mixed models accounting for partial repeated measures were used to analyze the effects of experimental treatment, sex, side of brain, trial number (first versus second) and estrous phase among females. Mixed models were run on percentage change of serotonin, either on specific time points after the onset of a stimulus or on the averages from the two to three usable measurements taken while a stimulus was present, depending on the time course of change of serotonin. Because not every trial had data for each time point, when mixed models were run on particular time points, sample sizes are specified in the Results. These vary from test to test based on where missing values occurred.
Spearman's tests were used to assess correlations between behaviors and average change of serotonin. For these tests, an average per individual was used for those individuals given the experimental treatment twice. Spearman's tests were also used to assess correlations among age or mass and serotonergic fluctuation and behavior of test subjects.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Sarah Keesom and Adam Smith for comments on the manuscript, Ian Hall for valuable training and guidance in the voltammetric technique used to collect these data, and Gabrielle Sell for analyzing videos of mouse behavior.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This research was supported in part by the Graduate Research Fellowship Program of the National Science Foundation (J.L.H.), the Center for the Integrative Study of Animal Behavior (Bloomington, IN, USA), and by the National Institute on Deafness and other Communication Disorders (DC006608 to L.M.H.). Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.087627/-/DC1
References
- Abizaid A., Mezei G., Thanarajasingam G., Horvath T. L. (2005). Estrogen enhances light-induced activation of dorsal raphe serotonergic neurons. Eur. J. Neurosci. 21, 1536-1546 [DOI] [PubMed] [Google Scholar]
- Adell A., Trullas R., Gelpi E. (1988). Time course of changes in serotonin and noradrenaline in rat brain after predictable or unpredictable shock. Brain Res. 459, 54-59 [DOI] [PubMed] [Google Scholar]
- Adell A., Casanovas J. M., Artigas F. (1997). Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology 36, 735-741 [DOI] [PubMed] [Google Scholar]
- Aubert Y., Allers K. A., Sommer B., de Kloet E. R., Abbott D. H., Datson N. A. (2013). Brain region-specific transcriptomic markers of serotonin-1a receptor agonist action mediating sexual rejection and aggression in female marmoset monkeys. J. Sex Med. 10, 1461-1475 [DOI] [PubMed] [Google Scholar]
- Bell A. M., Backström T., Huntingford F. A., Pottinger T. G., Winberg S. (2007). Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol. Behav. 91, 15-25 [DOI] [PubMed] [Google Scholar]
- Biegon A., Bercovitz H., Samuel D. (1980). Serotonin receptor concentration during the estrous cycle of the rat. Brain Res. 187, 221-225 [DOI] [PubMed] [Google Scholar]
- Blaustein J. D. (2008). Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu. Rev. Psychol. 59, 93-118 [DOI] [PubMed] [Google Scholar]
- Byatt S., Nyby J. (1986). Hormonal regulation of chemosignals of female mice that elicit ultrasonic vocalizations from males. Horm. Behav. 20, 60-72 [DOI] [PubMed] [Google Scholar]
- Caramaschi D., de Boer S. F., Koolhaas J. M. (2007). Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol. Behav. 90, 590-601 [DOI] [PubMed] [Google Scholar]
- Chabout J., Serreau P., Ey E., Bellier L., Aubin T., Bourgeron T., Granon S. (2012). Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS ONE 7, e29401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F., Berton O., Mormède P. (1999). Serotonin and stress. Neuropsychopharmacology 21 Suppl., 28S-32S [DOI] [PubMed] [Google Scholar]
- Cheeta S., Kenny P. J., File S. E. (2000). Hippocampal and septal injections of nicotine and 8-OH-DPAT distinguish among different animal tests of anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 24, 1053-1067 [DOI] [PubMed] [Google Scholar]
- Cologer-Clifford A., Simon N. G., Lu S.-F., Smoluk S. A. (1997). Serotonin agonist-induced decreases in intermale aggression are dependent on brain region and receptor subtype. Pharmacol. Biochem. Behav. 58, 425-430 [DOI] [PubMed] [Google Scholar]
- Cransac H., Cottet-Emard J. M., Hellström S., Peyrin L. (1998). Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear. Res. 118, 151-156 [DOI] [PubMed] [Google Scholar]
- Dacks A. M., Green D. S., Root C. M., Nighorn A. J., Wang J. W. (2009). Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J. Neurogenet. 23, 366-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruna J. H., Kent E. W. (1976). Comparison of regional serotonin levels and turnover in the brain of naturally high and low aggressive rats. Brain Res. 101, 489-501 [DOI] [PubMed] [Google Scholar]
- De Souza E. B., Van Loon G. R. (1986). Brain serotonin and catecholamine responses to repeated stress in rats. Brain Res. 367, 77-86 [DOI] [PubMed] [Google Scholar]
- Delay R. J., Kinnamon S. C., Roper S. D. (1997). Serotonin modulates voltage-dependent calcium current in Necturus taste cells. J. Neurophysiol. 77, 2515-2524 [DOI] [PubMed] [Google Scholar]
- Dias B. G., Crews D. (2006). Serotonergic modulation of male-like pseudocopulatory behavior in the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Horm. Behav. 50, 401-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U., Ostwald J. (1992). Serotonin modulates auditory information processing in the cochlear nucleus of the rat. Neurosci. Lett. 145, 51-54 [DOI] [PubMed] [Google Scholar]
- Ehret G., Schmid C. (2009). Reproductive cycle-dependent plasticity of perception of acoustic meaning in mice. Physiol. Behav. 96, 428-433 [DOI] [PubMed] [Google Scholar]
- Ehret G., Koch M., Haack B., Markl H. (1987). Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften 74, 47-47 [DOI] [PubMed] [Google Scholar]
- Goldman J. M., Murr A. S., Cooper R. L. (2007). The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B Dev. Reprod. Toxicol. 80, 84-97 [DOI] [PubMed] [Google Scholar]
- Goodson J. L., Kabelik D. (2009). Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front. Neuroendocrinol. 30, 429-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene T. B., Talmud J., Jonak G. J., Schneider F., Siegel S. J. (2009). Predator odor modulates auditory event-related potentials in mice. Neuroreport 20, 1260-1264 [DOI] [PubMed] [Google Scholar]
- Hall I. C., Rebec G. V., Hurley L. M. (2010). Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. J. Exp. Biol. 213, 1009-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. C., Sell G. L., Hurley L. M. (2011). Social regulation of serotonin in the auditory midbrain. Behav. Neurosci. 125, 501-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. C., Sell G. L., Chester E. M., Hurley L. M. (2012). Stress-evoked increases in serotonin in the auditory midbrain do not directly result from elevations in serum corticosterone. Behav. Brain Res. 226, 41-49 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K., Radyushkin K., Ehrenreich H., Fischer J. (2009). Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol. Lett. 5, 589-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. L., Hurley L. M. (2012). Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS ONE 7, e40782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke K. L., Pitts N. L. (2012). Modulation of sensory-motor integration as a general mechanism for context dependence of behavior. Gen. Comp. Endocrinol. 176, 465-471 [DOI] [PubMed] [Google Scholar]
- Huang Y. J., Maruyama Y., Lu K. S., Pereira E., Plonsky I., Baur J. E., Wu D., Roper S. D. (2005). Mouse taste buds use serotonin as a neurotransmitter. J. Neurosci. 25, 843-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull E. M., Dominguez J. M. (2007). Sexual behavior in male rodents. Horm. Behav. 52, 45-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L. M., Pollak G. D. (2001). Serotonin effects on frequency tuning of inferior colliculus neurons. J. Neurophysiol. 85, 828-842 [DOI] [PubMed] [Google Scholar]
- Hurley L. M., Pollak G. D. (2005). Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. J. Comp. Physiol. A 191, 535-546 [DOI] [PubMed] [Google Scholar]
- Hurley L. M., Thompson A. M., Pollak G. D. (2002). Serotonin in the inferior colliculus. Hear. Res. 168, 1-11 [DOI] [PubMed] [Google Scholar]
- Ito T., Oliver D. L. (2012). The basic circuit of the IC: tectothalamic neurons with different patterns of synaptic organization send different messages to the thalamus. Front. Neural Circuits 6, 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B. L., Azmitia E. C. (1992). Structure and function of the brain serotonin system. Physiol. Rev. 72, 165-229 [DOI] [PubMed] [Google Scholar]
- Jacobs B. L., Fornal C. A. (1999). Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology 21 Suppl., 9S-15S [DOI] [PubMed] [Google Scholar]
- Ji W., Suga N. (2007). Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J. Neurosci. 27, 4910-4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitsuki S., Kimura F., Funabashi T., Takahashi T., Mitsushima D. (2009). Sex-specific 24-h profile of extracellular serotonin levels in the medial prefrontal cortex. Brain Res. 1260, 30-37 [DOI] [PubMed] [Google Scholar]
- Johansen J. A., Clemens L. G., Nunez A. A. (2008). Characterization of copulatory behavior in female mice: evidence for paced mating. Physiol. Behav. 95, 425-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H., Yoshida M., Yokoo H., Nishi M., Tanaka M. (1993). Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci. Lett. 162, 81-84 [DOI] [PubMed] [Google Scholar]
- Kaya N., Shen T., Lu S. G., Zhao F. L., Herness S. (2004). A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am. J. Physiol. 286, R649-R658 [DOI] [PubMed] [Google Scholar]
- Klepper A., Herbert H. (1991). Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 557, 190-201 [DOI] [PubMed] [Google Scholar]
- Kloppenburg P., Mercer A. R. (2008). Serotonin modulation of moth central olfactory neurons. Annu. Rev. Entomol. 53, 179-190 [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., de Boer S. F., Buwalda B., van Reenen K. (2007). Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav. Evol. 70, 218-226 [DOI] [PubMed] [Google Scholar]
- Lin M.-T. (1997). Heatstroke-induced cerebral ischemia and neuronal damage. Involvement of cytokines and monoamines. Ann. New York Acad. Sci. 813, 572-580 [DOI] [PubMed] [Google Scholar]
- Liu R. C., Linden J. F., Schreiner C. E. (2006). Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur. J. Neurosci. 23, 3087-3097 [DOI] [PubMed] [Google Scholar]
- Mangiamele L. A., Burmeister S. S. (2011). Auditory selectivity for acoustic features that confer species recognition in the tungara frog. J. Exp. Biol. 214, 2911-2918 [DOI] [PubMed] [Google Scholar]
- Mas M., Fumero B., González-Mora J. L. (1995). Voltammetric and microdialysis monitoring of brain monoamine neurotransmitter release during sociosexual interactions. Behav. Brain Res. 71, 69-79 [DOI] [PubMed] [Google Scholar]
- Maswood S., Truitt W., Hotema M., Caldarola-Pastuszka M., Uphouse L. (1999). Estrous cycle modulation of extracellular serotonin in mediobasal hypothalamus: role of the serotonin transporter and terminal autoreceptors. Brain Res. 831, 146-154 [DOI] [PubMed] [Google Scholar]
- Matragrano L. L., Sanford S. E., Salvante K. G., Beaulieu M., Sockman K. W., Maney D. L. (2012). Estradiol-dependent modulation of serotonergic markers in auditory areas of a seasonally breeding songbird. Behav. Neurosci. 126, 110-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill T. E. (1962). Sexual behavior in three inbred strains of mice. Behaviour 19, 341-350 [Google Scholar]
- Miranda J. A., Wilczynski W. (2009a). Sex differences and androgen influences on midbrain auditory thresholds in the green treefrog, Hyla cinerea. Hear. Res. 252, 79-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda J. A., Wilczynski W. (2009b). Female reproductive state influences the auditory midbrain response. J. Comp. Physiol. A 195, 341-349 [DOI] [PubMed] [Google Scholar]
- Mize A. L., Alper R. H. (2000). Acute and long-term effects of 17beta-estradiol on G(i/o) coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPgammaS binding. Brain Res. 859, 326-333 [DOI] [PubMed] [Google Scholar]
- Moles A., Costantini F., Garbugino L., Zanettini C., D'Amato F. R. (2007). Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav. Brain Res. 182, 223-230 [DOI] [PubMed] [Google Scholar]
- Newman S. W. (1999). The medial extended amygdala in male reproductive behavior – a node in the mammalian social behavior network. In Advancing from the Ventral Striatum to the Extended Amygdala: Implications for Neuropsychiatry and Drug Abuse: In Honor of Lennart Heimer, Vol. 877 (ed. McGinty J. F.), pp. 242-257 New York, NY: New York Academy of Sciences; [DOI] [PubMed] [Google Scholar]
- Nyby J. (1983). Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav. Neural Biol. 39, 128-134 [DOI] [PubMed] [Google Scholar]
- Nyby J., Wysocki C. J., Whitney G., Dizinno G., Schneider J. (1979). Elicitation of male-mouse (Mus musculus) ultrasonic vocalizations. 1. Urinary cues. J. Comp. Physiol. Psychol. 93, 957-975 [Google Scholar]
- O'Connell L. A., Hofmann H. A. (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599-3639 [DOI] [PubMed] [Google Scholar]
- Petzold G. C., Hagiwara A., Murthy V. N. (2009). Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat. Neurosci. 12, 784-791 [DOI] [PubMed] [Google Scholar]
- Pollak G. D. (2013). The dominant role of inhibition in creating response selectivities for communication calls in the brainstem auditory system. Hear. Res. 305, 86-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak G. D., Xie R., Gittelman J. X., Andoni S., Li N. (2011). The dominance of inhibition in the inferior colliculus. Hear. Res. 274, 27-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors C. V. (2007). Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. 46, 28-34 [PubMed] [Google Scholar]
- Price M. L., Curtis A. L., Kirby L. G., Valentino R. J., Lucki I. (1998). Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology 18, 492-502 [DOI] [PubMed] [Google Scholar]
- Raleigh M. J., McGuire M. T., Brammer G. L., Pollack D. B., Yuwiler A. (1991). Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 559, 181-190 [DOI] [PubMed] [Google Scholar]
- Rubinow D. R., Schmidt P. J., Roca C. A. (1998). Estrogen-serotonin interactions: implications for affective regulation. Biol. Psychiatry 44, 839-850 [DOI] [PubMed] [Google Scholar]
- Shepard K. N., Liu R. C. (2011). Experience restores innate female preference for male ultrasonic vocalizations. Genes Brain Behav. 10, 28-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y. S., Sawarynski L. E., Dabiri P. D., Choi W. R., Andrews A. M. (2011). Head-to-head comparisons of carbon fiber microelectrode coatings for sensitive and selective neurotransmitter detection by voltammetry. Anal. Chem. 83, 6658-6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C. H., Korzan W. J., Lukkes J. L., Watt M. J., Forster G. L., Øverli Ø., Höglund E., Larson E. T., Ronan P. J., Matter J. M., et al. (2005). Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol. Biochem. Zool. 78, 679-694 [DOI] [PubMed] [Google Scholar]
- Terleph T. A., Lu K., Vicario D. S. (2008). Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS ONE 3, e2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiki K., Takada A., Nagahiro S., Grdiša M., Diksic M., Pappius H. M. (1995). Synthesis of serotonin in traumatized rat brain. J. Neurochem. 64, 1319-1325 [DOI] [PubMed] [Google Scholar]
- Waterhouse B. D., Azizi S. A., Burne R. A., Woodward D. J. (1990). Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 514, 276-292 [DOI] [PubMed] [Google Scholar]
- White N. R., Prasad M., Barfield R. J., Nyby J. G. (1998). 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol. Behav. 63, 467-473 [DOI] [PubMed] [Google Scholar]
- Wood W. E., Lovell P. V., Mello C. V., Perkel D. J. (2011). Serotonin, via HTR2 receptors, excites neurons in a cortical-like premotor nucleus necessary for song learning and production. J. Neurosci. 31, 13808-13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. E., Roseberry T. K., Perkel D. J. (2013). HTR2 receptors in a songbird premotor cortical-like area modulate spectral characteristics of zebra finch song. J. Neurosci. 33, 2908-2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. J., Yamaguchi A. (2009). 5-HT2C-like receptors in the brain of Xenopus laevis initiate sex-typical fictive vocalizations. J. Neurophysiol. 102, 752-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J. K., Fernandez A. A., Gosselink K. L. (2011). Female responses to acute and repeated restraint stress differ from those in males. Physiol. Behav. 104, 215-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.