Abstract
Background
Research in bipolar disorder (BD) implicates fronto-limbic-striatal dysfunction during face emotion processing but it is unknown how such dysfunction varies by task demands, face emotion and patient age.
Method
During functional magnetic resonance imaging (fMRI), 181 participants, including 62 BD (36 children and 26 adults) and 119 healthy comparison (HC) subjects (57 children and 62 adults), engaged in constrained and unconstrained processing of emotional (angry, fearful, happy) and non-emotional (neutral) faces. During constrained processing, subjects answered questions focusing their attention on the face; this was processed either implicitly (nose width rating) or explicitly (hostility; subjective fear ratings). Unconstrained processing consisted of passive viewing.
Results
Pediatric BD rated neutral faces as more hostile than did other groups. In BD patients, family-wise error (FWE)-corrected region of interest (ROI) analyses revealed dysfunction in the amygdala, inferior frontal gyrus (IFG), anterior cingulate cortex (ACC) and putamen. Patients with BD showed amygdala hyperactivation during explicit processing (hostility ratings) of fearful faces and passive viewing of angry and neutral faces but IFG hypoactivation during implicit processing of neutral and happy faces. In the ACC and striatum, the direction of dysfunction varied by task demand: BD demonstrated hyperactivation during unconstrained processing of angry or neutral faces but hypoactivation during constrained processing (implicit or explicit) of angry, neutral or happy faces.
Conclusions
Findings suggest amygdala hyperactivation in BD while processing negatively valenced and neutral faces, regardless of attentional condition, and BD IFG hypoactivation during implicit processing. In the cognitive control circuit involving the ACC and putamen, BD neural dysfunction was sensitive to task demands.
Keywords: Attention, bipolar disorder, face emotion, fronto-limbic-striatal dysfunction, imaging, pediatric
Introduction
Research in bipolar disorder (BD) implicates fronto-limbic-striatal dysfunction, with meta-analyses generally suggesting prefrontal cortex (PFC) hypoactivation and limbic hyper-activation during face emotion processing paradigms (Chen et al. 2011; Delvecchio et al. 2012; Houenou et al. 2011; Kupferschmidt & Zakzanis, 2011; Blond et al. 2012; Strakowski et al. 2012). However, the precise nature of the dysfunction varies; differing task demands and face emotions are used across studies and may contribute to discrepancies in the literature. Moreover, there is limited research on how abnormalities differ developmentally, i.e. in pediatric versus adult patients (Kim et al. 2012). We report data from a relatively large sample [62 BD, 119 healthy comparisons (HC)] in which we compared neural activity across different attention conditions and face emotions, using a paradigm that was brief enough to be tolerated by affected children and adults.
In the current emotional face viewing task, as in other tasks, there are three types of attentional conditions: (1) implicit, (2) passive viewing and (3) explicit. During implicit paradigms, subjects focus on a stimulus feature other than the face emotion. Amygdala hyperactivity has been reported consistently in both pediatric and adult BD patients during such tasks, including gender labeling (Lawrence et al. 2004; Kalmar et al. 2009; Surguladze et al. 2010; Ladouceur et al. 2011; Garrett et al. 2012; Kim et al. 2012; Thomas et al. 2013), face age labeling (Pavuluri et al. 2009) and face image color labeling (Chen et al. 2006; Keener et al. 2012; Perlman et al. 2012). However, the precise direction of dysfunction in the PFC and striatum during implicit face emotion processing has been variable, with some reports indicating hyperactivation in BD (Lawrence et al. 2004; Wessa et al. 2007; Hassel et al. 2008, 2009; Surguladze et al. 2010; Ladouceur et al. 2011; Keener et al. 2012) and others reporting hypoactivation (Lawrence et al. 2004; Hassel et al. 2009; Pavuluri et al. 2009; Garrett et al. 2012).
While some researchers have included passive viewing as a form of implicit face processing, subjects’ attention during passive viewing is completely unconstrained and the cognitive demands are unclear. As in implicit paradigms, amygdala hyperactivation has been reported in BD during passive viewing (Blumberg et al. 2005; Pavuluri et al. 2007; Killgore et al. 2008), although amygdala hypoactivation has also been observed (Blumberg et al. 2005). Similarly, both PFC and striatal hyperactivation (Pavuluri et al. 2007) and hypoactivation (Blumberg et al. 2005; Pavuluri et al. 2007; Killgore et al. 2008) have been reported in passive viewing paradigms.
Explicit face emotion processing paradigms, in which the task directs attention toward the face emotion, have also elicited amygdala hyperactivity in BD (Yurgelun-Todd et al. 2000; Rich et al. 2006; Foland et al. 2008; Almeida et al. 2010; Chen et al. 2010; Versace et al. 2010; Hulvershorn et al. 2012), although some find hypoactivation (Lennox et al. 2004; Chen et al. 2006; Vizueta et al. 2012). These mixed findings in BD are consistent with meta-analyses in healthy adults (Sergerie et al. 2008), suggesting that explicit face emotion processing may probe the amygdala less effectively than do passive viewing paradigms, and may even be associated with decreased amygdala activation (Costafreda et al. 2008). In BD, as with implicit and passive viewing tasks, findings in the PFC and striatum include both hyperactivity (Rich et al. 2006; Foland et al. 2008; Robinson et al. 2008; Chen et al. 2010; Hulvershorn et al. 2012) and hypoactivity (Yurgelun-Todd et al. 2000; Lennox et al. 2004; Altshuler et al. 2008; Foland et al. 2008; Foland-Ross et al. 2012; Hulvershorn et al. 2012). Thus, it is difficult to determine the impact of attentional demands on BD neural dysfunction because conclusions in the literature are based on findings across studies that use different paradigms. Studies have yet to include multiple attention conditions, including constrained (implicit and explicit) and unconstrained (passive viewing) conditions within one task.
In both constrained and unconstrained attentional paradigms, a wide range of face emotions have elicited neural dysfunction in BD. These emotions include happy (Lawrence et al. 2004; Lennox et al. 2004; Blumberg et al. 2005; Chen et al. 2006; Pavuluri et al. 2007; Hassel et al. 2008, 2009; Almeida et al. 2009; Shah et al. 2009; Surguladze et al. 2010; Versace et al. 2010; Passarotti et al. 2011; Garrett et al. 2012; Keener et al. 2012; Mourao-Miranda et al. 2012; Perlman et al. 2012; Thomas et al. 2012), angry (Pavuluri et al. 2007; Altshuler et al. 2008; Passarotti et al. 2011; Keener et al. 2012; Perlman et al. 2012; Thomas et al. 2012, 2013), sad (Lawrence et al. 2004; Lennox et al. 2004; Chen et al. 2006; Jogia et al. 2008; Almeida et al. 2010; Versace et al. 2010; Garrett et al. 2012; Keener et al. 2012; Perlman et al. 2012), neutral (Rich et al. 2006; Altshuler et al. 2008; Hassel et al. 2009; Garrett et al. 2012; Thomas et al. 2012, 2013) and fearful (Yurgelun-Todd et al. 2000; Lawrence et al. 2004; Chen et al. 2006; Altshuler et al. 2008; Hassel et al. 2008; Killgore et al. 2008; Shah et al. 2009; Surguladze et al. 2010; Keener et al. 2012; Perlman et al. 2012; Thomas et al. 2012). However, similar to conclusions drawn about BD dysfunction across different attention conditions, it is difficult to determine the impact of specific face emotions on neural function in BD because most studies use only one face emotion or collapse analyses across face emotions. To date, no study has included multiple face emotions and attention states (including both implicit and explicit conditions, and passive viewing) in one statistical model.
We examined neural dysfunction during face emotion processing across several emotions and attention states in pediatric and adult BD patients. We used a face emotion processing paradigm because behavioral deficits in face emotion labeling have been found in BD patients irrespective of age of onset (Kohler et al. 2011). Face processing deficits are also present during euthymia (Schenkel et al. 2007; Rich et al. 2008), representing a potential endophenotype of the illness (Brotman et al. 2008; Olsavsky et al. 2012). However, it remains unclear whether the neural correlates of such behavioral deficits differ in pediatric and adult BD. Meta-analyses report that decreased amygdala volume is found more consistently in youth than in adults with BD (Pfeifer et al. 2008; Chen et al. 2011), suggesting that functional amygdala abnormalities may be more prominent in BD youth than in adults (Kim et al. 2012).
We used a paradigm that included both implicit (nose width) and explicit (hostility, subjective fear) ratings, in addition to an unconstrained attention condition (passive viewing). The task included multiple face emotions (angry, happy, fearful, neutral). Four regions of interest (ROIs) were selected, including the amygdala, anterior cingulate cortex (ACC), inferior frontal gyrus (IFG) and putamen; these regions have been consistently implicated in BD (Chen et al. 2011; Delvecchio et al. 2012; Houenou et al. 2011). Our primary ROI analyses included, in one statistical model, all attention conditions, face emotion, subject age and diagnosis. We expected between-group diagnostic differences to emerge in all ROIs. We also examined main effects of diagnosis and diagnosis by age group interactions.
Specifically, given the relatively consistent literature on amygdala hyperactivation in BD across face emotions (Chen et al. 2011; Delvecchio et al. 2012; Houenou et al. 2011; Kupferschmidt & Zakzanis, 2011; Blond et al. 2012), we hypothesized that, relative to HC, both adult and pediatric BD patients would demonstrate increased amygdala activity to constrained (implicit, explicit ratings) and unconstrained (passive viewing) attention tasks across all face emotions (angry, fearful, happy, neutral). Consistent with a recent study using a gender identification paradigm (Kim et al. 2012), we expected amygdala dysfunction to be more pronounced in BD youth than BD adults, particularly during the implicit task. Based on meta-analyses (Chen et al. 2011; Delvecchio et al. 2012; Houenou et al. 2011), we also anticipated BD IFG hypoactivation. Finally, we expected that dysfunction in the cognitive control circuit composed of the ACC and striatum would be sensitive to attentional demands.
Method
Subjects
Usable functional magnetic resonance imaging (fMRI) data were obtained from 181 participants, including 36 pediatric BD (9–18 years old), 26 adult BD (24–58 years old), 57 HC children (9–18 years old) and 62 HC adults (20–53 years old). From these 181 subjects, data from 101 have been published previously, including 32 pediatric BD patients (Rich et al. 2006; Brotman et al. 2010; Olsavsky et al. 2012), 56 HC children (Rich et al. 2006; Guyer et al. 2008; Beesdo et al. 2009; Brotman et al. 2010; Olsavsky et al. 2012) and 13 HC adults (Guyer et al. 2008). The adult BD data (n=26) and data from 54 other subjects (four pediatric BD, one HC child, 49 HC adults) have not been published previously.
Pediatric BD patients were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime version (K-SADS-PL; Kaufman et al. 1997). To evaluate mood, clinicians administered the Children’s Depression Rating Scale (CDRS; Poznanski et al. 1984) and the Young Mania Rating Scale (YMRS; Young et al. 1978) to parent and child within 48 h of scanning.
Adult BD (BD-I or BD-II) was assessed using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders – Patient Edition (SCID-I/P; First et al. 2002) or the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al. 1994). The Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD; Williams, 1988) and the YMRS (Young et al. 1978) were used to evaluate mood state in BD adults.
Exclusion criteria included: IQ<70, history of head trauma, neurological disorder, pervasive developmental disorder, unstable medical illness, or substance abuse/dependence in the past 3 months. Medicated patients were included. For details, see online Supplementary Material.
Behavioral paradigm
For task details, see Supplementary Material and Rich et al. 2006; Guyer et al. 2008; Beesdo et al. 2009; Brotman et al. 2010; Olsavsky et al. 2012. In brief, subjects viewed adult faces displaying happy, fearful, angry or neutral expressions. Attention conditions included one implicit (‘How wide is the nose?’), two explicit (‘How hostile is the face?’; ‘How afraid are you?’) and one passive viewing condition.
Behavioral data analysis
We used a 2 (diagnosis: BD, HC)×2 (age group: child, adult)×4 (face emotion: angry, happy, fearful, neutral) × 3 (attention state: fear, hostility, nose width) Greenhouse–Geisser-corrected repeated-measures ANOVA, with face emotion and attention state as within-subject variables. To understand the significant interactions, separate post-hoc 2 (diagnosis) ×3 (attention state) and 2 (diagnosis) ×4 (face emotion) ANOVAs were used. We used t tests to further decompose these post-hoc analyses, and Pearson correlations to assess relationships between mood ratings and behavioral performance.
fMRI data
For scanning acquisition and preprocessing, see Supplementary Material.
We conducted separate left and right ROI analyses with a small volume correction (SVC), using family-wise error (FWE) correction at a statistical threshold of p<0.05. The primary analysis consisted of a four-way ANOVA [2 (diagnosis) ×2 (age group) ×4 (face emotion)× 4 (attention state)]. There were no supra-threshold voxels in any of the ROIs in the four-way ANOVA (see Supplementary Material for a trend).
Given our primary interest in differences between BD and HC, analyses then focused on the significant three-way interactions that included diagnosis. A 2 (diagnosis) ×4 (face emotion)× 4 (attention state) ANOVA three-way interaction yielded the highest number of significant clusters. No other significant three-way interactions (i.e. diagnosis ×age group×attention state and diagnosis×age group×face emotion) in any of the ROIs survived FWE correction. We also examined two-way interactions of diagnosis ×age group and main effects of diagnosis in each ROI.
In post-hoc analyses, age, behavioral ratings and reaction time were included as covariates. We performed a 2 (diagnosis) ×4 (face emotion) ×4 (attention state) repeated-measures ANCOVA, with age as a continuous covariate; a 2 (diagnosis) ×4 (face emotion) ×4 (attention state) repeated-measures ANCOVA, with ratings (hostility ratings of neutral faces) as a covariate; and a 2 (diagnosis) ×4 (face emotion) ×4 (attention state) repeated-measures ANCOVA, with reaction time as a covariate.
To test the effects of potentially confounding variables on our results (see Supplementary Material), post-hoc t tests compared: (1) euthymic (n=36) versus non-euthymic (n=24) patients, (2) patients with (n= 34) versus without a co-morbid diagnosis (n=28), and (3) unmedicated (n=12) versus medicated (n=47) patients. Finally, we used Pearson correlations to examine associations between neural activation and mood, along with neural activation and psychotropic medications.
In addition to ROI analyses, we conducted an exploratory whole-brain analysis, using a statistical threshold of p<0.001, uncorrected, voxel-wise extent threshold of k ≥20 (Lieberman & Cunningham, 2009). We obtained results consistent with the ROI analyses (see Supplementary Material and Table S4).
Results
Demographic and clinical characteristics
For demographic and clinical data, see Supplementary Material and Table 1.
Table 1.
Subject characteristics
| Characteristics | Pediatric BD (n=36) | Adult BD (n=26) | Pediatric HC (n=57) | Adult HC (n =62) |
|---|---|---|---|---|
| Age (years)a | 14.77±2.55 | 41.70±10.30 | 14.30±2.57 | 34.24±9.54 |
| WASI full-scale IQb | 110.06±13.38 | 115.60±11.69 | 112.30±13.33 | 116.09±11.63 |
| Age of onset (years)c | 10.74±3.30 | 21.44±9.18 | – | – |
| YMRSd | 9.39±5.98 | 4.92±5.19 | – | – |
| CDRS | 27.78±8.90 | – | – | – |
| SIGH-SADe | – | 16.79±10.91 | – | – |
| No. of medications | 2.21±1.50 | 2.11±1.55 | 0 | 0 |
| Male | 19/36 (52.8) | 8/26 (30.8) | 27/57 (47.4) | 24/62 (38.7) |
| Bipolar typef | ||||
| BD-I | 31/36 (86.1) | 13/26 (50.0) | – | – |
| BD-II | 5/36 (13.9) | 13/26 (50.0) | – | – |
| Mood stated,e | ||||
| Euthymic | 22/36 (61.1) | 14/24 (58.3) | – | – |
| Depressedg | 3/36 (8.3) | 7/24 (29.2) | – | – |
| Hypo/manich | 9/36 (25.0) | 1/24 (4.2) | – | – |
| Mixed | 2/36 (5.6) | 2/24 (8.3) | – | – |
| Co-morbid conditionsi,j | ||||
| Any co-morbidity | 25/36 (69.4) | 9/15 (60.0) | – | – |
| Any anxiety disorderk | 14/36 (38.9) | 9/26 (34.6) | – | – |
| ADHDj | 15/36 (41.7) | 0/9 (0) | ||
| ODD or CDl | 11/36 (30.6) | 0/26 (0) | – | – |
| Any substance abuse/dependence | 0/36 (0) | 1/26 (3.8) | – | – |
| Medicationm | ||||
| Unmedicated | 9/35 (25.7) | 3/24 (12.5) | 57 (100) | 62 (100) |
| Atypical antipsychotic | 16/35 (45.7) | 6/24 (25.0) | – | – |
| Lithium | 12/35 (34.3) | 6/24 (25.0) | – | – |
| Antiepilepticn | 18/35 (51.4) | 20/24 (83.3) | – | – |
| Antidepressant | 10/35 (28.6) | 10/24 (41.7) | – | – |
| Stimulantso | 8/35 (22.9) | 0 (0) | – | – |
Adult groups were older than children groups (p’s<0.001). Adult BD patients were older than adult HC (p<0.005).
Missing data from one adult BD, one child HC and seven adult HC.
Missing data from one pediatric BD. Pediatric BD patients had an earlier age of onset than adult BD patients (p<0.001).
Missing data from two adult BD patients. Higher YMRS scores in pediatric than in adult BD patients (p<0.005).
Missing data from two adult BD patients.
Pediatric BD were more likely to be type I compared to adult BD (p<0.005).
Adult BD were more likely to be depressed compared to pediatric BD (p<0.05).
Pediatric BD were more likely to be hypomanic compared to adult BD (p<0.05).
Missing co-morbid diagnosis data from one pediatric BD.
Missing co-morbid ADHD diagnostic data from 17 adult BD.
Includes generalized anxiety disorder, separation anxiety disorder, social phobia, panic disorder, post-traumatic stress disorder and obsessive–compulsive disorder.
Higher rates of ODD/CD in pediatric than in adult BD (p<0.005).
Missing medication data from one pediatric and two adult BD patients.
Adult BD patients were more likely to be taking antiepileptic medications compared to pediatric patients (p<0.01).
Pediatric BD patients were more likely to be taking stimulant medications compared to adult patients (p<0.01).
Behavioral analyses
Ratings
A diagnosis ×age group×face emotion ×attention state interaction (F6,1026 =2.30, p<0.05) revealed that pediatric patients rated neutral faces as more hostile than did other groups (p’s <0.01) (see Supplementary Material and Table S1).
Reaction time
A diagnosis ×face emotion interaction (F3,513 =4.41, p< 0.01) revealed that patients responded slowest to fearful faces and HC responded slowest to angry faces (p’s <0.05) (see Supplementary Material and Table S2).
fMRI analyses
Diagnosis ×face emotion ×attention state
This three-way interaction yielded numerous significant FWE-corrected SVCs in the ROIs (Table 2).
Table 2.
Region of interest (ROI) results of the diagnosis×face emotion×attention state analysis
| Area of activation | BA | Side | Cluster size | MNI coordinates x, y, z | F | p FWE corrected | Attention condition, face emotion versus fixation | Between-group differences |
|---|---|---|---|---|---|---|---|---|
| Amygdala | L | 215 | −22, 0, −18 | 3.58 | 0.006 | Explicit (Hostility) | BD>HC* | |
| Fearful | ||||||||
| Passive | BD>HC* | |||||||
| Anger | ||||||||
| Amygdala | R | 147 | 28, 0, −12 | 3.43 | 0.009 | Passive | BD>HC** | |
| Anger | ||||||||
| Passive | BD>HC* | |||||||
| Neutral | ||||||||
| ACC | 32 | R | 972 | 4, 26, 34 | 3.63 | 0.029 | Explicit (Afraid) | HC>BD* |
| Anger | ||||||||
| Implicit (Nose width) | HC>BD*** | |||||||
| Anger | ||||||||
| Implicit (Nose width) | HC>BD* | |||||||
| Happy | ||||||||
| Implicit (Nose width) | HC>BD** | |||||||
| Neutral | ||||||||
| Passive | BD>HC** | |||||||
| Anger | ||||||||
| IFG | 45/47 | R | 159 | 34, 24, −12 | 3.59 | 0.021 | Implicit (Nose width) | HC>BD* |
| Happy | ||||||||
| Implicit (Nose width) | HC>BD* | |||||||
| Neutral | ||||||||
| Putamen | L | 471 | −30, −8, −8 | 3.72 | 0.009 | Explicit (Hostility) | HC>BD** | |
| Happy | ||||||||
| Passive | BD>HC* | |||||||
| Anger | ||||||||
| Passive | BD>HC* | |||||||
| Neutral |
ACC, Anterior cingulate cortex; IFG, inferior frontal gyrus; BA, Brodmann area; BD, bipolar disorder; HC, healthy comparisons; R, right; L, left.
p<0.05,
p<0.01,
p<0.001.
Amygdala
Compared to HC, patients showed left amygdala hyperactivation during explicit (hostility) ratings of fearful faces (t =2.27, p =0.03) and passive viewing of angry faces (t =2.06, p=0.04) (Fig. 1). Patients also showed right amygdala hyperactivation versus HC during passive viewing of angry and neutral faces (t =2.82, p=0.005 and t = 2.34, p=0.02 respectively).
Fig. 1.
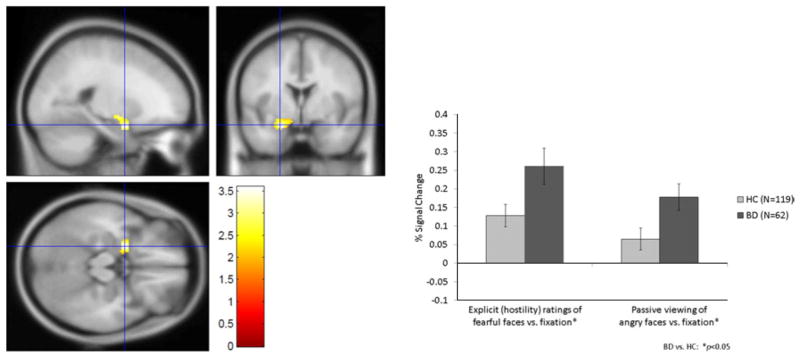
Left amygdala hyperactivity during explicit (hostility) ratings of fearful faces and passive viewing of angry faces in patients with bipolar disorder (BD) and healthy comparisons (HC).
ACC
In response to angry faces during passive viewing, patients demonstrated hyperactivation in the right ACC (t =2.76, p=0.006). However, during explicit (subjective fear) ratings of angry faces (t =2.46, p=0.02) and during implicit (nose width) ratings of angry, happy and neutral faces, patients demonstrated right ACC hypoactivation (t’s= 2.17–3.38, p’s =0.03–0.001) (Fig. 2).
Fig. 2.
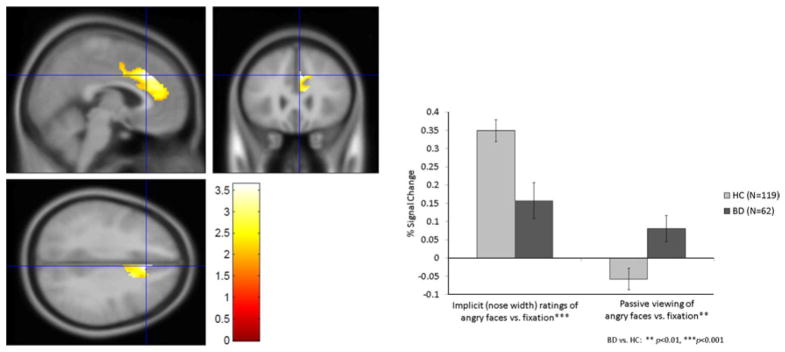
Right anterior cingulate cortex (ACC) activation during angry faces: hypoactivity during implicit (nose width) ratings but hyperactivity during passive viewing in patients with bipolar disorder (BD) and healthy comparisons (HC).
IFG
Patients demonstrated hypoactivation in the right IFG during implicit (nose width) ratings of happy and neutral faces (t =1.96, p=0.05 and t =2.31, p=0.02 respectively) (Fig. 3).
Fig. 3.
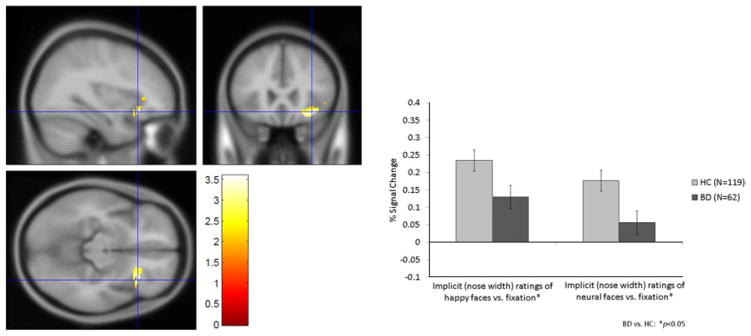
Right inferior frontal gyrus (IFG) hypoactivation during implicit (nose width) ratings of happy and neutral faces in patients with bipolar disorder (BD) and healthy comparisons (HC).
Putamen
Patients showed left putamen hyperactivation during passive viewing of angry and neutral faces (t =2.38, p=0.02 and t =2.19, p=0.03 respectively) and left putamen hypoactivation during explicit (hostility) ratings of happy faces (t =2.71, p= 0.007).
Post-hoc analyses
Covarying age, ratings and reaction time
The three-way interactions remained significant when covarying age, hostility ratings of neutral faces and reaction time (p’s <0.05).
Mood state, co-morbid illnesses and medication
Post-hoc t tests comparing euthymic versus non-euthymic patients, patients with and without a co-morbid diagnosis, and unmedicated versus medicated patients indicated that mood state, co-morbidity and medication were unlikely to be driving the findings in most ROIs (p’s >0.07; see Supplementary Material). There was no association between activation in any ROI and number of medications, YMRS, CDRS or SIGH-SAD scores.
Diagnosis ×age group
This two-way interaction was significant (p< 0.001, uncorrected, k=152; p<0.001, FWE corrected) in the left amygdala (−16, −4, −18) (F1,177 =5.40, p =0.02), with child BD demonstrating greater activation than adult BD (p=0.03).
Main effects of diagnosis
We found main effects of diagnosis in all ROIs (p’s≤0.001, FWE corrected) except the left amygdala (p=0.26, FWE corrected) (see Supplementary Material and Table S3). Compared to HC, BD showed hyper-activation in the right amygdala (t =−2.20, p= 0.03), left putamen (t =−1.94, p=0.06) and right putamen (t =−1.78, p=0.08). Also compared to HC, BD showed hypoactivation in the left (t =2.39, p=0.02) and right (t =2.27, p=0.03) ACC and left (t =2.30, p=0.02) and right (t =2.79, p=0.006) IFG.
Discussion
To our knowledge, this is the first study to compare neural activation in response to multiple face emotions and attentional conditions in adults and children with and without BD. We found that, relative to HC, both pediatric and adult patients demonstrated amygdala hyperactivity during both explicit ratings (hostility of fearful faces) and unconstrained processing (passive viewing of angry and neutral faces). BD hypoactivation in the IFG was present during implicit processing (nose width ratings) of neutral and happy faces. ACC and putamen abnormalities were present in response to angry, happy and neutral faces; however, the direction of dysfunction depended on attentional demands. BD patients showed ACC and putamen hyperactivation to angry and neutral faces when attention was unconstrained but ACC and putamen hypoactivation when attention was constrained, either with an implicit (nose width rating) or explicit (subjective fear or hostility rating) task. Neural dysfunction did not differ between euthymic versus non-euthymic, medicated versus unmedicated BD patients, and those with versus those without co-morbidities. Behaviorally, consistent with work in a partially overlapping sample (Rich et al. 2006; Brotman et al. 2010), pediatric BD patients rated neutral faces as more hostile than did other groups.
In the context of multiple face emotions and attention conditions, patients had pervasive overactivity in the amygdala in response to negatively valenced and neutral faces during both unconstrained attention and explicit face emotion processing. Main effects of diagnosis also showed BD hyperactivity in the right amygdala across the entire task (i.e. all attention conditions and face emotions). The amygdala is crucial to effective emotional appraisal and regulation (Davis & Whalen, 2001), and our results are consistent with BD patients’ dysfunction in these domains (Pavuluri & Passarotti, 2008; Kohler et al. 2011). Our findings add to the existing literature by demonstrating amygdala hyperactivation in both pediatric and adult BD across several attentional conditions and in response to several face emotion types.
Consistent with the literature (Chen et al. 2011; Delvecchio et al. 2012; Kupferschmidt & Zakzanis, 2011; Foland-Ross et al. 2012), we also observed IFG hypoactivation in BD during implicit ratings of happy and neutral faces; main effects of diagnosis revealed general BD hypoactivation during the task. The IFG is also crucial in the integration of emotional information (Cabeza & Nyberg, 2000) and emotion regulation (Quirk & Beer, 2006), and plays a role in effective modulation of the amygdala. Indeed, some studies suggest that IFG dysfunction is unique to BD relative to other mood disorders (Delvecchio et al. 2012).
In contrast to amygdala hyperactivity and IFG hypoactivation, dysfunction in the cognitive control circuit comprising the ACC and putamen was sensitive to task demands. The relevant literature in BD is mixed, demonstrating both frontostriatal hyperactivity (Lawrence et al. 2004; Rich et al. 2006; Pavuluri et al. 2007; Wessa et al. 2007; Foland et al. 2008; Hassel et al. 2008, 2009; Robinson et al. 2008; Chen et al. 2010; Surguladze et al. 2010; Ladouceur et al. 2011; Hulvershorn et al. 2012; Keener et al. 2012) and hypoactivity (Yurgelun-Todd et al. 2000; Lawrence et al. 2004; Lennox et al. 2004; Blumberg et al. 2005; Pavuluri et al. 2007, 2009; Altshuler et al. 2008; Foland et al. 2008; Killgore et al. 2008; Pochon et al. 2008; Hassel et al. 2009; Garrett et al. 2012; Hulvershorn et al. 2012) in BD. Our work may clarify the previous findings by indicating that, compared to HC, unconstrained viewing was associated with hyperactivity in BD whereas directed attention (either explicit or implicit ratings) elicited hypoactivation in these regions.
The ACC and putamen are important components of the emotional processing and cognitive control circuits, with involvement in attention allocation, conflict detection and cognitive interference mediation (Devinsky et al. 1995; Bush et al. 2000). When attention was unconstrained during passive viewing of angry or neutral faces, BD patients demonstrated ACC, amygdala and putamen hyperactivation. It is difficult to interpret activation patterns during passive viewing because we do not know the cognitive processes engaged. However, in the absence of attentional instructions, viewing emotional faces while continuing to comply with the demands of being in the scanning environment may require greater engagement of cognitive control regions in patients than in healthy subjects.
In contrast to hyperactivity during passive viewing of angry and neutral faces, BD patients showed hypoactivation in the ACC and putamen when asked to attend to either nose width or their subjective fear while viewing a face. Of note, the pattern of activation in these areas during constrained attention tasks was similar to that in the IFG. This suggests that, compared to healthy subjects, patients did not engage the cognitive control circuit effectively in the context of these task demands. The nose width rating task is, in essence, a cognitive interference task because subjects are asked to rate nose width while not attending to the face emotion. Healthy subjects recruited the cognitive control circuit during this task whereas BD patients showed relative hypoactivity in the circuit, consistent with prior work demonstrating executive (Fleck et al. 2008) and cognitive control (Strakowski et al. 2005; Drevets et al. 2008) dysfunction in BD. Similarly, when rating their subjective fear of an angry or happy face, BD patients did not engage this circuit. Despite these differences in neural activity, there were no group differences in performance, possibly because imaging measures may be more sensitive to group differences than behavioral measures. Other studies also find decreased PFC activation in BD patients without group differences in performance (Foland et al. 2008; Hassel et al. 2008; Foland-Ross et al. 2012).
We expected, but did not observe, three additional findings in the amygdala: (1) that the implicit task (nose width), like the explicit and passive viewing tasks, would elicit amygdala hyperactivity; (2) that during implicit processing amygdala dysfunction would be more marked in pediatric versus adult BD; and (3) that BD would show amygdala hyperactivity to happy faces.
While multiple reasons, including Type II error, may have contributed to not observing amygdala hyperactivity during implicit processing, one potential explanation is that our implicit task involves nose width rating rather than the more commonly used gender labeling (Lawrence et al. 2004; Kalmar et al. 2009; Surguladze et al. 2010; Ladouceur et al. 2011; Kim et al. 2012). We used nose width, as opposed to gender labeling (a binary option), to parallel the 1–5 ratings in the explicit rating conditions. If subjects had been allowed to process the face globally within the context of an implicit task such as gender labeling, rather than being asked to direct their attention to a specific region of the face (nose), we may have detected amygdala hyperactivity in BD. In fact, recent eye tracking work indicates dysfunctional gaze patterns in BD, whereby patients focus less on the eyes and more on the nose relative to HC subjects (Kim et al., in press). In the nose width condition, subjects were instructed to focus their eye gaze to the nose. This task instruction may have obfuscated between-group differences in amygdala processing during this implicit processing condition. In addition, it has been suggested that tasks that impose fewer attentional demands (e.g. gender labeling, passive viewing) are more likely to elicit amygdala activation (Costafreda et al. 2008); judging nose width requires a relatively high level of effortful attention and thus may be less likely to elicit amygdala activation. Consistent with this, in the current study we found amygdala hyperactivation during passive viewing of angry and neutral faces but not during the implicit task (nose width).
Second, we did not detect differences in amygdala function between pediatric and adult BD specifically during the implicit rating condition. However, across all attention conditions and face emotions, diagnosis by age group interactions showed that left amygdala hyperactivation was more pervasive in pediatric than in adult BD patients. The one prior study comparing pediatric versus adult BD used a gender labeling paradigm; across emotions, there was amygdala hyperactivity in pediatric BD versus adult BD and healthy subjects (Kim et al. 2012). Consistent with those results, our findings suggests that, relative to adult BD, pediatric patients’ amygdala dysfunction may be particularly pervasive and less sensitive to attentional demands and face emotions.
Third, although angry, fearful and neutral faces elicited amygdala hyperactivity, we did not observe amygdala hyperactivation to happy faces. Others have noted amygdala hyperactivation to happy faces in both BD children and adults (Lawrence et al. 2004; Blumberg et al. 2005; Chen et al. 2006; Pavuluri et al. 2007, 2009; Surguladze et al. 2010). Those paradigms included varying intensities of happiness in the faces, with some studies demonstrating amygdala hyperactivity to mild, and others to intense, happiness (Lawrence et al. 2004; Surguladze et al. 2010). Here, we used only one face emotion intensity. Future studies are needed to examine the neural correlates of subtle changes in emotional expressions and corresponding patterns of neural modulation.
These findings should be considered in light of additional limitations. First, our BD patients were in a variety of mood states at the time of testing, which may influence neural activity (Foland-Ross et al. 2012; Liu et al. 2012; Townsend & Altshuler, 2012). However, findings did not differ between euthymic (n=36) and non-euthymic (n=24) BD patients, and there was no relationship between mood rating scores and activation in any ROIs, suggesting that mood state did not account for our results. Second, most BD patients had co-morbid diagnoses (n=34), but they did not differ from BD patients without a co-morbid diagnosis (n=28) in blood oxygen level-dependent (BOLD) activity in most ROIs. Of note, BD patients with comorbidities showed higher amygdala activity during passive viewing of neutral faces compared to BD without co-morbidities, suggesting that co-morbid diagnoses may be associated with more severe amygdala dysfunction. Third, most patients were medicated; although activation did not differ between medicated (n=47) and unmedicated (n=12) patients, these post-hoc analyses were underpowered and thus susceptible to Type II error. Studies indicate that neural dysfunction may normalize with treatment, suggesting that medication may be unlikely to be driving the between-group differences that we observed (Chang et al. 2008; Phillips et al. 2008; Passarotti et al. 2010, 2011; Hafeman et al. 2012; Pavuluri et al. 2012). Although our sample sizes compare favorably to those in the literature, future studies with even larger samples are needed to clarify the role of psychotropic medication, co-morbidity and mood state on the neural correlates of face emotion processing. Finally, although this face viewing paradigm enabled us to examine multiple face emotions and attention states, each is sampled relatively sparsely (eight replicates for each attentional condition). Although this design maintained short task duration and increased tolerance for youth and adults with severe psychopathology, it precluded connectivity analyses. The task is also prone to Type II error, particularly in the context of complicated models with multiple within- and between-subject variables. Thus, the findings we observed are likely to be particularly robust.
In sum, our results demonstrate fronto-limbic-striatal dysfunction in both pediatric and adult BD. In the context of multiple face emotions and attention conditions, we observed amygdala hyperactivation during both explicit (i.e. hostility ratings of fearful faces) and unconstrained processing (i.e. passive viewing of angry and neutral faces), and IFG hypoactivation during implicit ratings of happy and neutral faces. By contrast, ACC and striatal dysfunction were most sensitive to task demands. We observed hypoactivation in BD in these regions when attention was directed during either an implicit or an explicit rating, but hyperactivation when attention was unconstrained. Future imaging studies should continue to examine stimulus valence and attentional demands in the pathophysiology of pediatric and adult BD. Moreover, work is needed to explore the functional connectivity of the frontal-limbic-striatal circuit in the development and maintenance of the illness.
Supplementary Material
Acknowledgments
Funding for this study was provided by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH). We thank the staff of the Emotion and Development and Experimental Therapeutics and Pathophysiology Branches at NIMH and the patients and their parents for their participation.
Footnotes
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S003329171300202X.
Declaration of Interest
Dr Olsavsky’s research was made possible through the Clinical Research Training Program, a public–private partnership supported jointly by the NIH and Pfizer Inc. (through a grant to the Foundation for NIH from Pfizer Inc.). Dr Zarate is listed as a co-inventor on a patent application for the use of ketamine in major depression. Dr Zarate has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government. All other authors have no conflicts to disclose.
References
- Almeida JR, Mechelli A, Hassel S, Versace A, Kupfer DJ, Phillips ML. Abnormally increased effective connectivity between parahippocampal gyrus and ventromedial prefrontal regions during emotion labeling in bipolar disorder. Psychiatry Research. 2009;174:195–201. doi: 10.1016/j.pscychresns.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, Cohen MS. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disorders. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond BN, Fredericks CA, Blumberg HP. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disorders. 2012;14:340–355. doi: 10.1111/j.1399-5618.2012.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berlin) 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, Pine DS, Leibenluft E. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. American Journal of Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chang KD, Wagner C, Garrett A, Howe M, Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disorders. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disorders. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Ooi C, Jacob R, Lupson V, Bullmore ET, Lennox BR. A longitudinal fMRI study of the manic and euthymic states of bipolar disorder. Bipolar Disorders. 2010;12:344–347. doi: 10.1111/j.1399-5618.2010.00801.x. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, Hietala J, Lawrie SM, Martinot JL, McIntosh AM, Meisenzahl E, Frangou S. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. European Neuropsychopharmacology. 2012;22:100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research Department, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fleck DE, Shear PK, Madore M, Strakowski SM. Wisconsin Card Sorting Test performance in bipolar disorder: effects of mood state and early course. Bipolar Disorders. 2008;10:539–545. doi: 10.1111/j.1399-5618.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, Torrisi S, Penfold C, Madsen SK, Thompson PM, Altshuler LL. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. NeuroImage. 2012;59:738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Research. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Reiss AL, Howe ME, Kelley RG, Singh MK, Adleman NE, Karchemskiy A, Chang KD. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:821–831. doi: 10.1016/j.jaac.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disorders. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Frank E, Versace A, Nau SA, Klein CR, Kupfer DJ, Phillips ML. Prefrontal cortical and striatal activity to happy and fear faces in bipolar disorder is associated with comorbid substance abuse and eating disorder. Journal of Affective Disorders. 2009;118:19–27. doi: 10.1016/j.jad.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, Wessa M. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. Journal of Affective Disorders. 2011;132:344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, Karne H, Gunn AD, Hartwick SL, Wang Y, Hummer TA, Anand A. Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biological Psychiatry. 2012;71:603–610. doi: 10.1016/j.biopsych.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogia J, Haldane M, Cobb A, Kumari V, Frangou S. Pilot investigation of the changes in cortical activation during facial affect recognition with lamotrigine monotherapy in bipolar disorder. British Journal of Psychiatry. 2008;192:197–201. doi: 10.1192/bjp.bp.107.037960. [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT, Blumberg HP. Relation between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keener MT, Fournier JC, Mullin BC, Kronhaus D, Perlman SB, LaBarbara E, Almeida JC, Phillips ML. Dissociable patterns of medial prefrontal and amygdala activity to face identity versus emotion in bipolar disorder. Psychological Medicine. 2012;42:1913–1924. doi: 10.1017/S0033291711002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Gruber SA, Yurgelun-Todd DA. Abnormal corticostriatal activity during fear perception in bipolar disorder. Neuroreport. 2008;19:1523–1527. doi: 10.1097/WNR.0b013e328310af58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Arizpe JM, Rosen BH, Razdan V, Haring CT, Jenkins SE, Deveney CM, Brotman MA, Blair RJR, Pine DS, Baker CI, Leibenluft E. Impaired fixation to eyes during face emotion labeling in children with bipolar disorder or severe mood dysregulation. Journal of Psychiatry and Neuroscience. doi: 10.1503/jpn.120232. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Thomas LA, Rosen BH, Moscicki AM, Brotman MA, Zarate CA, Jr, Blair RJR, Pine DS, Leibenluft E. Differing amygdala responses to facial expressions in children vs. adults with bipolar disorder. American Journal of Psychiatry. 2012;169:642–649. doi: 10.1176/appi.ajp.2012.11081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Research. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: quantitative evidence from the neuroimaging literature. Psychiatry Research. 2011;193:71–79. doi: 10.1016/j.pscychresns.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, Birmaher B, Phillips ML. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:1275–1289. e2. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Medicine. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, Blumberg HP. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disorders. 2012;14:432–441. doi: 10.1111/j.1399-5618.2012.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourão-Miranda J, Oliveira L, Ladouceur CD, Marquand A, Brammer M, Birmaher B, Axelson D, Phillips ML. Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents. PLoS One. 2012;7:e29482. doi: 10.1371/journal.pone.0029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:294–303. doi: 10.1016/j.jaac.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berlin) 2011;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti A. Neural bases of emotional processing in pediatric bipolar disorder. Expert Review of Neurotherapeutics. 2008;8:1381–1387. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Fitzgerald JM, Wegbreit E, Sweeney JA. Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: a pharmacological functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:157–170. e5. doi: 10.1016/j.jaac.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Almeida JR, Kronhaus DM, Versace A, Labarbara EJ, Klein CR, Phillips ML. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar Disorders. 2012;14:162–174. doi: 10.1111/j.1399-5618.2012.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1289–1298. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. Journal of Neuroscience. 2008;28:3468–3473. doi: 10.1523/JNEUROSCI.4195-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. Journal of the American Academy of Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, Leibenluft E. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Monkul ES, Tordesillas-Gutiérrez D, Franklin C, Bearden CE, Fox PT, Glahn DC. Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Research. 2008;164:106–113. doi: 10.1016/j.pscychresns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shah MP, Wang F, Kalmar JH, Chepenik LG, Tie K, Pittman B, Jones MM, Constable RT, Gelernter J, Blumberg HP. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. 2009;34:1301–1310. doi: 10.1038/npp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, Delbello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disorders. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. American Journal of Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Marshall N, Schulze K, Hall MH, Walshe M, Bramon E, Phillips ML, Murray RM, McDonald C. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. NeuroImage. 2010;53:58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- Thomas LA, Brotman MA, Muhrer EJ, Rosen BH, Bones BL, Reynolds RC, Deveney CM, Pine DS, Leibenluft E. Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Archives of General Psychiatry. 2012;69:1257–1266. doi: 10.1001/archgenpsychiatry.2012.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, Kim P, Bones BL, Hinton KE, Milch HS, Reynolds RC, Adleman NE, Marsh AA, Blair RJR, Pine DS, Leibenluft E. Elevated amygdala responses to emotional faces in youths with chronic irritability or bipolar disorder. NeuroImage: Clinical. 2013;2:637–645. doi: 10.1016/j.nicl.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disorders. 2012;14:326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, Kupfer DJ, Phillips ML. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biological Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizueta N, Rudie JD, Townsend JD, Torrisi S, Moody TD, Bookheimer SY, Altshuler LL. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. American Journal of Psychiatry. 2012;169:831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Houenou J, Paillère-Martinot ML, Berthoz S, Artiges E, Leboyer M, Martinot JL. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. American Journal of Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


