Abstract
Background
To assess the association of P2RX7 gene rs2230912 polymorphism with mood disorders using a meta-analysis.
Methods
Data were collected from the following electronic databases: PubMed, Excerpta Medica Database, Elsevier Science Direct, Cochrane Library, and Chinese Biomedical Literature Database, with the last report up to April 1, 2013. Odds ratio (OR) with 95% confidence interval (CI) was used to assess the strength of the association. Dependent on the results of heterogeneity test among individual studies, the fixed effect model (Mantel–Haenszel) or random effect model (DerSimonian–Laird) was selected to summarize the pooled OR.
Results
We identified 13 separate studies using search (6,962 cases and 9,262 controls). We detected significant between-study heterogeneity. No significant association of this polymorphism with mood disorders was found (P>0.05). We also performed disease-specific meta-analysis in unipolar depression and bipolar disorder. No significant association of this polymorphism with unipolar depression or bipolar disorder was found (P>0.05). Additionally, we performed subgroup analysis by different types of cases. No significant association of this polymorphism with mood disorders in clinical cohorts or population-based cohorts (P>0.05). A significant association of this polymorphism with mood disorders was found for the allele contrast in family-based cohorts (OR = 1.26, 95%CI = 1.05–1.50, P = 0.01).
Conclusions
Overall, our meta-analysis suggests that P2RX7 gene rs2230912 polymorphism may not contribute to the risk of developing mood disorders using a case-control design. Given the discordance in the subgroup analysis by different types of cases, further studies based on larger sample size are still needed.
Introduction
Mood disorders define a large group of human psychiatric disorders with a high phenotypic complexity but all characterized by consistent, pervasive alterations in mood, which affect thoughts, emotions and behaviours, and they are among the most prominent causes of disability as well as the second leading source of disease burden [1], [2]. Among them, unipolar disorder and bipolar disorder are two main categories with lifetime prevalence rates of 16% and 1%, respectively [3]. The etiology of mood disorders has not yet been fully described but believed to be multifactorial, and genetic factor plays a role in the pathogenesis of these disorders. Heritability of bipolar disorder is estimated at 90%, whereas for unipolar disorder it is around 40% [4], [5].
The P2X7 receptor is an ATP-gated non-selective cation channel activated by high concentrations of ATP (>100 µM), expressed as homo-oligomeric assemblies of individual subunits, and is widely distributed at immunocompetent cells of the central and peripheral nervous system [6]. Evidence indicates that the P2X 7 receptors may affect neuronal cell death through their ability to regulate the processing and release of interleukin-1β, which is a key mediator in chronic inflammation, neurodegeneration and chronic pain [7]. Moreover, P2X 7 receptor-deficient mice have substantially attenuated inflammatory responses [8]. The excessive secretion of proinflammatory cytokines from activated macrophages has been suggested to play a role in the pathogenesis of mood disorders [9]. Recently, the potential role of P2X 7 receptors in neuronal functions have received considerable attention, and many studies indicate that they are involved in the regulation of diverse neural functions [6]. P2X 7 receptors have been proposed to be potential therapeutic target sites in disorders of the nervous system [6].
P2RX7 (purinergic receptor P2X, ligand-gated ion channel, 7) gene located on chromosome 12q24.31 encodes the P2X7 receptor [10]. The P2RX7 gene was selected as a candidate gene in the first genetic studies of mood disorders based on the results of linkage studies and subsequent detailed studies of the 12q22–24 region [11]–[14]. There are extensive single nucleotide polymorphisms (SNPs) in the P2RX7 gene. Of these SNPs, rs2230912 is located in exon 13 of P2RX7 and results in a change of the amino acid glutamine to arginine at 460 position (Gln460Arg) [15]. In the past six years, a number of studies have investigated the association of this polymorphism with mood disorders, but findings are not always consistent [16]–[25]. There are several possible explanations for this discordance, such as small sample size, ethnic background, different types of mood disorders, and publication bias. Meta-analysis is a statistical procedure for combining the results of several studies to produce a single estimate of the major effect with enhanced precision, and it is considered a powerful tool for summarizing inconsistent results from different studies [26]. The aim of the present study is to perform a comprehensive meta-analysis to evaluate the association between P2RX7 gene rs2230912 polymorphism and mood disorders.
Methods
Identification of eligible studies
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Checklist S1) [27]. We searched the following electronic databases: PubMed, Excerpta Medica Database (EMBASE), Elsevier Science Direct, Cochrane Library, and Chinese Biomedical Literature Database (CBM). The search stratrgy was based on the key words “P2RX7”, “P2X7”, “affective”, “depression”, “depressive”, “depressed”, “mood” “unipolar”, “bipolar”, “mania”, “manic”, “gene”, “allele”, “polymorphism”, and “variation”. Additional studies were identified by a hand search of references of original studies and review articles on the association between P2RX7 gene polymorphisms and mood disorders. No language restrictions were applied. Abstracts, case reports and editorials were excluded. A study was included in the current meta-analysis if the publications met all of the following criteria: (1) it was published up to April 1, 2013; (2) it was a case-control study of P2RX7 gene rs2230912 polymorphism and mood disorders. When there were multiple publications from the same population, only the largest study was included. When a study reported the results on different subpopulations, we treated them separately. Two reviewers independently searched the electronic databases.
Data extraction
Two reviewers independently extracted the data with the standard protocol, and the result was reviewed by a third reviewer. Discrepancies were resolved by discussion with our research team. From each study, we extracted the first author, year of publication, journal, ethnicity, types of mood disorders, numbers of cases and controls, and the available genotype and allele frequencies of P2RX7 gene rs2230912 polymorphism. If original data were unavailable in relevant articles, a request for additional data were sent to the corresponding author.
Statistical analyses
We assessed the relationship between the allele, as well as genotypes and susceptibility to mood disorders. The odds ratio (OR) and its 95% confidence interval (95%CI) were estimated for each study. The heterogeneity between the study results was assessed by the Chi square-test based Q-statistic [28]. A significant Q-statistic (P<0.10) indicated heterogeneity across studies. The degree of heterogeneity was further assessed with the I2 statistics (I2 = 100%×(Q-df)/Q) [29]. Meta-regression was performed to detect the source of heterogeneity. Dependent on the results of heterogeneity test among individual studies, the fixed effect model (Mantel–Haenszel) or random effect model (DerSimonian–Laird) was selected to summarize the pooled OR [30], [31]. Publication bias was evaluated by visual inspection of the funnel plot. Funnel plot asymmetry was further evaluated by the method of Egger's linear regression test [32]. Additionally, Chi square-test was used to determine if observed frequencies of genotypes conformed to Hardy-Weinberg equilibrium (HWE) expectations. Analyses were performed using the software Review Manager (v4.2; Oxford, England) and Stata statistical software (v10.0; StataCorp, College Station, TX, USA). P<0.05 (two-tailed) was considered statistically significant.
Results
Study characteristics
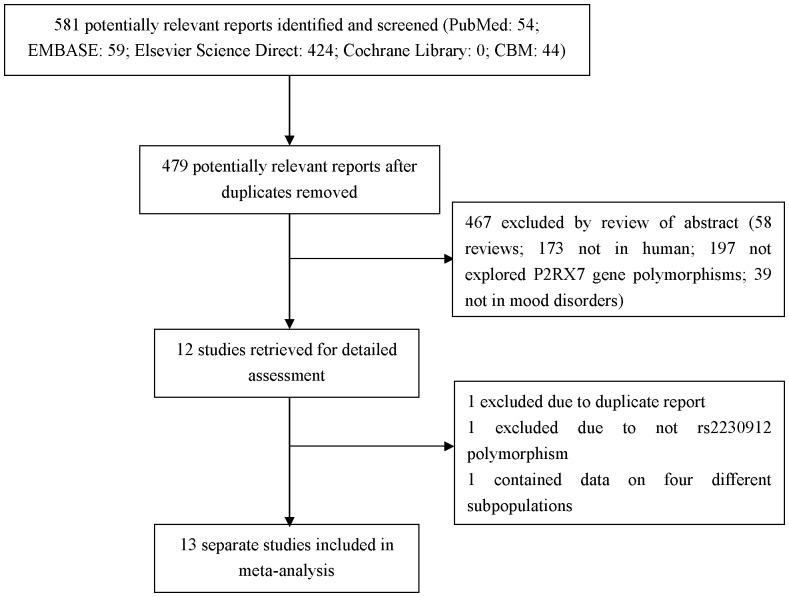
The characteristics of studies included in the current meta-analysis are presented in Table 1. The study selection process is shown in Figure 1. There were 581 studies relevant to the searching terms (Pubmed: 54; Embase: 59; Elsevier Science Direct: 424; Cochrane Library: 0; CBM: 44). Totally, 12 studies examined the association between P2RX7 gene polymorphisms and mood disorders [16]–[25], [33], [34]. Of these, 2 studies were excluded (1 was excluded due to duplicate report; 1 was excluded due to not rs2230912 polymorphism) [33], [34]. All of the eligible studies were conducted in Caucasian population. The results of HWE test for the distribution of the genotype in control population are shown in Table 1. The genotype distribution in one study was not in agreement with HWE [24]. It was unavailable for another study to perform HWE test [16]. Of 10 eligible studies, one contained data on four different subpopulations, and we treated them separately [19]. Thus, a total of 13 separate studies were included in the meta-analysis [16]–[25].
Table 1. Characteristics of studies investigating the association of P2RX7 gene rs2230912 polymorphism with mood disorders.* .
| ID | Study (first author, year, reference) | Ethnicity | Types of mood disorders | Types of case | Sample size | Frequencies of genotypes | HWE (P-value) | Results | |
| Case | Control | ||||||||
| 1 | Soronen 2011 [16] | Caucasian | UD/BD | Family cohorts | 450 | 1322 | NA | NA | S |
| 2 | Viikki 2011 [17] | Caucasian | UD | Clinical cohorts | 218 | 391 | available | >0.05 | NS |
| 3 | Lavebratt 2010 [18] | Caucasian | UD/dysthymia/mixed anxiety depression | Population cohorts | 435 | 2215 | available | >0.05 | NS |
| 4 | Grigoroiu-Serbanescu 2009 [19] | Caucasian(German/Romanian/Polish/Russian) | UD/BD | Clinical cohorts | 2085 | 2006 | available | >0.05 | NS |
| 5 | Green 2009 [20] | Caucasian | UD/BD | Population cohorts | 1710 | 1179 | NA | >0.05 | NS |
| 6 | Hejjas 2009 [21] | Caucasian | UD/BD | Clinical cohorts | 171 | 178 | available | >0.05 | NS |
| 7 | McQuillin 2009 [22] | Caucasian | BD | Population cohorts | 587 | 546 | available | >0.05 | S |
| 8 | Yosifova 2009 [23] | Caucasian | BD | Clinical cohorts | 94 | 184 | NA | >0.05 | NS |
| 9 | Lucae 2006 [24] | Caucasian | UD | Clinical cohorts | 999 | 1029 | available | <0.05 | S |
| 10 | Barden 2006 [25] | Caucasian | BD | Family cohorts | 213 | 212 | available | >0.05 | S |
*HWE: Hardy–Weinberg equilibrium; UD: unipolar depression; BD: bipolar disorder; NA: not available; S: significant; NS: not significant.
Figure 1. Flow diagram of the study selection process.
Meta-analysis results
Table 2 summarizes the main results of this meta-analysis and the heterogeneity test. We detected significant between-study heterogeneity in the contrasts of G versus A, AG+GG versus AA and AG versus AA. No significant association of P2RX7 gene rs2230912 polymorphism with mood disorders was found (P>0.05). A sensitivity analysis was performed in those studies fulfilling HWE. The result showed that there was still no significant association of P2RX7 gene rs2230912 polymorphism with mood disorders (data not shown).
Table 2. Meta-analysis of P2RX7 gene rs2230912 polymorphism and mood diorders association.* .
| Comparsions | Sample size | No. of Studies | Test of association | Test of heterogeneity | ||||||||
| Case | Control | OR (95%CI) | Z | P-value | Model | χ2 | P-value | I2(%) | ||||
| Overall | G vs A | 13924 | 18524 | 13 | 1.05(0.96–1.15) | 1.04 | 0.30 | R | 22.49 | 0.03 | 46.6 | |
| AG+GG vs AA | 4708 | 6577 | 10 | 1.08(0.94–1.24) | 1.09 | 0.28 | R | 19.69 | 0.02 | 54.3 | ||
| GG vs AA+AG | 4708 | 6577 | 10 | 0.87(0.68–1.11) | 1.12 | 0.26 | F | 7.32 | 0.60 | 0.0 | ||
| GG vs AA | 3367 | 4858 | 10 | 0.90(0.70–1.15) | 0.85 | 0.39 | F | 7.52 | 0.58 | 0.0 | ||
| AG vs AA | 4589 | 6397 | 10 | 1.10(0.95–1.27) | 1.27 | 0.20 | R | 20.55 | 0.01 | 56.2 | ||
| UD | G vs A | 6540 | 10388 | 6 | 1.01(0.87–1.18) | 0.17 | 0.86 | R | 13.21 | 0.02 | 62.2 | |
| AG+GG vs AA | 1964 | 2693 | 4 | 0.98(0.76–1.27) | 0.14 | 0.89 | R | 9.42 | 0.02 | 68.1 | ||
| GG vs AA+AG | 1964 | 2693 | 4 | 0.71(0.49–1.04) | 1.77 | 0.08 | F | 0.26 | 0.97 | 0.0 | ||
| GG vs AA | 1431 | 2023 | 4 | 0.73(0.50–1.07) | 1.62 | 0.11 | F | 0.17 | 0.98 | 0.0 | ||
| AG vs AA | 1921 | 2540 | 4 | 0.92(0.63–1.33) | 0.44 | 0.66 | R | 17.97 | 0.0004 | 83.3 | ||
| BD | G vs A | 6514 | 11254 | 10 | 1.08(0.99–1.17) | 1.74 | 0.08 | F | 13.20 | 0.15 | 31.8 | |
| AG+GG vs AA | 2309 | 2942 | 7 | 1.09(0.91–1.30) | 0.92 | 0.36 | R | 11.48 | 0.07 | 47.7 | ||
| GG vs AA+AG | 2309 | 2942 | 7 | 0.99(0.71–1.38) | 0.05 | 0.96 | F | 5.47 | 0.48 | 0.0 | ||
| GG vs AA | 1629 | 2149 | 7 | 1.02(0.73–1.42) | 0.12 | 0.90 | F | 5.38 | 0.44 | 0.0 | ||
| AG vs AA | 2242 | 2857 | 7 | 1.10(0.92–1.32) | 1.02 | 0.31 | R | 11.27 | 0.08 | 46.8 | ||
| Clinical cohorts | G vs A | 7134 | 7576 | 8 | 0.96(0.84–1.11) | 0.51 | 0.61 | R | 14.53 | 0.04 | 51.8 | |
| AG+GG vs AA | 3473 | 3604 | 7 | 1.00(0.83–1.20) | 0.03 | 0.97 | R | 16.65 | 0.01 | 64.0 | ||
| GG vs AA+AG | 3473 | 3604 | 7 | 0.78(0.58–1.04) | 1.72 | 0.08 | F | 2.72 | 0.84 | 0.0 | ||
| GG vs AA | 2510 | 2674 | 7 | 0.79(0.59–1.06) | 1.57 | 0.12 | F | 2.71 | 0.84 | 0.0 | ||
| AG vs AA | 3389 | 3492 | 7 | 1.02(0.83–1.25) | 0.17 | 0.86 | R | 18.16 | 0.006 | 67.0 | ||
| Population cohorts | G vs A | 5464 | 7880 | 3 | 1.07(0.97–1.18) | 1.31 | 0.19 | F | 3.24 | 0.20 | 38.2 | |
| Family cohorts | G vs A | 1326 | 3068 | 2 | 1.26(1.05–1.50) | 2.55 | 0.01 | F | 0.01 | 0.99 | 0.0 | |
*UD: unipolar depression; BD: bipolar disorder; vs: versus; R: random effect model; F: fixed effect model.
We also performed disease-specific meta-analysis in unipolar depression (six studies, 3,270 cases and 5,194 controls) and bipolar disorder (ten studies, 3,257 cases and 5,627 controls). In the analysis of unipolar depression, significant between-study heterogeneity was found in the contrasts of G versus A, AG+GG versus AA and AG versus AA. We did not detect significant association of P2RX7 gene rs2230912 polymorphism with unipolar depression (P>0.05). In the analysis of bipolar disorder, significant between-study heterogeneity was found in the contrasts of AG+GG versus AA and AG versus AA. Similarly, no significant association of P2RX7 gene rs2230912 polymorphism with bipolar disorder was found (P>0.05).
Additionally, we performed subgroup analysis by different types of cases (clinical cohorts: eight studies including 3,567 cases and 3,788 controls; population-based cohorts: three studies including 2,732 cases and 3,940 controls; family-based cohorts: two studies including 663 cases and 1,534 controls). Significant between-study heterogeneity was found for the contrasts of G versus A, AG+GG versus AA and AG versus AA in clinical cohorts, but not for other contrasts. No significant association of this polymorphism with mood disorders in clinical cohorts or population-based cohorts (P>0.05). A significant association of this polymorphism with mood disorders was found for the allele contrast in family-based cohorts (OR = 1.26, 95%CI = 1.05–1.50, P = 0.01).
Evaluation of publication bias and heterogeneity
Funnel plot and Egger's linear regression test were performed to estimate the publication bias of literatures. The shapes of the funnel plots did not reveal any evidence of obvious asymmetry (funnel plots not shown). The results of Egger's linear regression test are shown in Table 3. The intercept a provides a measure of asymmetry, and the larger its deviation from zero the more pronounced the asymmetry. It was shown that there was no publication bias for most of comparisons. However, Egger's linear regression test was not applied for some comparisons due to the small number of studies. As shown in Table 2, significant heterogeneity was found, thus we performed meta-regression analysis for predefined underlying sources of heterogeneity, including year of publication, region, types of mood disorders, types of cases and sample size. Meta-regression indicated that types of cases (P = 0.051) may contribute to heterogeneity.
Table 3. Egger's linear regression test to measure the funnel plot asymmetric.* .
| Comparsions | Y axis intercept: a (95%CI) | ||||
| G vs A | AG+GG vs AA | GG vs AA+AG | GG vs AA | AG vs AA | |
| Overall | −1.33(−3.63–0.96) | −1.91(−5.08–1.25) | 0.02(−2.48–2.54) | −0.20(−2.74–2.32) | −1.89(−5.12–1.32) |
| UD | −1.37(−7.46–4.72) | −3.58(−10.99–3.83) | 0.23(−2.01–2.48) | −0.21(−2.04–1.61) | −3.67(−11.91–4.56) |
| BD | −1.79(−4.16–0.56) | −1.74(−5.98–2.50) | −1.46(−5.22–2.29) | −1.66(−5.44–2.11) | −1.45(−5.71–2.79) |
| Clinical cohorts | −2.48(−5.06–0.08) | −2.72(−6.58–1.14) | 0.26(−2.81–3.35) | −0.33(−2.61–1.93) | −2.77(−6.82–1.28) |
| Population cohorts | 4.92(−12.73–22.58) | – | – | – | – |
*All P>0.05; UD: unipolar depression; BD: bipolar disorder; vs:versus.
Discussion
There is compelling evidence from family, twin and adoption studies for the existence of genes influencing susceptibility to mood disorders [4], [5]. The enormous public health importance of mood disorders has stimulated much work aimed at identifying susceptibility genes. Association studies in mood disorders over recent years have focussed attention on several candidate genes [35]. However, findings are not always consistent. Large samples of subjects are necessary to obtain suffcient power of detection [36]. Small sample sized association studies lack statistical power and may result in contradicting findings [37]. Meta-analysis has become important in psychiatric disorders because of rapid increases in the number and size of datasets. In the meta-analysis, we retrieved 10 studies that included data from 6,962 cases and 9,262 controls to evaluate the association between P2RX7 gene rs2230912 polymorphism and mood disorders. Overall, we did not find significant association of this polymorphism with mood disorders. To our knowledge, the present meta-analysis is the first to assess the association between P2RX7 gene rs2230912 polymorphism and mood disorders.
In the central and peripheral nervous system, the P2X7 receptors are expressed on microglial cells, neurons and astrocytes. They take part in inflammatory responses and the cross-talk between glia and neurons, and are proposed to promote neurotransmitter release at presynaptic sites [7]. The P2X7 receptors may have a role to play in susceptibility to mood disorders. Genetic linkage studies have previously identified many single non-synonymous nucleotide polymorphisms in the human P2RX7 gene in individuals with mood disorders. The non-synonymous SNP rs2230912 is located in the C-terminal domain of the P2X7 ion channel, suggesting a role in receptor function, and protein-protein or protein-lipid interaction [38]. However, there is some controversy concerning the functionality of rs2230912, and, recently, a well-conducted study showed that this polymorphism did not give rise to profound effects on the P2X7 receptors function [39]. In the present study, we found no support for the specific hypothesis that P2RX7 gene rs2230912 polymorphism influences susceptibility to mood disorders in overall analysis and disease-specific analysis. Cases from clinical cohorts, population-based cohorts and family-based cohorts may have different clinical pictures of mood disorders. In the subgroup analysis by different types of cases, no significant association of this polymorphism with mood disorders was found in clinical cohorts or population-based cohorts. A significant association of this polymorphism with mood disorders was found for the allele contrast in family-based cohorts. However, in family-based cohorts, only two studies were included the meta-analysis, and further studies based on larger sample size are still needed to verify the finding. Additionally, molecular biological studies are required to identify the functional importance of this polymorphism on receptor function, and neurobiological investigations are needed to shed light on the connection between P2X7 receptor functioning and mood disorders.
Several specific details merit consideration in the current meta-analysis. A first consideration is that only published studies were included in this meta-analysis, and Egger's linear regression test was not applied for some comparisons due to the small number of studies. Thus, publication bias may occur. A second consideration is that significant between-study heterogeneity was detected in some comparisons, and may be distorting the results of the meta-analysis. Further studies based on refined phenotypes may help to elucidate the association of P2RX7 gene rs2230912 polymorphism with mood disorders. A third consideration is that our reulsts are based on unadjusted estimates, and a more precise analysis stratified by age and sex could be performed if individual data were available. Finally, all of the eligible studies were conducted in Cauasian population. Further studies are still needed in non-Cauasian population.
Overall, our meta-analysis suggests that P2RX7 gene rs2230912 polymorphism may not contribute to the risk of developing mood disorders using a case-control design. Given the discordance in the subgroup analysis by different types of cases, further studies based on larger sample size are still needed.
Supporting Information
PRISMA checklist.
(DOC)
Funding Statement
This work was supported by grant from the Youth Scientific Research Foundation of Shanxi Provincial Health Department (2012E9). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lee S, Jeong J, Kwak Y, Park SK (2010) Depression research: where are we now? Mol Brain 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Lopez AD (1996) Evidence-based health policy-lessons from the Global Burden of Disease Study. Science 274: 740–743. [DOI] [PubMed] [Google Scholar]
- 3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, et al. (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan PF, Neale MC, Kendler KS (2000) Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 157: 1552–1562. [DOI] [PubMed] [Google Scholar]
- 5. Kieseppä T, Partonen T, Haukka J, Kaprio J, Lönnqvist J (2004) High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry 161: 1814–1821. [DOI] [PubMed] [Google Scholar]
- 6. Sperlágh B, Vizi ES, Wirkner K, Illes P (2006) P2X7 receptors in the nervous system. Prog Neurobiol 78: 327–346. [DOI] [PubMed] [Google Scholar]
- 7. Skaper SD, Debetto P, Giusti P (2010) The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24: 337–345. [DOI] [PubMed] [Google Scholar]
- 8. Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, et al. (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114: 386–396. [DOI] [PubMed] [Google Scholar]
- 9. Leonard BE (2001) The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 25: 767–780. [DOI] [PubMed] [Google Scholar]
- 10. Rassendren F, Buell GN, Virginio C, Collo G, North RA, et al. (1997) The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem 272: 5482–5486. [DOI] [PubMed] [Google Scholar]
- 11. Morissette J, Villeneuve A, Bordeleau L, Rochette D, Laberge C, et al. (1999) Genome-wide search for linkage of bipolar affective disorders in a very large pedigree derived from a homogeneous population in quebec points to a locus of major effect on chromosome 12q23–q24. Am J Med Genet 88: 567–587. [DOI] [PubMed] [Google Scholar]
- 12. Curtis D, Kalsi G, Brynjolfsson J, McInnis M, O′Neill J, et al. (2003) Genome scan of pedigrees multiply affected with bipolar disorder provides further support for the presence of a susceptibility locus on chromosome 12q23–q24, and suggests the presence of additional loci on 1p and 1q. Psychiatr Genet 13: 77–84. [DOI] [PubMed] [Google Scholar]
- 13. Abkevich V, Camp NJ, Hensel CH, Neff CD, Russell DL, et al. (2003) Predisposition locus for major depression at chromosome 12q22–12q23.2. Am J Hum Genet 73: 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGuffin P, Knight J, Breen G, Brewster S, Boyd PR, et al. (2005) Whole genome linkage scan of recurrent depressive disorder from the depression network study. Hum Mol Genet 14: 3337–3345. [DOI] [PubMed] [Google Scholar]
- 15. Denlinger LC, Coursin DB, Schell K, Angelini G, Green DN, et al. (2006) Human P2X7 pore function predicts allele linkage disequilibrium. Clin Chem 52: 995–1004. [DOI] [PubMed] [Google Scholar]
- 16. Soronen P, Mantere O, Melartin T, Suominen K, Vuorilehto M, et al. (2011) P2RX7 gene is associated consistently with mood disorders and predicts clinical outcome in three clinical cohorts. Am J Med Genet B Neuropsychiatr Genet 156B: 435–447. [DOI] [PubMed] [Google Scholar]
- 17. Viikki M, Kampman O, Anttila S, Illi A, Setälä-Soikkeli E, et al. (2011) P2RX7 polymorphisms Gln460Arg and His155Tyr are not associated with major depressive disorder or remission after SSRI or ECT. Neurosci Lett 439: 127–130. [DOI] [PubMed] [Google Scholar]
- 18. Lavebratt C, Aberg E, Sjöholm LK, Forsell Y (2010) Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J Affect Disord 125: 249–255. [DOI] [PubMed] [Google Scholar]
- 19. Grigoroiu-Serbanescu M, Herms S, Mühleisen TW, Georgi A, Diaconu CC, et al. (2009) Variation in P2RX7 candidate gene (rs2230912) is not associated with bipolar I disorder and unipolar major depression in four European samples. Am J Med Genet B Neuropsychiatr Genet 150B: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 20. Green EK, Grozeva D, Raybould R, Elvidge G, Macgregor S, et al. (2009) P2RX7: A bipolar and unipolar disorder candidate susceptibility gene? Am J Med Genet B Neuropsychiatr Genet 150B: 1063–1069. [DOI] [PubMed] [Google Scholar]
- 21. Hejjas K, Szekely A, Domotor E, Halmai Z, Balogh G, et al. (2009) Association between depression and the Gln460Arg polymorphism of P2RX7 gene: a dimensional approach. Am J Med Genet B Neuropsychiatr Genet 150B: 295–299. [DOI] [PubMed] [Google Scholar]
- 22. McQuillin A, Bass NJ, Choudhury K, Puri V, Kosmin M, et al. (2009) Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol Psychiatry 14: 614–620. [DOI] [PubMed] [Google Scholar]
- 23. Yosifova A, Mushiroda T, Stoianov D, Vazharova R, Dimova I, et al. (2009) Case-control association study of 65 candidate genes revealed a possible association of a SNP of HTR5A to be a factor susceptible to bipolar disease in Bulgarian population. J Affect Disord 117: 87–97. [DOI] [PubMed] [Google Scholar]
- 24. Lucae S, Salyakina D, Barden N, Harvey M, Gagné B, et al. (2006) P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet 15: 2438–2445. [DOI] [PubMed] [Google Scholar]
- 25. Barden N, Harvey M, Gagné B, Shink E, Tremblay M, et al. (2006) Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet 141B: 374–382. [DOI] [PubMed] [Google Scholar]
- 26. Munafo MR, Flint J (2004) Meta-analysis of genetic association studies. Trends Genet 20: 439–444. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10: 101–129. [Google Scholar]
- 29. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 30. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Backlund L, Lavebratt C, Frisén L, Nikamo P, Hukic Sudic D, et al. (2012) P2RX7: expression responds to sleep deprivation and associates with rapid cycling in bipolar disorder type 1. PLoS One 7: e43057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman OA, Sasvari-Szekely M, Szekely A, Faludi G, Guttman A, et al. (2010) Analysis of a polymorphic microRNA target site in the purinergic receptor P2RX7 gene. Electrophoresis 31: 1790–1795. [DOI] [PubMed] [Google Scholar]
- 35. Craddock N, Forty L (2006) Genetics of affective (mood) disorders. Eur J Hum Genet 14: 660–668. [DOI] [PubMed] [Google Scholar]
- 36. Risch N, Merikangas K (1996) The future of genetic studies of complex human diseases. Science 273: 1516–1517. [DOI] [PubMed] [Google Scholar]
- 37. Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN (2003) Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 33: 177–182. [DOI] [PubMed] [Google Scholar]
- 38. Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, et al. (2001) Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol 167: 1871–1876. [DOI] [PubMed] [Google Scholar]
- 39. Roger S, Mei ZZ, Baldwin JM, Dong L, Bradley H, et al. (2010) Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J Psychiatr Res 44: 347–355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)