Abstract
People with sensorineural hearing loss often have substantial difficulty understanding speech under challenging listening conditions. Behavioral studies suggest that reduced sensitivity to the temporal structure of sound may be responsible, but underlying neurophysiological pathologies are incompletely understood. Here, we investigate the effects of noise-induced hearing loss on coding of envelope (ENV) structure in the central auditory system of anesthetized chinchillas. ENV coding was evaluated noninvasively using auditory evoked potentials recorded from the scalp surface in response to sinusoidally amplitude modulated tones with carrier frequencies of 1, 2, 4, and 8 kHz and a modulation frequency of 140 Hz. Stimuli were presented in quiet and in three levels of white background noise. The latency of scalp-recorded ENV responses was consistent with generation in the auditory midbrain. Hearing loss amplified neural coding of ENV at carrier frequencies of 2 kHz and above. This result may reflect enhanced ENV coding from the periphery and/or an increase in the gain of central auditory neurons. In contrast to expectations, hearing loss was not associated with a stronger adverse effect of increasing masker intensity on ENV coding. The exaggerated neural representation of ENV information shown here at the level of the auditory midbrain helps to explain previous findings of enhanced sensitivity to amplitude modulation in people with hearing loss under some conditions. Furthermore, amplified ENV coding may potentially contribute to speech perception problems in people with cochlear hearing loss by acting as a distraction from more salient acoustic cues, particularly in fluctuating backgrounds.
Keywords: Amplitude modulation, auditory evoked potential (AEP), background noise, chinchilla, envelope coding, sensorineural hearing loss
1. Introduction
People with sensorineural hearing loss (SNHL) often have substantial difficulty understanding speech in their daily lives, even with amplification from a hearing aid. The severity of the problem typically depends on the listening environment, with many individuals reporting great difficulty understanding speech in noisy or reverberant environments with more than one source of sound.
Degraded speech perception in people with SNHL may be caused by diminished sensitivity to the temporal structure of sound. Acoustic signals contain two types of temporal information: rapidly varying temporal fine structure (TFS) and slower changes in the overall amplitude envelope (ENV). Recent studies have shown that with SNHL, the ability to use envelope cues remains intact while the ability to use temporal fine structure information is drastically reduced (Lorenzi et al., 2006; Moore et al., 2006). Moreover, listeners with high frequency hearing loss and normal-hearing thresholds at low frequencies still appear to experience deficits in TFS sensitivity (Lorenzi et al., 2009). While information from the ENV alone may be enough to discern speech in quiet conditions, the inability to use TFS information in people with SNHL could underlie their degraded ability to perceive speech in fluctuating background noise (Hopkins et al., 2008; Zeng et al., 2005).
The physiological contributors to this phenomenon have not yet been elucidated. One theory is that in cases of SNHL, neurons in the peripheral auditory system lose their ability to phase lock, or to discharge synchronously with the stimulus TFS. However, studies of phase locking to pure tones in animals with SNHL have provided conflicting results. One study found that the loss of outer hair cells, a major component of SNHL, does not affect the ability of auditory nerve fibers to encode pure tone signals in guinea pigs (Harrison and Evans, 1979). Similarly, noise induced hearing loss in cats does not affect phase locking to tones (Miller et al., 1997). However, another study in chinchillas showed that outer hair cell loss decreases both the strength of phase locking and the range of frequencies over which phase locking occurs (Woolf et al., 1981). More recent attempts to clarify these conflicting results suggest that individual auditory nerve fibers do not lose the ability to encode TFS information of narrowband sounds, at least in quiet conditions (Kale and Heinz, 2010; Henry and Heinz, 2012). A second theory, on which we focus here, is that amplified coding of ENV information in the cochlea with SNHL leads to a “relative” deficit in TFS coding. Enhanced ENV coding, which is expected with reduced compression of the basilar membrane input-output function (Sellick et al., 1982; Ruggero and Rich, 1991; Glasberg and Moore, 1992; Moore et al. 1996), has been observed in auditory-nerve fiber responses to amplitude modulated tones in chinchillas with SNHL (Kale and Heinz, 2010, 2012).
Previous studies of temporal coding in animals with SNHL are limited in several regards. Most studies were conducted in quiet settings while deficits in speech perception in people with SNHL are most prominent in noisy environments. A recent study of chinchillas illustrated the importance of this difference by demonstrating that phase locking in auditory nerve fibers to tones degrades more rapidly with the addition of background noise in animals with noise-induced SNHL than in normal-hearing control animals (Henry and Heinz, 2012). Second, most studies have examined temporal coding of auditory-nerve fibers at the level of the peripheral auditory system. It has recently been proposed that the degradation of speech perception may be due to deficits in more central processing (Moore, 2008), which suggests a need for studies of the central auditory processing system.
Auditory evoked potentials (AEPs) recorded from the scalp surface can be used to non-invasively study central processing of acoustic stimuli. AEPs reflect the summed neural response generated by populations of neurons along the auditory pathway. AEP waveforms can exhibit phase locked components to both the ENV and low frequency TFS of sustained acoustic stimuli (Krishnan, 2006). Using latency calculations, previous studies suggest that AEPs originate primarily from the auditory brainstem and midbrain (Galbraith, 1994; Galbraith et al., 2000; Glaser et al., 1976; Smith et al., 1975). Furthermore, AEPs are absent in human subjects with upper brain stem lesions (Sohmer et al., 1977) and in cats when the inferior colliculus is cooled (Smith et al., 1975). The non-invasive nature of AEPs allows for assessment of central auditory processing in humans and for comparisons of auditory processing before and after induction of hearing loss in the same animal subject.
In the present study, we used AEPs evoked by sinusoidally amplitude-modulated (SAM) tones to examine the effects of noise-induced SNHL on neural coding of the ENV structure in the central auditory system of chinchillas. Previous studies of amplitude modulation evoked AEPs in another rodent, the Mongolian gerbil, show that ENV responses to modulation frequencies from 50-200 Hz have a group delay of approximately 6 ms, consistent with generation in the auditory midbrain or brainstem (Dolphin and Mountain, 1992). In a more direct study in cats, lesions were used to demonstrate that the inferior colliculus is the major contributor to responses to amplitude-modulation frequencies from 20-200 Hz (Kiren et al., 1994). ENV responses recorded in the current study of chinchillas were consistent with generation in the auditory midbrain. Responses to TFS were also typically observed but not examined further because they had short latency consistent with generation by outer hair cells of the cochlea (i.e. the cochlear microphonic; Chimento and Schreiner, 1990) rather than neurons of the central auditory pathway. Responses to SAM tones were recorded under both quiet and noisy conditions to determine if changes in processing of ENV information with SNHL depend on the listening environment. We predicted that noise-induced SNHL would (1) amplify coding of ENV information in the central auditory system, at least under quiet conditions, and (2) lead to a stronger adverse effect of increasing masker intensity on coding of ENV information.
2. Methods
AEP responses to SAM tones were recorded from a total of 14 chinchillas weighing between 0.4 and 0.6 kg. In 7 of the animals, AEPs were recorded both before the induction of hearing loss and after its stabilization (i.e. 27-40 days after noise exposure). In the remaining animals, recordings were made either before (N=4) or after (N=3) induction of hearing loss. All procedures were conducted with approval from the Purdue Animal Care and Use Committee.
Noise Exposure
Animals were anesthetized using xylazine (1-1.5 mg/kg subcutaneous) followed by ketamine (50-60 mg/kg intraperitoneal). Atropine (0.1 mg/kg intramuscular) was given to control mucous secretions and eye ointment was applied to prevent drying of the eyes. Hearing loss was induced by exposing animals to a 116 dB SPL, octave band of noise with a center frequency of 500 Hz for two hours. In an electrically shielded, double walled sound attenuating chamber (Industrial Acoustics Company, Bronx, NY), noise was presented through an enclosed woofer (Selenium 10PW3) raised 25 cm above the animal's head, and calibrated at the ear with a type 2 sound level meter (Simpson 886-2, Elgin, IL). Anesthesia was maintained with supplemental ketamine injections (20-30 mg/kg intraperitoneal). Body temperature was maintained at 37°C with a feedback controlled heating pad (Harvard Apparatus 50-7220F).
AEP recordings
Animals were anesthetized using xylazine (1-1.5 mg/kg subcutaneous) followed by ketamine (50-60 mg/kg intraperitoneal). Atropine (0.1 mg/kg intramuscular) was given and eye ointment was applied. A sealed microphone (Etymotic ER-10B) and integral transducer (Etymotic ER-2) were inserted into the right ear canal to calibrate and present acoustic stimuli, respectively.
SAM tone stimuli were generated with carrier frequencies of 1, 2, 4, and 8 kHz and an amplitude modulation frequency of fm = 140 Hz (Fig. 1; top trace). This modulation frequency was selected based on pilot experiments showing robust ENV responses at all carrier frequencies. Furthermore, this modulation frequency falls within the range of fundamental frequencies (and hence, modulation frequencies) observed in voiced speech (Titze, 1994). Modulation depth was 100%. Stimulus duration was 90 ms with 5 ms cosine squared onset and offset ramps. The silent interval between stimuli was 60 ms. Stimuli were presented with alternating polarity at an SPL 30 dB above the animal's threshold at the same frequency, which was determined using auditory brainstem responses (ABRs) to tone burst stimuli. ABR thresholds were quantified using a previously described template based cross correlation procedure that estimates by linear regression the SPL at which the ABR waveform falls into the noise floor (Henry et al. 2011).
Fig. 1.
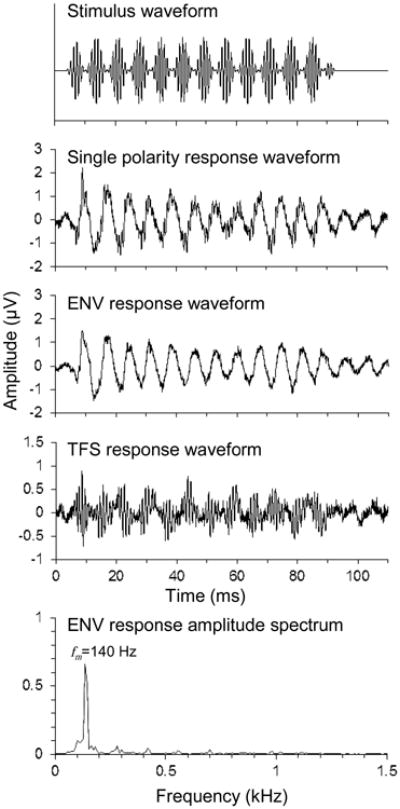
SAM tone stimulus waveform and representative AEP responses from a normal hearing chinchilla. The stimulus carrier and modulation frequencies were 1 kHz and 140 Hz, respectively, presented at 52 dB SPL. AEP responses to stimuli of opposite polarity (e.g. second trace from top) were halved and then added to yield the ENV response (third trace) and subtracted to yield the TFS response (fourth trace). The amplitude of the ENV response at fm = 140 Hz was measured from its Fourier transform (bottom panel).
SAM tone responses were recorded in quiet and in three levels of Gaussian masking noise (bandwidth: 24.4 kHz). Noise maskers were gated on and off with the SAM tone stimuli (i.e., same duration and ramping). The overall SPLs of the noise maskers, expressed in dB relative to the SPL of the SAM tone stimulus, were 0, 5, and 10 dB for 1 kHz stimuli, -5, 0, and 5 dB for 2 kHz stimuli, and -10, -5, and 0 dB for 4 and 8 kHz stimuli. For example, in an animal with a threshold of 20 dB SPL at 1 kHz, SAM tone stimuli would be presented at 50 dB SPL while noise maskers would be presented at 50, 55, and 60 dB SPL. These overall SPLs correspond to noise spectrum levels of 6.1, 11.1, and 16.1 dB/Hz, respectively. Lower noise levels were used for stimuli with higher carrier frequencies because responses to these stimuli were found to be more susceptible to masking, consistent with broader tuning bandwidth (measured in Hz) in the base of the cochlea.
Responses were recorded from the scalp using needle electrodes inserted at the dorsal midline between the eyes (non-inverting), posterior to the right pinna (inverting), and at the bridge of the nose (ground). Individual responses were collected for 110 ms, amplified 20,000 times (World Precision Instruments ISO-80, Sarasota, FL; Dagan 2400A, Minneapolis, MN), band pass filtered from 0.1 to 6 kHz (Krohn-Hite 3550, Brockton, MA), and digitally sampled at a rate of 48828.125 Hz (TDT RP2.1, Alachua, FL). 500 responses of each polarity were averaged to obtain the response at a given stimulus carrier frequency and noise level (Fig. 1; second trace from top).
AEP analysis
The ENV response was calculated by adding the responses to the two stimulus polarities and dividing by two (Fig 1; third trace from top). Recordings were cropped from 19 to 86 ms to isolate the steady-state portion of the response and transformed with a normalized Hamming window. The signals were discrete time Fourier transformed. The magnitude of the spectral peak at the modulation frequency was taken as the amplitude of the ENV response (Fig 1; bottom panel).
To determine a threshold amplitude for a statistically significant ENV response, TFS responses were calculated by subtracting the responses to the two stimulus polarities and dividing by two (Fig. 1; fourth trace from top). TFS responses do not contain a peak at the modulation frequency, and therefore provide a measurement of the amplitude of physiological noise at the modulation frequency. The 95th percentile of the noise amplitude, computed from 192 responses of 6 animals, was -23.8 dB μV (i.e., 0.0646 μV; amplitude in dB μV is computed as 20*log10[observed voltage {in μV}/1 μV]). This value was taken as the threshold amplitude for a statistically significant ENV response.
Statistical Analyses
We used repeated-measures mixed models to analyze changes in ENV coding with noise-induced SNHL (MIXED Procedure; SAS). Repeated-measures analyses correct for covariance of observations made within the same experimental subject (Littell et al., 2006). Within-subject covariance structure was specified with a random subject effect and random effects of masking condition (categorical variable; 4 levels) and exposure-status (categorical variable; 2 levels) nested within the subject effect. This structure corresponds to a “split-block” design with two within-subject factors. Statistical inferences were drawn based on F tests and T tests with degrees of freedom calculated based on the Kenward-Rogers algorithm, as suggested for repeated measures designs with relatively few subjects (Littell et al. 2006).
3. Results
Noise-induced threshold elevation
ABR thresholds measured prior to the noise exposure in normal-hearing control animals generally fell between 15 and 25 dB SPL at all test frequencies (Fig. 2). The noise exposure caused substantial average threshold elevation at all frequencies. Threshold elevation following the noise exposure varied widely across individuals from near 0 dB in animals with “tough ears” to 40 dB in more “tender-eared” animals, consistent with previous studies of noise-induced hearing loss (e.g. Cody and Robertson, 1983; Maison and Liberman, 2000).
Fig. 2.
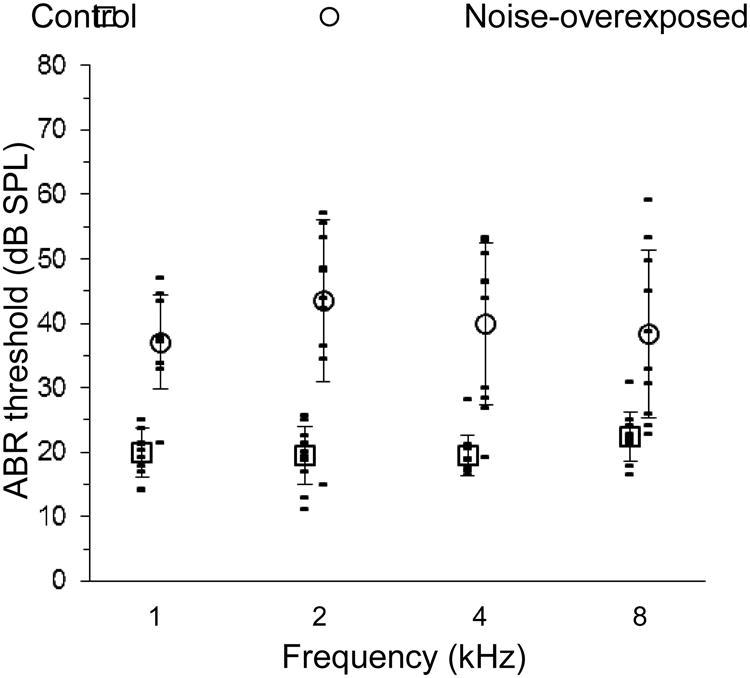
Auditory brainstem response (ABR) threshold as a function of stimulus frequency in chinchillas with normal hearing and noise-induced sensorineural hearing loss (see legend, top). Data collected after the noise-exposure are offset to the right for clarity. Open symbols and error bars indicate means and standard deviations, respectively.
SAM tone responses of control animals in quiet and in background noise
AEP responses to SAM tones in normal-hearing control animals contained a synchronized component to the ENV of the signal, i.e., at the 140 Hz AM frequency (Fig. 1). The ENV response was greatest in amplitude for the 1 kHz stimulus and decreased in amplitude with both increasing carrier frequency and increasing masking level (Fig. 3).
Fig. 3.
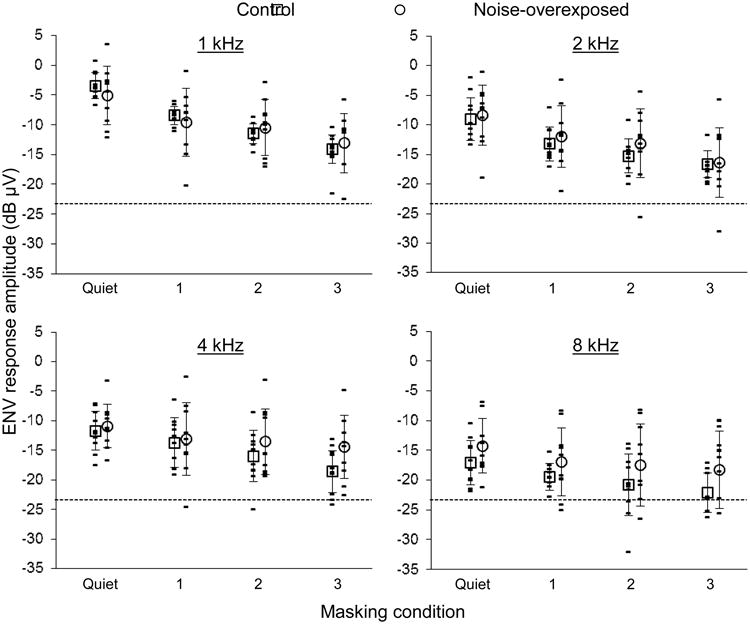
ENV response amplitude under four masking conditions in chinchillas with normal hearing and noise-induced hearing loss (see legend, top). Stimulus carrier frequency is given at the top of each panel. Data collected after the noise exposure are offset to the right for clarity. Masking conditions 1, 2 and 3 correspond to progressively higher sound pressure levels of white background noise (see text). Open symbols and error bars indicate means and standard deviations, respectively. The dashed line corresponds to the noise floor (i.e. -23.8 dB μV).
To confirm that ENV responses originated in the central auditory system, as in other small mammal species, we estimated the latency of the ENV response to stimuli with 1 and 4 kHz carrier frequencies in a single animal using a group delay approach (Bode, 1945; Kuwada et al., 1986). Response latency was calculated as the slope of the response phase by modulation frequency function for modulation frequencies ranging between 110 and 200 Hz (Fig. 4). Delay estimates at 1 and 4 kHz were 6.82 ±0.26 and 6.10 ±0.76 ms (means ±SE), respectively. These values are consistent with a midbrain origin of the ENV response (e.g. Langner et al. 2002).
Fig. 4.
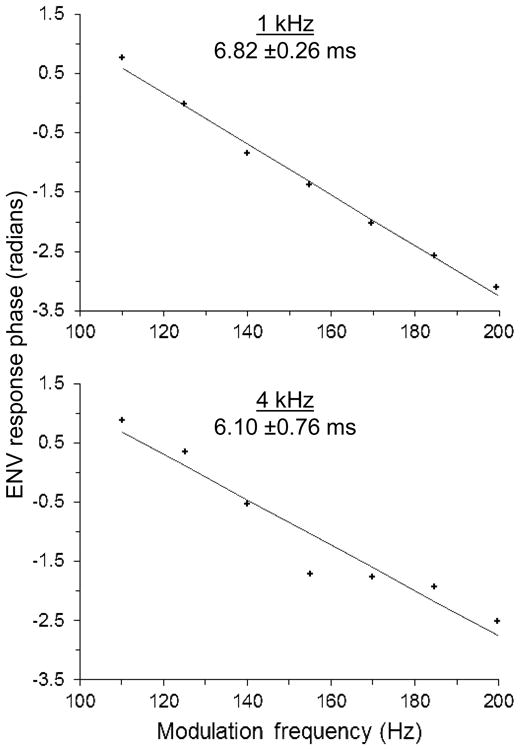
The phase of the ENV response in a single chinchilla as a function of the stimulus amplitude-modulation frequency. Stimulus carrier frequency is given at the top of each panel, followed by the estimate of the group delay (predicted mean and standard error). The group delay estimate was calculated from the slope of the linear regression (solid line).
Effects of noise-induced SNHL on ENV responses
Considerable overlap was observed in the distributions of ENV response amplitude measured before and after the onset of noise-induced SNHL (Fig. 3). We reasoned that this overlap could in part reflect variability in the extent of SNHL induced by the noise exposure (Fig. 2). For example, tender-eared animals with relatively greater noise-induced physical damage to the cochlea might show greater changes in ENV coding than tough-eared individuals. To investigate changes in ENV coding with varying degrees of SNHL, we focused on data from the seven animals for which AEP recordings were available both before and after the noise exposure. We analyzed ENV response amplitude at each stimulus carrier frequency with a repeated-measures mixed model that included main effects of ABR threshold (continuous variable; our metric of SNHL) and masking condition (categorical variable; 4 levels) and the interaction between ABR threshold and masking condition.
SNHL amplified the coding of ENV information as predicted at the higher stimulus carrier frequencies (Fig. 5). At stimulus frequencies of 2 kHz and above, the effect of ABR threshold on ENV response amplitude was significantly positive (2 kHz: F1,6.8=10.00, P=0.017; 4 kHz: F1,6.4=20.14, P=0.004; 8 kHz: F1,6.0=32.35, P=0.001). At 1 kHz in contrast, there was no consistent effect of SNHL on ENV coding (ABR threshold effect: F1,8.3=0.18, P=0.68). These patterns are shown separately for each stimulus frequency in Figure 5, which plots mean ENV response amplitude as a function of ABR threshold.
Fig. 5.
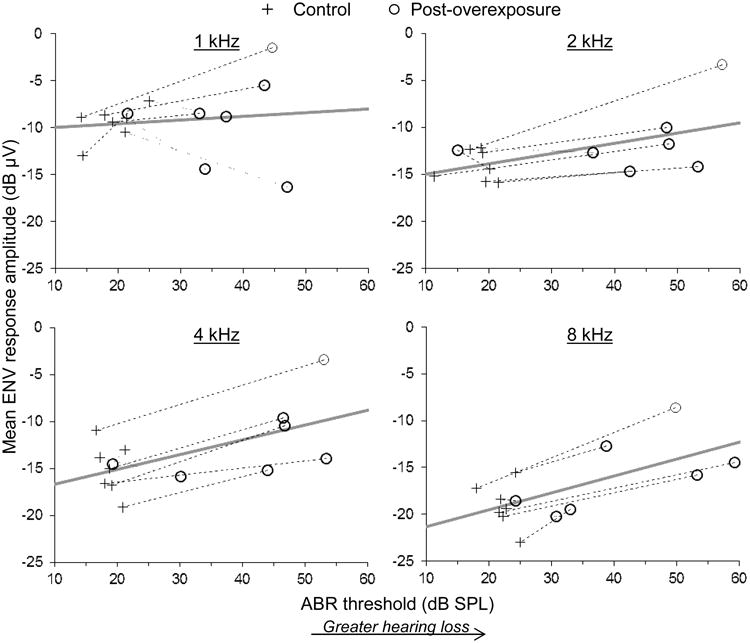
Mean ENV response amplitude averaged across masking conditions as a function of ABR threshold in seven chinchillas for which physiological data were available both before and after acoustic overexposure (see legend, top). Stimulus carrier frequency is given at the top of each panel. Dashed and dotted lines connect measurements made from the same animal with increasing and decreasing ENV response amplitude following acoustic overexposure, respectively. Thick gray lines show the predicted relationship from a repeated measures mixed model analysis (see text).
In contrast to predictions, noise-induced SNHL was not associated with a stronger adverse effect of increasing masker intensity on ENV coding at any stimulus carrier frequency (Fig. 6; more negative values along the vertical axis indicate a greater adverse effect of increasing masker level on ENV coding). At stimulus frequencies of 2 kHz and above, the interaction between ABR threshold and masking condition was insignificant (2 kHz: F3,35.1=0.28, P=0.84; 4 kHz: F3,35.1=1.58, P=0.21; 8 kHz: F3,23=0.62, P=0.61). A significant ABR threshold by masking condition interaction was found at 1 kHz (F3,22.4=6.93, P=0.002), but was opposite of predictions. The effect of increasing masker intensity on ENV coding was weaker (i.e. less negative) in cases of more pronounced SNHL. These patterns are shown separately for each stimulus frequency in Figure 6, which plots the mean change in ENV response amplitude per increase in masking condition level as a function of the ABR threshold.
Fig. 6.
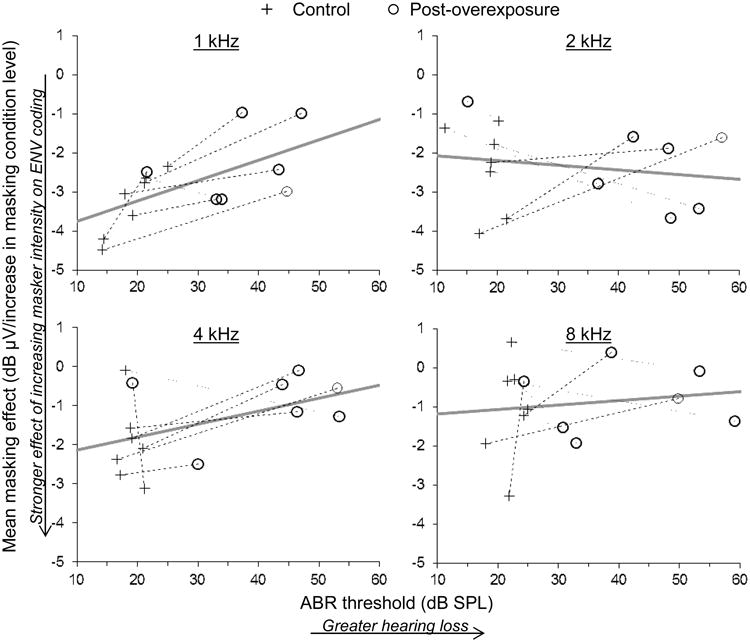
Mean change in ENV response amplitude per increase in masking condition level as a function of ABR threshold. More negative values along the vertical axis correspond to a stronger adverse effect of increasing masker intensity on ENV coding. Stimulus carrier frequency is given at the top of each panel. Dashed and dotted lines connect measurements made from the same animal with increasing and decreasing rates of change following acoustic overexposure, respectively. Thick gray lines show the predicted relationship from a repeated measures mixed model analysis (see text).
4. Discussion
In summary, we found that noise-induced SNHL amplifies neural responses to the ENV of SAM tones at carrier frequencies of 2 kHz and above at the level of the auditory midbrain in chinchillas. Furthermore, we found no evidence that the negative effect of masking noise on ENV coding increases in magnitude with SNHL.
Amplified neural coding of ENV information in the central auditory system may reflect a variety of potential sources including inheritance of physiological changes from the peripheral auditory system. Recent studies show that noise-induced SNHL in chinchillas enhances phase locking of auditory nerve fibers to the ENV of both SAM tones and single formant stimuli (Kale and Heinz, 2010). Amplification of ENV coding extends over a broad range of amplitude modulation frequencies and does not appear to alter the corner frequency of the temporal modulation rate transfer function derived for SAM tones (Kale and Heinz, 2012). Stronger ENV coding in the peripheral auditory system with SNHL, though perhaps surprising in light of associated perceptual difficulties, is consistent with the increase in the slope of the input-output function of the basilar membrane associated with outer hair cell dysfunction (reduced compression; Sellick et al., 1982; Ruggero and Rich, 1991; Glasberg and Moore, 1992; Moore et al., 1996). That is, a given modulation of the stimulus amplitude ENV with outer hair cell dysfunction should lead to greater variation in basilar membrane velocity and hence, greater variation in auditory nerve fiber firing rate with the stimulus amplitude ENV (i.e. enhanced neural synchrony). In these studies of noise-induced cochlear damage, amplification of ENV coding could have occurred because the increase in the slope of the cochlear input-output function due to outer hair cell dysfunction exceeded any decrease in slope due to inner hair cell dysfunction (Heinz and Young, 2004; Kale and Heinz, 2010). However, enhanced ENV coding in these evoked responses may also reflect contributions of high-threshold impaired AN fibers with steeper than normal rate-level functions associated with AN-fiber high-level irregularities (i.e. component-1/component-2 interactions; Liberman and Kiang, 1984; Heinz and Young, 2004; Kale and Heinz, 2010), or the contributions of altered temporal dynamics in AN-fiber response following SNHL (Scheidt et al, 2010).
Our results may also reflect an increase in the gain of central auditory neurons. While SNHL due to acoustic overexposure increases the amplitude of ENV coding in individual peripheral neurons as described above, it also damages and/or eliminates neural synapses with sensory inner hair cells inside the cochlea and leads to degeneration of spiral ganglion neurons (e.g. Kujawa and Liberman, 2009), ultimately reducing the total number of neural channels carrying information to the central auditory system. Emerging evidence points to a scenario in which reduced neural input to the central nervous system leads to a net increase in the excitability of central neurons due to homeostatic regulation of excitatory and inhibitory synapses (Turrigiano et al., 1998; Kilman et al, 2002). These homeostatic mechanisms putatively act to stabilize mean firing rate over extended time scales (Turrigiano, 1999). Neurophysiological studies in animals have shown increased neural activity in the central auditory system following acoustic trauma in various regions including the cochlear nucleus, inferior colliculus, and auditory cortex (e.g. Finlayson and Kaltenbach, 2009; Mulders and Robertson, 2009; Seki and Eggermont, 2003; Niu et al. 2013). Recent studies in humans also point to an increase in the gain of central auditory neurons with auditory pathology. Compared to normal hearing subjects without tinnitus, individuals with tinnitus and normal-hearing thresholds had auditory brainstem responses with lower wave I amplitude, similar wave V amplitude, and thus lower wave I to wave V amplitude ratios (Schaette and McAlpine, 2011). These results are consistent with both reduced neural output from the periphery and a larger increase in neural response amplitude from the level of the cochlea to the level of the inferior colliculus.
The physiological increase in ENV coding demonstrated here in the central auditory system of noise-overexposed chinchillas helps explain the results of human studies showing enhanced perception of ENV cues with hearing loss under some listening conditions. In individuals with unilateral hearing loss, sensation of equal amplitude modulation depth between the two ears requires less modulation depth in the impaired ear than the normal hearing ear, suggesting that the representation of ENV cues is amplified in the damaged ear (Moore et al. 1996). Studies examining minimum detectable modulation depth in listeners with SNHL have produced mixed results, with some studies showing lower thresholds with hearing loss (i.e. sensitivity to smaller modulations; Bacon and Gleitman, 1992; Moore and Glasberg, 2001; Füllgrabe et al., 2003) and others showing no difference in the minimum detectable modulation depth between groups (e.g. Moore et al. 1992).
We were surprised to find no consistent change in the effect of masking noise on ENV coding with SNHL at most stimulus frequencies. We expected to see a stronger, negative effect of masking noise on ENV coding with SNHL due to the broader bandwidth of auditory frequency tuning associated with hearing loss (Young, 2012). Broader frequency tuning allows more background noise into the receptive field of the neuron, and should therefore decrease the amplitude of phase locking to the temporal structure of the signal to a greater degree. Consistent with this hypothesis, phase locking to the TFS of masked tones in the peripheral auditory system of chinchillas degrades more rapidly with increasing noise level in animals with noise-induced SNHL than in control animals (Henry and Heinz, 2012). The absence of a similar result in the present study may reflect the use of population level neural responses that are more readily attenuated by low-level background noise than responses of single neurons with characteristic frequencies closely matched to the stimulus frequency. Population neural responses recorded in quiet contain contributions from neurons with characteristic frequencies both near the stimulus frequency and far removed in some cases (i.e. off-frequency neurons). Presumably, these off-frequency neurons drop out of the population neural response with the addition of low-level background noise while neurons with characteristic frequencies near the stimulus frequency can remain faithfully locked to the temporal structure of the stimulus. Consistent with this general idea, the population level neural responses studied here could be masked to some degree by background noise with spectrum levels as far as 45-55 dB below the stimulus SPL depending on the stimulus frequency. Another potential contributing factor is that the narrower cochlear filters associated with normal hearing produce significant ENV modulations at lower modulation rates (Joris, 2003; Dau et al 1999), which could mask the normal modulation coding of the SAM tone. With SNHL, degraded frequency selectivity could actually reduce this modulation masking effect, and thus could contribute to the observation of greater susceptibility to noise at 1 kHz for normal hearing than for the SNHL fibers (Fig. 6). Future studies of TFS and ENV coding in single neurons of the peripheral and central auditory system will help to clarify the extent to which SNHL exacerbates the negative effects of background noise on auditory processing.
In conclusion, the results of the present study show that noise-induced SNHL amplifies the neural representation of ENV information at the level of the auditory midbrain in anesthetized chinchillas. Together with other neurophysiological changes following SNHL, including reduced synchrony capture of vowel formants (Miller et al. 1997), diminished representation of TFS in background noise (Henry and Heinz, 2012), reduced neural output from the periphery (Kujawa and Liberman, 2009), and fewer independent neural channels carrying information to the central auditory system (Heinz et al. 2010), the amplified representation of ENV information shown here may contribute to problems with speech perception in people with hearing loss. Indeed, several behavioral studies have shown that artificial enhancement of ENV information in normal hearing listeners reduces speech intelligibility, particularly in fluctuating background noise and in the presence of competing talkers (Moore and Glasberg, 1993; Moore et al. 1995). Potentially, the heightened representation of ENV cues may act as an auditory distraction from more salient acoustic cues necessary for fine-scale discrimination of speech sounds. Future research should examine whether the ENV of masked speech signals can be manipulated in ways that improve speech performance in people with hearing loss.
Highlights.
Effects of cochlear hearing loss on central processing are not well understood
Central processing was studied in noise-exposed chinchillas using scalp potentials
Hearing loss amplified central responses to the envelope of modulated signals
Response susceptibility to masking by background noise was unaffected by hearing loss
Amplified envelope processing may contribute to speech perception problems in humans
Acknowledgments
This work was supported by NIH grants R01-DC009838 to MGH and F32-DC012236 to KSH from the National Institute on Deafness and other Communication Disorders.
Abbreviations
- ABR
auditory brainstem response
- AEP
auditory evoked potential
- ENV
envelope
- SAM
sinusoidally amplitude modulated
- SNHL
sensorineural hearing loss
- TFS
temporal fine structure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacon SP, Gleitman RM. Modulation detection in subjects with relatively flat hearing losses. J Speech Hear Res. 1992;35:642–653. doi: 10.1044/jshr.3503.642. [DOI] [PubMed] [Google Scholar]
- Bode HW. Network Analysis and Feedback Amplifier Design. Van Nostrand; Toronto: 1945. [Google Scholar]
- Chimento TC, Schreiner CE. Selectively eliminating cochlear microphonic contamination from the frequency following response. Electroencephalogr Clin Neurophysiol. 1990;75:88–96. doi: 10.1016/0013-4694(90)90156-e. [DOI] [PubMed] [Google Scholar]
- Cody AR, Roberston D. Variability of noise-induced damage in the guinea-pig cochlea: electro-physiological and morphological correlates after strictly controlled exposures. Hear Res. 1983;9:55–70. doi: 10.1016/0378-5955(83)90134-x. [DOI] [PubMed] [Google Scholar]
- Dau T, Verhey J, Kohlrausch A. Intrinsic envelope fluctuations and modulation-detection thresholds for narrow-band noise carriers. J Acoust Soc Am. 1999;106:2752–2760. doi: 10.1121/1.428103. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllgrabe C, Meyer B, Lorenzi C. Effect of cochlear damage on the detection of complex temporal envelopes. Hear Res. 2003;178:35–43. doi: 10.1016/s0378-5955(03)00027-3. [DOI] [PubMed] [Google Scholar]
- Galbraith GC. Two-channel brain-stem frequency-following responses to pure tone and missing fundamental stimuli. Electroen Clin Neuro. 1994;92:321–30. doi: 10.1016/0168-5597(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Galbraith GC, Hemsley J, Salour K, Songdej N, Threadgill MR, Ton J, Cheung L. Putative measure of peripheral and brainstem frequency-following in humans. Neurosci Lett. 2000;292:123–7. doi: 10.1016/s0304-3940(00)01436-1. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ. Effects of envelope fluctuations on gap detection. Hear Res. 1992;64:81–92. doi: 10.1016/0378-5955(92)90170-r. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Dasheiff R, Goldberg A, Suter CM. The human frequency-following response: its behavior during continuous tone and tone burst stimulation. Electroen Clin Neuro. 1976;40:25–32. doi: 10.1016/0013-4694(76)90176-0. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Evans EF. Some aspects of temporal coding by single cochlear fibres from regions of cochlear hair cell degeneration in the guinea pig. Arch Oto-Rhino-Laryn. 1979;224:71–8. doi: 10.1007/BF00455226. [DOI] [PubMed] [Google Scholar]
- Heinz MG, Young ED. Response growth with sound level in auditory-nerve fibers after noise-induced hearing loss. J Neurophysiol. 2004;91:784–795. doi: 10.1152/jn.00776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz MG, Swaminathan J, Boley JD, Kale S. Across-fiber coding of temporal fine-structure: Effects of noise-induced hearing loss on auditory-nerve responses. In: Lopez-Poveda EA, Palmer AR, Meddis R, editors. The Neurophysiological Bases of Auditory Perception. Springer; New York: 2010. pp. 621–630. [Google Scholar]
- Henry KS, Heinz MG. Diminished temporal coding with sensorineural hearing loss emerges in background noise. Nat Neurosci. 2012;15:1362–1364. doi: 10.1038/nn.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Kale S, Scheidt RE, Heinz MG. Auditory brainstem responses predict auditory nerve fiber thresholds and frequency selectivity in hearing impaired chinchillas. Hear Res. 2011;280:236–244. doi: 10.1016/j.heares.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins K, Moore BCJ, Stone MA. Effects of moderate cochlear hearing loss on the ability to benefit from temporal fine structure information in speech. J Acoust Soc Am. 2008;123:1140–1153. doi: 10.1121/1.2824018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX. Interaural time sensitivity dominated by cochlea-induced envelope patterns. J Neurosci. 2003;23:6345–6350. doi: 10.1523/JNEUROSCI.23-15-06345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale S, Heinz MG. Envelope coding in auditory nerve fibers following noise-induced hearing loss. J Assoc Res Oto. 2010;11:657–73. doi: 10.1007/s10162-010-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale S, Heinz MG. Temporal modulation transfer functions measured from auditory-nerve responses following sensorineural hearing loss. Hear Res. 2012;286:64–75. doi: 10.1016/j.heares.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA-A receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. Frequency-Following Responses. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory Evoked Potentials: Basic Principles and Clinical Applications. Lippincott, Williams & Williams; 2006. pp. 313–333. [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Batra R, Mahrer VL. Scalp potentials of normal and hearing-impaired subjects in response to sinusoidally amplitude-modulated tones. Hear Res. 1986;21:179–192. doi: 10.1016/0378-5955(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Langner G, Albert M, Briede T. Temporal and spatial coding of periodicity information in the inferior colliculus of awake chinchilla (Chinchilla laniger) Hear Res. 2002;168:110–130. doi: 10.1016/s0378-5955(02)00367-2. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NYS. Single-neuron labeling and chronic cochlear pathology. IV. Stereocilia damage and alterations in rate- and phase-level functions. Hear Res. 1984;16:75–90. doi: 10.1016/0378-5955(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. Second. SAS Publishing; Cary, NC: 2006. [Google Scholar]
- Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BCJ. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proc Natl Acad Sci USA. 2006;103:18866–9. doi: 10.1073/pnas.0607364103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi C, Debruille L, Garnier S, Fleuriot P, Moore BCJ. Abnormal processing of temporal fine structure in speech for frequencies where absolute thresholds are normal. J Acoust Soc Am. 2009;125:27–30. doi: 10.1121/1.2939125. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Schilling JR, Franck KR, Young ED. Effects of acoustic trauma on the representation of the vowel/ε/in cat auditory nerve fibers. J Acoust Soc Am. 1997;101:3602–16. doi: 10.1121/1.418321. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. The role of temporal fine structure processing in pitch perception, masking, and speech perception for normal-hearing and hearing-impaired people. J Assoc Res Oto. 2008;9:399–406. doi: 10.1007/s10162-008-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ, Shailer MJ, Schooneveldt GP. Temporal modulation transfer functions for band-limited noise in subjects with cochlear hearing loss. Br J Audiol. 1992;26:229–237. doi: 10.3109/03005369209076641. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR. Simulation of the effects of loudness recruitment and threshold elevation on the intelligibility of speech in quiet and in a background of speech. J Acoust Soc Am. 1993;94:2050–2062. doi: 10.1121/1.407478. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg GR, Vickers DA. Simulation of the effects of loudness recruitment on the intelligibility of speech in noise. Br J Audiol. 1995;29:131–143. doi: 10.3109/03005369509086590. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Wojtczak M, Vickers DA. Effect of loudness recruitment on the perception of amplitude modulation. J Acoust Soc Am. 1996;100:481–489. [Google Scholar]
- Moore BCJ, Glasberg BR. Temporal modulation transfer functions obtained using sinusoidal carriers with normally hearing and hearing impaired listeners. J Acoust Soc Am. 2001;110:1067–1073. doi: 10.1121/1.1385177. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR, Hopkins K. Frequency discrimination of complex tones by hearing-impaired subjects: Evidence for loss of ability to use temporal fine structure. Hear Res. 2006;222:16–27. doi: 10.1016/j.heares.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Mulders WHAM, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Niu Y, Kumaraguru A, Wang R, Sun W. Hyperexcitability of inferior colliculus neurons caused by acute noise exposure. J Neurosci Res. 2013;91:292–299. doi: 10.1002/jnr.23152. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Furosemide alters organ of Corti mechanics - evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991;11:1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RE, Kale S, Heinz MG. Noise-induced hearing loss alters the temporal dynamics of auditory-nerve responses. Hear Res. 2010;269:23–33. doi: 10.1016/j.heares.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Sellick PM, Patuzzi R, Johnstone BM. Measurement of basilar-membrane motion in the Guinea-pig using the Mossbauer technique. J Acoust Soc Am. 1982;72:131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- Smith JC, Marsh JT, Brown WS. Far-field recorded frequency-following responses: evidence for the locus of brainstem sources. Electroen Clin Neuro. 1975;39:465–72. doi: 10.1016/0013-4694(75)90047-4. [DOI] [PubMed] [Google Scholar]
- Sohmer H, Pratt H, Kinarti R. Sources of frequency following responses (FFR) in man. Electroen Clin Neuro. 1977;42:656–64. doi: 10.1016/0013-4694(77)90282-6. [DOI] [PubMed] [Google Scholar]
- Titze IR. Principles of Voice Production. Prentice Hall; Englewood Cliffs, N.J: 1994. [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF, Bone RC. Neural phase-locking properties in the absence of cochlear outer hair cells. Hear Res. 1981;4:335–46. doi: 10.1016/0378-5955(81)90017-4. [DOI] [PubMed] [Google Scholar]
- Young ED. Neural Coding of Sound with Cochlear Damage. In: Henderson D, LePrell CG, editors. Noise-Induced Hearing Loss: Scientific Advances. Springer; New York: 2012. pp. 87–135. [Google Scholar]
- Zeng FG, Nie K, Stickney GS, Kong YY, Vongphoe M. Speech recognition with amplitude and frequency modulations. Proc Natl Acad Sci USA. 2005;102:2293–2298. doi: 10.1073/pnas.0406460102. [DOI] [PMC free article] [PubMed] [Google Scholar]


