Abstract
The structure of a novel indigoid component was characterized by X-ray crystallography. This compound exhibited excellent anti-tuberculosis activity against Mycobacterium tuberculosis H37Rv in whole cell culture showing a submicromolar minimum inhibitory concentration (MIC). A synthesis of this molecule was designed and carried out to produce sufficient material for further testing. The in vitro profile, structure, and first synthesis of this indigoid component is reported.
Keywords: Anti-tuberculosis, Indigoid, X-ray
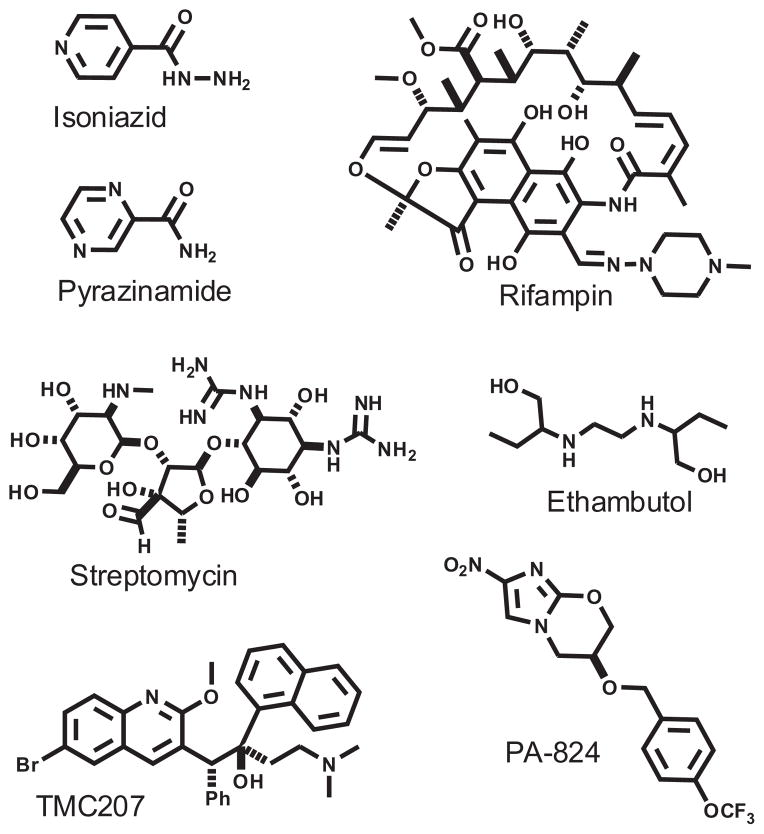
There exists great demand for new agents capable of combating infections associated with Mycobacterium tuberculosis (M.tb), the causative bacterium of tuberculosis (TB). This organism afflicts over a third of the world’s population with an annual death rate in excess of 2 million.1 Current first-line therapy involves use of a combination of isoniazid, rifampicin, pyrazinamide, and ethambutol or streptomycin (Fig. 1). These drugs were identified decades ago, and while a number of molecules are currently under study as new agents2 (e.g., PA-824, OPC-67683), only one (TMC-207)3 has successfully completed phase III clinical-stage development.
Figure 1.
Anti-TB agents.
A standard therapeutic course4 of 6–8 months is required to kill this slow-growing bacteria and to allow for sterilization of the persistent phenotype of M.tb. Furthermore, the infected populations are generally found in developing countries where such dosing dynamics and logistics often lead to poor patient compliance. As a result, the non-compliance can exacerbate development of drug resistance; for example, in 2006, 500,000 cases of multi-drug resistant TB (MTR-TB) were estimated with 6.6% of these cases carrying the extensively-drug resistant TB (XTR-TB) strains.5 Considering all infectious diseases, TB is the #1 cause of death of HIV-infected populations, and the epidemic has now become an urgent global health problem.
High-throughput screening (HTS) of compound libraries in search of new anti-TB actives is fraught with problems related to the unique lipophilic cell wall6 of M.tb serving as a barrier to some structural types, along with the relative abundance of cytochrome P450 enzymes7 (20 isoforms) which inactivate functionalized molecules.
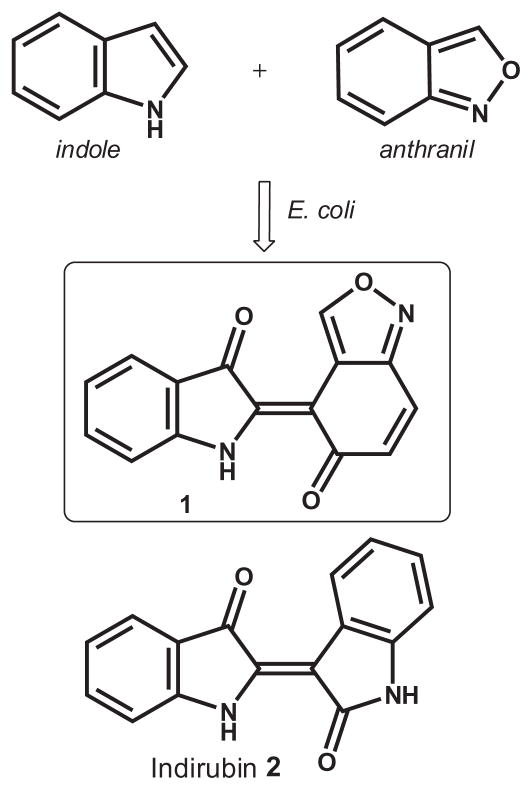
Recently, scientists from The Shaw Group, Inc. have successfully employed genetic engineering to modify the active site of the oxidative enzyme, toluene-4-monooxygenase(T4MO).8 The wild type T4MO enzyme is able to hydroxylate a wide range of aromatic and aliphatic chemicals, and through their efforts, the substrate range has been expanded by creating new isoforms with different substrate specificities and product distributions. This approach, termed ‘combinatorial biocatalysis’,9 proceeds by incubating the organism (Escherichia coli) expressing the cloned T4MO isoform, with the substrate(s), and the resultant microbial processing of these substrates is followed by HPLC analysis. Indole-based substrates were selected for this experiment due to their status as a privileged core in medicinal chemistry, their propensity for oxidation and subsequent dimerization under these culture conditions. Following the microbial processing of this modified oxidative system, novel secondary metabolites were produced and isolated.22 Upon screening these indigoid libraries from the aforementioned bacterial culture for antimicrobial bioactivities, several structural sub-sets typified by compound 1 were found to exhibit potent anti-TB activity.10 Herein, we report the anti-TB profile, structural characterization of 1,12 and the first chemical synthesis of this compound.11
The in vitro profile of 1 is listed in Table 1 along with that of the anti-TB agents, isoniazid and rifampin. The biocatalytic reaction described above utilized indole and anthranil as the lone substrates (Fig. 2) and produced multiple components from which 1 was isolated. Compound 1 exhibits good potency and is not greatly affected by protein-shift assays 4 and 5 (Table 1) showing a 1- and 3.7-fold increase in the MIC, respectively. This compound has excellent selectivity in the breadth of spectrum assays (6–9) with all MICs >50 μM. The Low Oxygen Recovery Assay (LORA), designed to test those mycobacteria in the non-replicating phenotype, shows approximately a 10-fold effect.
Table 1.
In vitro profile of compound 1 (μM)
| Assaya | (1) | Isoniazid | Rifampin |
|---|---|---|---|
| 1 MIC H37Rv | 0.57 | 0.22 | 0.038 |
| 2 Cytotoxicity Vero cell | >32 | >100 | >182 |
| 3 LORA MIC | 5.3 | >100 | 0.49 |
| 4 Protein shift MIC (4% BSA) | 2.1 | 0.45 | 0.089 |
| 5 Protein shift MIC (10% FBS) | 0.57 | 0.24 | 0.21 |
| 6 MIC M. smegmatis | >50 | 18.9 | 16.7 |
| 7 MIC C. albicans | >50 | ND | ND |
| 8 MIC S. aureus | >50 | ND | ND |
| 9 MIC E. coli | >50 | ND | ND |
Description of these assays are listed in Supplementary data section.
Figure 2.
Biocatalytic formation of 1 in E. coli and structure of indirubin (2).
Single crystal X-ray analysis,13 of 1 confirmed its novel structure (Fig. 3). Compound 1 is structurally related to indirubin (2), a dye-like indole dimer, such that both share a 3-indolone core; however, as indirubin lacked anti-TB activity,14 both the structural and biological novelty of 1 was established.15
Figure 3.
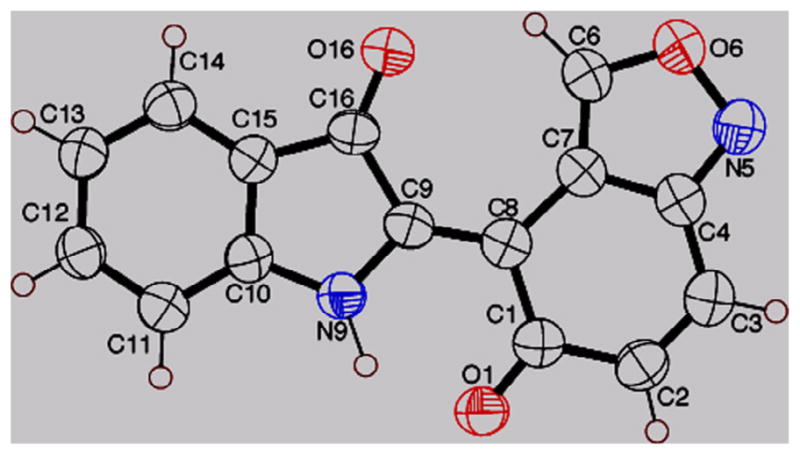
X-ray structure of indigoid 1 (CCDC 919159).
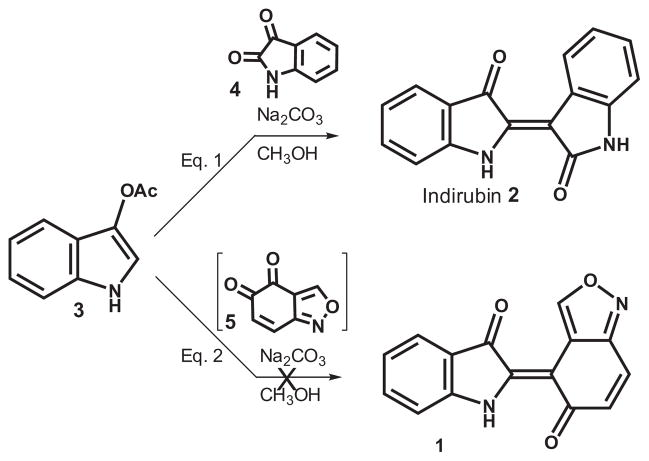
In order to determine the viability of compound 1 as an anti-TB agent, we required quantities much greater than the scale-limited cellular system could provide. Initially, synthetic routes were based upon the classical synthesis16 of indirubin utilizing 3-acetoxylindole (3) as the nucleophilic partner and isatin (4) as the electrophile (Scheme 1; Eq. 1). In a similar manner, coupling of 3 with the putative quinone 5 (attempted prep of 5 via oxidation17 of 5-hydroxybenzisoxazole; Scheme 1; Eq. 2) led to a complex mixture of reaction products none of which was the desired product.
Scheme 1.
Indoxyl route to indigoids.
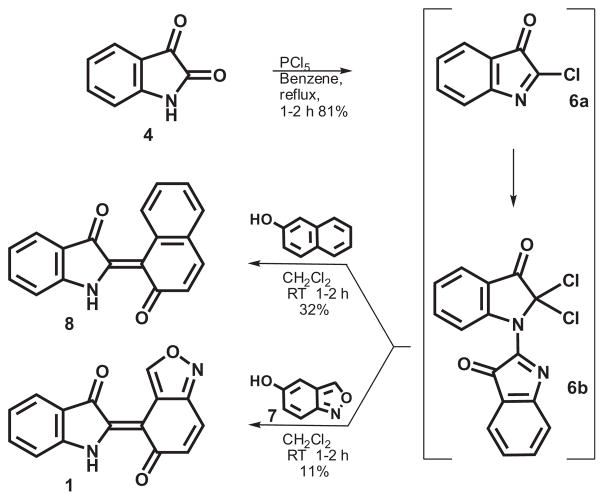
Alternatively, through the use of the polarity inversion concept, the 5-hydroxybenzisoxazole (7) was utilized as the nucleophilic partner with the indole-related fragment serving as the electrophile via its corresponding 2-chloroindolen-3-one (6a/b) (Scheme 2). As previously reported, treatment of isatin (4) with phosphorus pentachloride actually leads to dimeric structure 6b.18 Although this dimer is isolable via rapid chromatographic purification techniques, its instability to moisture encouraged direct use of the crude material in subsequent reactions. The addition of 6 to other aromatic systems has precedent; for example, reaction of 6 with 2-naphthol afforded adduct 819 which did not exhibit any anti-TB activity. Direct combination of 6 with 5-hydroxybenzisoxazole (7) at 25°C produced 1.20 Generally, these products were obtained in a pure state by filtration from the dichloromethane (DCM) reaction mixtures. Further purification, if necessary, was accomplished via silica gel chromatography using methanolic-DCM as eluting solvents.
Scheme 2.
Chemical synthesis of compound 1.
These compounds exhibit poor water solubility and are unstable in the presence of secondary amines. The possibility of this chemical instability being related to the poor metabolic stability21 is presently under investigation. Efforts to modify the solubility and stability characteristics and expand the structure–activity relationship is presently underway using new chemical approaches for functionalization. The excellent potency, ease of access, and low molecular weight of this novel anti-TB hit provides the encouragement for these efforts.
Supplementary Material
Acknowledgments
The project described herein was supported by Grant Number R21AI097670 from the National Institute of Allergy And Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health. We are indebted to K. McClay and R. J. Steffan (The Shaw Group) for their initial findings and collaboration in this project.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2013.11.024.
References and notes
- 1.Janin YL. Bioorg Med Chem. 2007;15:2479. doi: 10.1016/j.bmc.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 2.(a) Palomino JC, Ramos DF, da Silva PA. Curr Med Chem. 2009;16:1898. doi: 10.2174/092986709788186066. [DOI] [PubMed] [Google Scholar]; (b) Rivers EC, Mancera RL. Drug Discovery Today. 2008;13:1090. doi: 10.1016/j.drudis.2008.09.004. [DOI] [PubMed] [Google Scholar]; (c) Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Lancet. 2010 May 19; doi: 10.1016/S0140-6736(10)60359-9. http://dx.doi.org/10.1016/50140-6736(10)60359-9 (online) [DOI] [PubMed]
- 3.FDA to approve bedaquiline (TMC-207) 2012 Dec 11; http://www.sarpam.net/archives/2211.
- 4.Elzinga G, Raviglione MC, Maher D. Lancet. 2004;363:814. doi: 10.1016/S0140-6736(04)15698-5. [DOI] [PubMed] [Google Scholar]
- 5.Haydel SE. Pharmaceuticals. 2010;3:2268. doi: 10.3390/ph3072268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan PJ. Tuberculosis. 2003;83:91. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 7.McKean KJ, Munro AW. Drug Metab Rev. 2008;40:427. doi: 10.1080/03602530802186389. [DOI] [PubMed] [Google Scholar]
- 8.McClay K, Boss C, Keresztes I, Steffan RJ. Appl Environ Microbiol. 2005;71:5476. doi: 10.1128/AEM.71.9.5476-5483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rich JO, Michels PC, Khmelnitsky YL. Curr Opin Chem Biol. 2002;6:161. doi: 10.1016/s1367-5931(02)00299-5. [DOI] [PubMed] [Google Scholar]
- 10.Collins L, Franzblau SG. Antimicrob Agents Chemother. 1997;41:1004. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.1H NMR (400 MHz, DMSO-d6) δ ppm 6.76 (d, 1H, J = 9.85 Hz), 7.11 (dt, 1H, J = 7.38, 7.59 Hz), 7.49 (d, 1H, J = 7.79 Hz), 7.62 (dt, 1H, J = 7.38, 7.59 Hz), 7.70 (d, 1H, J = 7.38 Hz), 7.90 (d, 1H, J = 9.85), 10.06 (s, 1H), 12.40 (br s, 1H). 13C NMR (400 MHz, DMSO-d6) δ ppm 100.59, 112.16, 114.08, 119.00, 122.82, 124.82, 128.14, 134.10, 137.27, 140.74, 151.59, 153.60, 156.66, 187.87, 188.89. HRMS [ESI] calculated for C15H8O3N2 (M+H+) 265.0608; found 265.0603.
- 12.Pauli G, Lankin D. Several structures were proposed as possible matches for the 1-D and 2-D NMR data for the isolate, which included the correct structure. Institute for Tuberculosis Research, College of Pharmacy, University of Illinois at Chicago; 833 S. Wood Street, Chicago, IL 60612: [Google Scholar]
- 13.For X-ray data, see Supplementary data. The author has deposited atomic coordinates for 1 with the Cambridge Crystallographic Data Centre and allocated the deposition number: CCDC 919159. The coordinates can be obtained, on request, from the Director, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 lEZ, U.K.
- 14.Indirubin, indigo, and isoindigo were tested under standard conditions and found to show MICs of >32 μM, >32 μM, and 27 μM, respectively, against M.tb.
- 15.SciFinder (Chemical Abstracts Service) and Reaxys (Elsevier Properties) were analyzed via substructure, term, and patent searches.
- 16.(a) Baeyer A. Chem Ber. 1881;14:1741. [Google Scholar]; (b) Russell GA, Kaupp GJ. J Am Chem Soc. 1969;91:3851. [Google Scholar]
- 17.Attempted oxidation of 5-hydroxybenzoisoxazole afforded 76% of the putative quinone. 1H NMR (CDCl3) δ ppm 9.06 (s, 1H), 7.73 (d, 1H, J = 8.0 Hz), 6.74 (d, 1H, J = 8.0 Hz). Mass spectral analysis showed only a dimer signal [M+H] = 299 rather than the parent [M+H] = 150.
- 18.Cornforth J, Hitchcock PB, Rozos P. J Chem Soc, Perkin Trans 1. 1996:2787. [Google Scholar]
- 19.Begley WJ, Grimshaw J. J Chem Soc, Perkin Trans 1. 1975:1840. [Google Scholar]
- 20.A sample (25 mg) was purified for an analytical sample via silica gel chromatography using DCM as eluent to give 9 mg (11%) of the desired product, 1 whose 1H, 13C NMR data, HRMS and HPLC data agreed in total with material obtained from the incubation.11
- 21.Unpublished results, L.L. Klein, V. Petukhova; Treatment of 1 with secondary amines such as diethylamine, piperidine at room temperature led to slow loss of starting material and formation of blue-green products which are, as yet, unidentified.
- 22.(a) Baeyer A. Chem Ber. 1881;14:1741. [Google Scholar]; (b) McClay K, Wan B, Wang Y, Cho S, Yu J, Santarsiero B, Mehboob S, Johnson M, Franzblau S, Steffan R. US patent US2011/0082180 A1, 2013. Appl Microbiol Biotechnol. 2013;97:7151. doi: 10.1007/s00253-013-5012-9. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.