Abstract
Background
Pulmonary hypertension (PH) increases right ventricular (RV) pressure, resulting in septal shift and RV dilation. Few echocardiographic measures have been used to evaluate severity and outcomes in children with PH. The aims of this study were to compare the RV to left ventricular (LV) diameter ratio at end-systole (RV/LV ratio) in normal controls and patients with PH, to correlate the RV/LV ratio with invasive hemodynamic measures, and to evaluate its association with outcomes in children with PH.
Methods
The RV/LV ratio was compared retrospectively between 80 matched normal controls and 84 PH patients without shunts. Of the patients with PH, 49 children underwent 94 echocardiographic studies and cardiac catheterizations within 48 hours (13 patients had simultaneous measurements). The RV/LV ratio was correlated against hemodynamic measures. Kaplan-Meier curves and a Cox proportional-hazards regression model were used to assess relationships between RV/LV ratio and time until an adverse clinical event (initiation of intravenous prostacyclin therapy, atrial septostomy, death, or transplantation).
Results
RV/LV ratios were lower in controls compared with patients with PH (mean, 0.51 [95% confidence interval, 0.48–0.54] vs 1.47 [95% confidence interval, 1.25–1.70], P < .01). The RV/LV ratio correlated significantly with mean pulmonary artery pressure, systolic pulmonary artery pressure, systolic pulmonary artery pressure as a percentage of systemic pressure, and pulmonary vascular resistance index (r = 0.65 [P < .01], r = 0.6 [P < .01], r = 0.49 [P < .01], and r = 0.43 [P < .01], respectively). Twenty-two patients with PH with RV/LV ratios > 1 had adverse events within a median of 1.1 years from their earliest echocardiographic studies. Increasing RV/LV ratio was associated with an increasing hazard for a clinical event (hazard ratio, 2.49; 95% confidence interval, 1.92–3.24).
Conclusions
The RV/LV end-systolic diameter ratio can easily be obtained noninvasively in the clinical setting and can be used in the management of patients with PH. The RV/LV ratio incorporates both pathologic septal shift and RV dilation in children with PH and correlates with invasive measures of PH. An RV/LV ratio > 1 is associated with adverse clinical events.
Keywords: Pulmonary hypertension in children, RV/LV end-diastolic diameter ratio, Echocardiography, Outcomes
Pulmonary hypertension (PH) is defined as mean pulmonary artery pressure ≥ 25 mm Hg at rest.1 PH is a progressive disease associated with elevations of pulmonary artery pressure and pulmonary vascular resistance, resulting in right ventricular (RV) dilation and dysfunction. The effect of RV dilation on the interventricular septum leads to a septal shift toward the left ventricle in systole. This abnormal interventricular septal shift has been previously described in patients with PH.2–4 In those with severe PH, progressive RV dilation and dysfunction result in RV failure and death.5–7 Hemodynamic data obtained by cardiac catheterization are used as a gold standard to assess patients with PH and to determine the severity of PH, but cardiac catheterization is an invasive procedure.8 The inherent risks of cardiac catheterization limit its use as a surveillance tool for children with PH.
Transthoracic echocardiography is a noninvasive method used to evaluate pulmonary hemodynamics and right heart function.9–17 It is cost effective, accessible, and safe. Several echocardiographic indices have been described to evaluate PH and its association with clinical outcomes.3,7,17–19 Tricuspid regurgitation velocity is the most feasible noninvasive technique to estimate systolic pulmonary artery pressure (sPAP) in patients with PH; however, its precision has been debatable, and it cannot be assessed in all patients.20 We sought to evaluate a new index, the RV to left ventricular (LV) diameter ratio at end-systole (RV/LV ratio), with the following objectives: (1) evaluate the feasibility of this index, (2) compare normal controls and patients with PH, (3) correlate with invasively obtained hemodynamic measures in PH, and (4) evaluate the association between RV/LVratio and clinical outcomes in children with PH.
METHODS
Study Population
Normal Controls
The University of Colorado Children’s Hospital Colorado pediatric normal echocardiographic database (per an institutional review board–approved protocol) was used to retrospectively identify 80 normal controls with similar age and gender distributions as the PH cohort. All 80 normal children were evaluated for heart murmurs and had normal results on echocardiography. RV/LV ratios were obtained in normal controls and compared with RV/LV ratios in patients with PH.
Patients with PH
The University of Colorado Children’s Hospital Colorado pediatric PH database was used to retrospectively identify 84 patients with PH who underwent echocardiographic evaluation, cardiac catheterization, and had outcomes between March 2006 and November 2012. Inclusion criteria were adequate echocardiographic images in parasternal short-axis views at the level of the papillary muscles and patients who could have any category of PH. Exclusion criteria were inadequate-quality echocardiographic images in which the right ventricle was cut off in the parasternal short-axis view, presence of intracardiac shunts, or presence of RV outflow tract obstruction. PH patients’ clinical statuses were obtained from their medical charts. This study was approved by the institutional review board at the University of Colorado.
Transthoracic Echocardiography
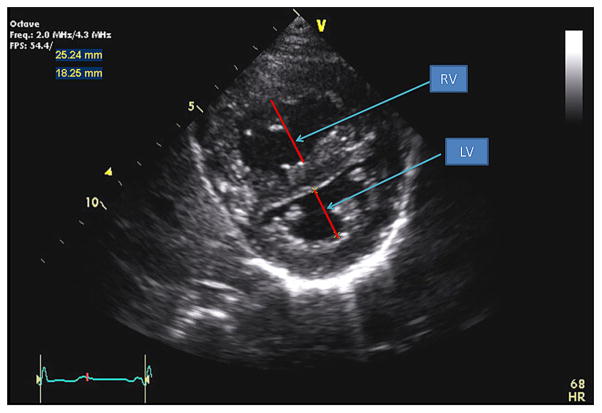
Echocardiography was performed on all normal controls and patients with PH using an iE33 (Philips Ultrasound, Bothell, WA) or a Vivid 7 (GE Medical Systems, Milwaukee, WI) ultrasound machine. Echocardiograms were digitally acquired using a standard protocol with appropriately sized transducers for patient size in all patients. RV/LV ratios were obtained offline from the parasternal short-axis two-dimensional view at the level of the papillary muscles with the RV free wall in view. RV and LV diameters were measured from the endocardial to endocardial surfaces at end-systole. RV/LV ratios were calculated (Figure 1).
Figure 1.
Parasternal short-axis view of the right and left ventricles. The RV/LV ratio was derived from RV diameter and LV diameter at end-systole.
Echocardiographic images were analyzed offline using the Agfa Heartlab system (Mortsel, Belgium). Feasibility of RV/LV ratios was assessed in normal controls and patients with PH. RV/LV ratios were compared between normal controls and patients with PH. Forty-nine of 84 patients with PH underwent echocardiography within 48 hours of cardiac catheterization. In these 49 patients, 94 echocardiographic studies and cardiac catheterizations were performed between March 2006 and November 2012. Echocardiographic images were analyzed within 48 hours of cardiac catheterization, and a small subset of patients (13 patients) had simultaneous image acquisition in the cardiac catheterization laboratory. Echocardiographic studies from all 84 patients with PH were analyzed for clinical outcomes.
Cardiac Catheterization
Forty-nine patients with PH underwent 94 standard right-sided heart catheterizations within 48 hours of their echocardiographic assessments. Of these 49 patients, 13 underwent simultaneous echocardiographic studies and cardiac catheterizations. General anesthesia was used in 62 of 94 studies (65%), and the rest were performed under conscious sedation. Eighty-three of 94 studies (88%) were done with the patients on room air. Three studies were performed with the patients on 100% oxygen and 8 studies with the patients on 30% oxygen. Data collected from the catheterization procedure included sPAP, sPAP as a percentage of systemic pressure (%PAP), mean pulmonary artery pressure, pulmonary vascular resistance index, pulmonary capillary wedge pressure, right atrial pressure, and cardiac index. Cardiac output was obtained using thermodilution, and cardiac index was calculated. Pulmonary vascular resistance index was calculated as (mean pulmonary artery pressure − mean pulmonary wedge pressure)/cardiac index.
Clinical Outcomes
Clinical outcomes were analyzed in all 84 patients with PH who underwent echocardiography between March 2006 and November 2012. Clinical outcomes were retrospectively obtained from medical charts in patients with PH. An adverse clinical event was defined as (1) disease progression requiring the initiation of intravenous prostacyclin, (2) clinically indicated creation of an atrial septostomy, (3) death, or (4) transplantation. The earliest echocardiographic studies available (defined as the earliest echocardiograms from 2006 onward for each patient) and the most recent echocardiographic studies (or those performed just before adverse events) were analyzed for outcome analysis. A subgroup analysis of earliest echocardiographic studies was also done to evaluate clinical outcomes of patients who underwent cardiac catheterization within 48 hours and patients who did not.
Statistical Analysis
Descriptive statistics were calculated using percentages and means and standard deviations for categorical and continuous variables, respectively. Intraobserver and interobserver variability for the RV/LV ratio was assessed in 10 randomly selected studies. Intraobserver variability was based on measurements by the same observer (P.-N.J.) at different times (6 months apart). Interobserver variability was evaluated by comparing the results obtained by two independent observers (P.-N.J. and J.H.). Both were blinded to the clip numbers and images of the echocardiograms. Intraobserver and interobserver variability was evaluated using mean percentage error, defined by the absolute difference between observations divided by the mean of the observations. The mean RV/LV ratio values across control subjects and patients with PH were compared using a two-sample t test. Pearson’s correlation coefficients were used to assess cross-sectional correlations between matched echocardiographic and cardiac catheterization measurements. Bivariate mixed models were used to estimate the correlations between RV/LV ratios and catheterization measurements for all paired observations while adjusting for repeated measures. Kaplan-Meier curves were used to assess the cross-sectional relationships between RV/LV ratio, categorized at 1, and time until a clinical event. Kaplan- Meier curves were also used to assess the cross-sectional relationship between patients who did and did not undergo cardiac catheterization within 48 hours of their echocardiographic studies and time until a clinical event. Cox proportional-hazards regression model was fit using all RV/LV ratio measurements as time varying explanatory variables to estimate the association with clinical events. P values< .05 were considered statistically significant. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
The data consist of 80 echocardiograms in 80 normal controls and 194 echocardiographic measurements in 84 patients with PH, with a median of two observations per patient with PH (range, 1–6). Clinical diagnoses and medications in patients with PH are shown in Table 1.
Table 1.
Clinical characteristics of patients
| Variable | Patients with PH (n = 84) | Normal controls (n = 80) |
|---|---|---|
| Age (y), mean ± SD | 8.7 ± 6.0 | 8.3 ± 5.5 |
| Male/female | 43/41 | 41/39 |
| Events | 22 (26%) | |
| IV prostacyclin | 11 | |
| Atrial septostomy | 6 | |
| Transplant | 1 | |
| Death | 4 | |
| IV medications | ||
| Treprostinil | 8 (9.5%) | |
| Epoprostenol | 3 (3.5%) | |
| Oral medications | ||
| Phosphodiesterase type 5 inhibitor | 41 (49%) | |
| Endothelin receptor antagonists | 20 (25%) | |
| Inhaled prostanoid | 7 (8.8%) | |
| Diagnosis | ||
| Idiopathic pulmonary arterial hypertension | 34 (40%) | |
| ASD s/p repair | 12 (14.2%) | |
| Pulmonary vein stenosis | 5 (6%) | |
| “Absent” LPA or RPA | 4 (5%) | |
| AVSD s/p repair | 4 (5%) | |
| DORV s/p repair | 1 (1.2%) | |
| TGA s/p repair | 1 (1.2%) | |
| Coarctation s/p repair | 1 (1.2%) | |
| PDA s/p closure | 1 (1.2%) | |
| Others (CDH, pulmonary fibrosis, altitude connective tissue disease, sickle cell) | 21 (25%) | |
ASD, Atrial septal defect; AVSD, atrioventricular septal defect; CDH, congenital diaphragmatic hernia; DORV, double-outlet right ventricle; IV, intravenous; LPA, left pulmonary artery; PDA, patent ductus arteriosus; RPA, right pulmonary artery; s/p, status post; TGA, transposition of the great arteries.
Feasibility of RV/LV Ratio
RV/LV ratios were obtained for all normal controls and all patients with PH. There were 3 of 194 echocardiograms (1%) in patients with PH for which RV/LVratios were not obtained, whereas tricuspid regurgitation velocity could not be estimated on 29 (18%) echocardiograms. The RV/LV ratio measured in the parasternal short-axis views in end-systole was highly reproducible, with low intraobserver and interobserver variability (3.4% and 5.2%).
Comparison across Normal Controls and Patients with PH
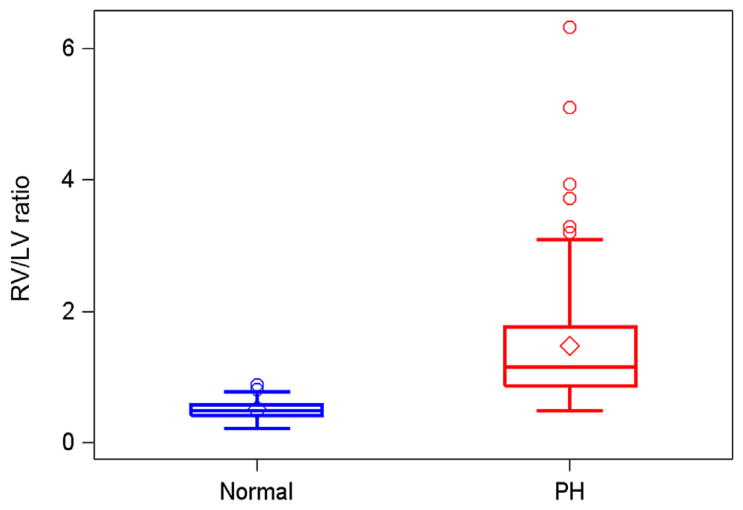
Normal subjects had similar gender and age range as patients with PH (Table 1). Eighty normal echocardiograms were compared with 84 earliest echocardiograms in patients with PH. The mean RV/LVratio for the matched group of normal subjects (0.51; 95% confidence interval, 0.48–0.54) was significantly lower compared with the mean of earliest RV/LV measurements (1.47; 95% confidence interval, 1.25–1.70) from patients with PH (P < .01; Figure 2).
Figure 2.
Distribution of RV/LV ratios between normal controls and patients with PH.
Correlation between Echocardiographic and Hemodynamic Variables
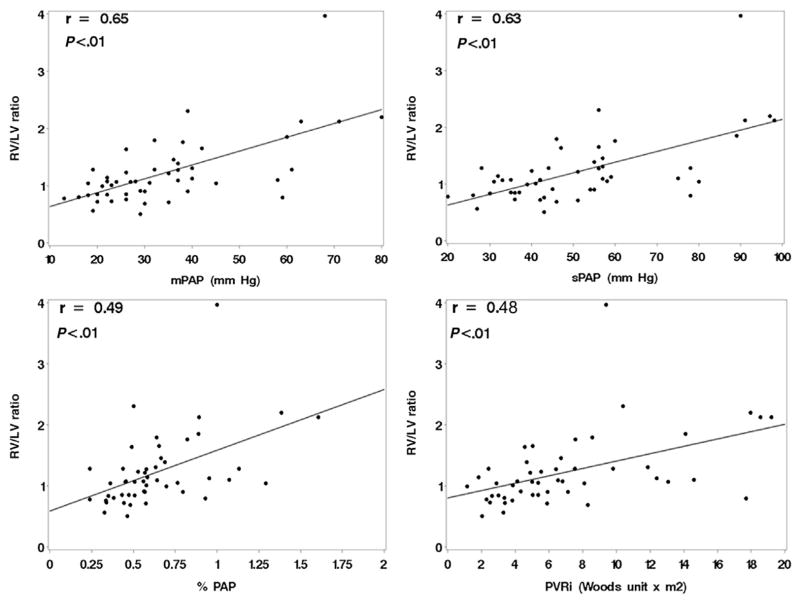
Forty-nine patients underwent 94 echocardiographic studies and cardiac catheterizations within 48 hours. Cross-sectional correlations between RV/LV ratios and hemodynamic variables were calculated using the most recent echocardiograms for patients obtained within 48 hours outside the catheterization laboratory. The RV/LV ratio correlated significantly with mean pulmonary artery pressure, sPAP, %PAP, and pulmonary vascular resistance index (Figure 3, Table 2) when evaluated using both cross-sectional and repeated-measures data. In patients with multiple echocardiograms (repeated measurements), there were no significant changes in RV/LV ratios over time on average (slope estimate, −0.01; standard error, 0.02). There was no statistically significant correlation of RV/LV ratio with pulmonary capillary wedge pressure, right atrial pressure, or cardiac index.
Figure 3.
Cross-sectional correlations of RV/LV ratio to hemodynamic data outside the cardiac catheterization laboratory. mPAP, Mean pulmonary artery pressure; PVRi, pulmonary vascular resistance index.
Table 2.
Correlations between RV/LV ratio and hemodynamic measurements out of the cardiac catheterization laboratory
| Hemodynamic variable | RV/LV ratio (single measure)
|
RV/LV ratio (repeated measures)
|
||||
|---|---|---|---|---|---|---|
| n | Pearson’s correlation | P | n | Pearson’s correlation | P | |
| mPAP | 49 | 0.65 | <.01 | 94 | 0.67 | <.01 |
|
| ||||||
| sPAP | 49 | 0.63 | <.01 | 94 | 0.67 | <.01 |
|
| ||||||
| %PAP | 49 | 0.49 | <.01 | 94 | 0.61 | <.01 |
|
| ||||||
| PVRi | 49 | 0.48 | <.01 | 94 | 0.70 | <.01 |
mPAP, Mean pulmonary artery pressure; PVRi, pulmonary vascular resistance index.
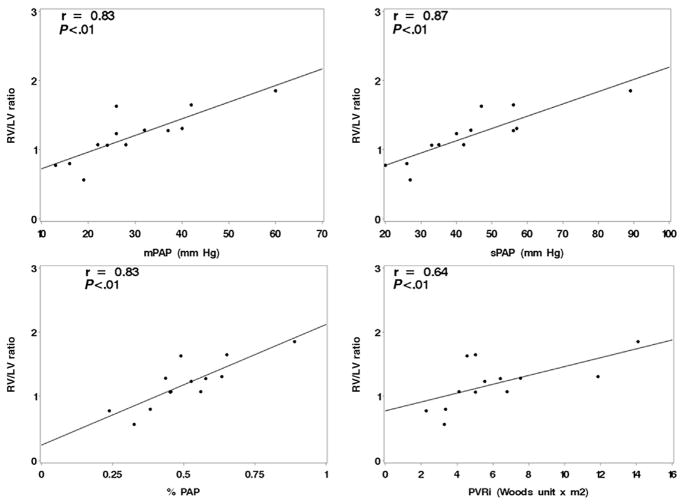
There were 13 patients with RV/LV ratio measurements obtained simultaneously in the catheterization laboratory while they were undergoing cardiac catheterizations. These measurements had improved correlations with the hemodynamic variables (Figure 4). Eight subjects had matched RV/LV ratio measurements collected both in the catheterization laboratory and within 48 hours previously. All but one of the values were lower in the catheterization laboratory, but the mean difference was only −0.1 ± 0.4.
Figure 4.
Correlations between RV/LV ratio and hemodynamic measurements obtained simultaneously in the cardiac catheterization laboratory. mPAP, Mean pulmonary artery pressure; PVRi, pulmonary vascular resistance index.
Outcomes in Patients with PH
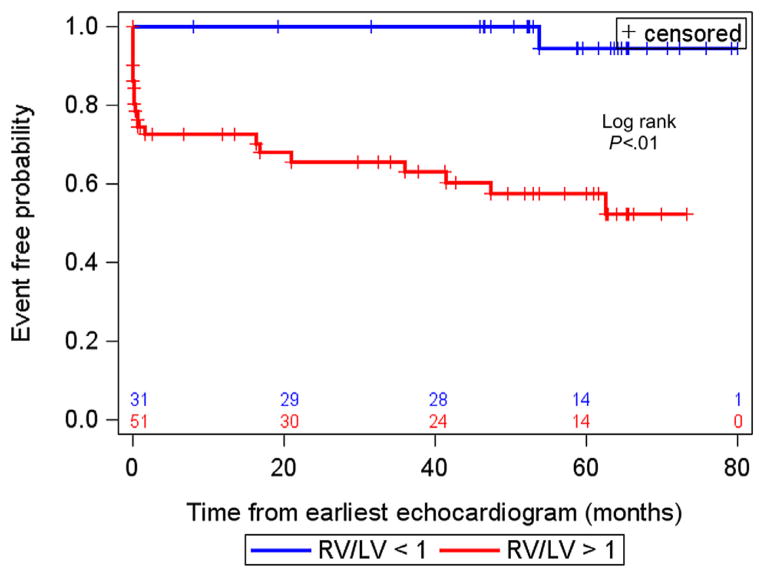
Twenty-two of 84 patients with PH had adverse events, including 11 patients initiated on intravenous prostacyclin, six with atrial septostomy, one transplantation, and four deaths (Table 1). The association between RV/LV ratio from the earliest echocardiographic study in a patient and time until an event was evaluated. Of those patients with adverse events, the events occurred within a median of 1.1 years from their earliest echocardiographic studies. The median follow-up time for those patients without events was 4.3 years from the earliest echocardiographic study. An RV/LVratio measurement > 1 was associated with an increased risk for an adverse event from the earliest echocardiographic study (log-rank P < .01; Figure 5). From the 84 patients used for the survival analysis (earliest echocardiographic study), 27 underwent matched catheterization within 48 hours. Those patients in whom the interval between catheterization and RV/LV ratio measurement was <48 hours had an adverse event rate of 19%, compared with 30%for those without such a short interval between catheterization and echocardiographic assessment of the RV/LV ratio. However, this difference was not statistically significant using a log-rank test (P = .29).
Figure 5.
Kaplan-Meier event curves for RV/LV ratio associated with adverse clinical events. RV/LV ratio > 1 was associated with an increased risk for adverse clinical events.
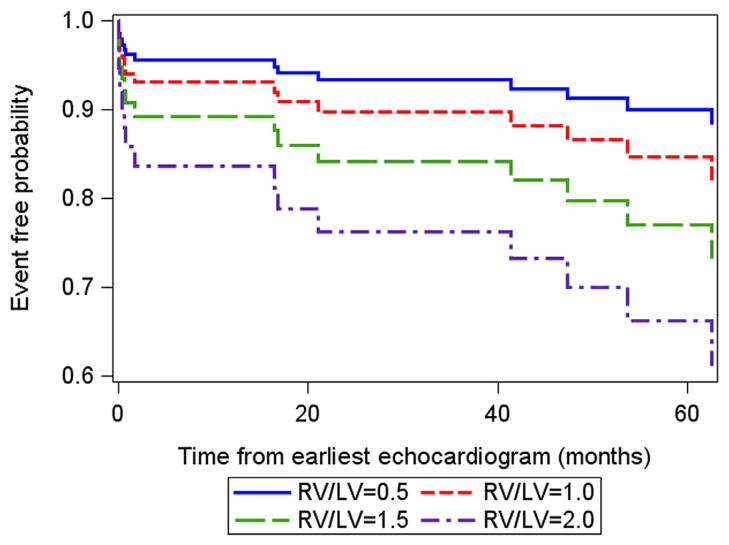
A time-varying covariates survival model was fit to all available RV/LV ratio measurements over 6 years. This analysis revealed that increasing RV/LV ratio was associated with an increasing hazard for a clinical event (hazard ratio, 2.49; 95% confidence interval, 1.92–3.24; Figure 6). When the RV/LVratio measurement increased by one unit, the proportional change in the hazard was 2.49. Alternatively, for every increase of 0.1 units in RV/LVratio, the hazard increased by 10%. This association was robust after adjustment for patient age and gender.
Figure 6.
Estimated survival curves for four possible RV/LV ratios estimated from the Cox varying coefficients regression corresponding to a hazard ratio of 2.49 for RV/LV ratio.
DISCUSSION
In this study, RV/LV ratio measured in the standard parasternal short-axis view was easily obtained in all subjects and feasible in 99% of all echocardiographic studies. RV/LV ratio was significantly higher in patients with PH compared with normal controls. RV/LV ratios correlated well with invasive hemodynamic measures, and increased RV/LV ratio was associated with adverse clinical events, making this new index a potentially clinical relevant parameter in patients with PH.
Because of the increase in available therapeutic options and improving survival in children with PH, reliable, noninvasive techniques are critical in the surveillance of these challenging patients. 12,19,21,22 Several noninvasive echocardiographic indices have been described to evaluate PH. Tricuspid regurgitation velocity is most often used to estimate sPAP in patients with PH; however, its precision has been debatable.20 Fisher et al.23 demonstrated that sPAP derived from tricuspid regurgitation velocity differed by more than ± 10mmHg from invasively measured sPAP by cardiac catheterization in 48% of patients. Yared et al.15 showed that 25.3% of their 500 patients did not have measurable tricuspid regurgitation velocities. In our study, we found that 18% of our PH patients’ echocardiograms did not have measureable tricuspid valve regurgitation to allow accurate estimation of systolic pressure.
We demonstrated that RV/LV ratio correlated significantly with invasive hemodynamic measures of PH. The RV/LV ratio demonstrated significant correlations both in (simultaneously) and out (within 48 hours) of the cardiac catheterization laboratory, indicating that the RV/LVratio can be used in the clinical setting as a reasonable noninvasive measure to evaluate the severity of PH. Of the 13 patients who had simultaneous measurements, eight had RV/LV ratios collected both in the catheterization laboratory and within 48 hours previously. The RV/LV ratios obtained in these eight patients were quite similar both in and out of the cardiac catheterization lab, indicating that the effect of anesthesia on the measurements was minimal.
As RV/LV ratio increased, the risk for an adverse clinical event increased in our patients with PH. As demonstrated in Figure 5, RV/LVratio > 1 was associated with increased risk for adverse events. We included initiation of intravenous prostacyclin and creation of an atrial septal defect as adverse clinical events because this represents escalation of therapy consistent with disease progression. The decision to start intravenous prostacyclin or to perform atrial septostomy was made by the treating cardiologists on the basis of patients’ heart failure in the presence of optimal therapy and worsening World Health Organization classification. The primary author (P.-N.J.) was blinded to this information when measuring RV/LV ratios and was not the treating cardiologist for any of these patients with PH, so selection bias and recall bias were avoided. The RV/LV ratio can be used as a noninvasive clinical tool to evaluate a patient’s disease progression, as demonstrated by the hazard ratio. With every increase of 0.1 units in RV/LV ratio, the hazard increases by 10%. Thus, the echocardiographic study before a patient is placed on intravenous prostacyclin or creation of atrial septal defect becomes very important in the patient’s management.
The RV/LVratio was derived to combine a measure of RV size with septal shift secondary to elevated RV pressure. RV pressure overload causes RV hypertrophy and dilation. Jurcut et al.24 demonstrated in their study that patients with PH respond to high afterload differently than in patients with pulmonary valve stenosis. In their study, patients with PH had more dilated right ventricles compared with those with pulmonary valve stenosis. This dilation not only affects RV performance but also results in more tricuspid valve regurgitation. With increased tricuspid valve regurgitation, RV dilation is further exacerbated, which in turn results in more interventricular septal shift. This phenomenon is demonstrated as an increase in the RV/LVratio and is associated with worse hemodynamic measures, consistent with more severe PH.
RV dilation results in RV diastolic and systolic dysfunction, which is associated with poor prognosis and increased mortality.7,18 Kassem et al.19 demonstrated that the RV end-systolic area increases significantly in nonsurvivors and reflects poor RV systolic performance in children with PH. As the right ventricle dilates, the geometry of the right ventricle is changed, and the inability of the right ventricle to adapt to increased afterload results in RV dysfunction.20 Increases in RV pressure and RV dilation result in higher transseptal pressures, which result in abnormal septal shift. Abnormal septal motion was first described on echocardiography by Popp et al.25 in 1969. Subsequent studies have demonstrated interventricular septal shift in patients with PH.2–4,26–28 This results in interventricular septum bowing into the left ventricle, resulting in the characteristic “D-shaped” left ventricle. RV size has been shown to be a strong predictor of outcomes, with its effects on the left ventricle.29 In adult patients with PH, early systolic anterior septal motion predicts worse clinical status and hemodynamic profile.26 The degree of septal shift reflects severity of RV failure in adult patients with PH and is associated with poor outcomes.3 Our analysis demonstrates that increasing RV/LVratio is associated with an increasing hazard for a clinical event, suggesting the prognostic value of this measurement (Figures 5 and 6).
We measured the RV/LV ratio in end-systole because septal flattening is greatest in end-systole because of increased RV afterload. King et al.2 observed that there is consistent leftward displacement and flattening of the interventricular septum with increases in RV afterload. The shift in the interventricular septum is greatest in end-systole. Ryan et al.4 derived an eccentricity index to differentiate between volume overload and pressure overload of the right heart. They found that LV deformation of the interventricular septum was greatest in end-systole in patients with RV pressure overload. Lammers et al.28 subsequently demonstrated that children with PH have systolic bowing of the interventricular septum more prominent than in diastole in their eccentricity index. Eccentricity index measures the deformity in the left ventricle but does not take into account the altered RV dimension. The interventricular septum is shared by both ventricles. The importance of ventricular-ventricular interactions is increasingly recognized in patients with PH in both adult and pediatric populations.13,26–28 Interventricular septal shift impairs LV diastolic filling, which results in decreased LV function.30–32 The RV/LV ratio measured in end-systole captures both RV dilation and the adverse ventricular-ventricular interaction seen in patients with PH.
Although cardiac catheterization is the gold standard for evaluating PH, it is an invasive procedure with associated risks. On the basis of our findings, the RV/LV ratio may serve as a useful serial surveillance tool for children with PH, as it is an easy and reproducible method performed on standard echocardiography. It can be used as a clinical tool in the management of patients with PH and may improve risk stratification in this challenging group of patients.
Study Limitations
Limitations of this study include those inherent to a retrospective study. We studied patients with all forms of PH but could not divide them into different causes of PH, because of the small sample size. However, the types of patients seen in our study mirror those of other larger PH programs.33,34 Because left-to-right shunt lesions can alter the size of the right ventricle, we did not include patients with shunts in this study. This could limit the use of the RV/LV ratio. We did not investigate the serial changes in the RV/LV ratio with the impact of therapy, but this can be analyzed in further studies. We chose echocardiograms in 2006 as the earliest echocardiograms for our patients with PH; therefore stages of disease and type of therapy were not consistent across this group. But our analysis showed that RV/LVratio at a given time point can be used to gauge a patient’s PH severity and to use this index to follow such patients over time.
CONCLUSIONS
The RV/LV end-systolic diameter ratio incorporates both pathologic septal shift and RV dilation in PH and correlates with invasive hemodynamic measures of PH. The RV/LV ratio can easily be obtained in the clinical setting and appears to be a strong predictor of outcome. The RV/LV ratio may be a valuable additional echocardiographic measure in the quest to find a surrogate technology for cardiac catheterization in children with PH.
Acknowledgments
This study was supported by the Frederick and Margaret L Weyerhaeuser Foundation, the Jayden DeLuca Foundation, the Leah Bult Foundation, Colorado Clinical Translational Science Institute (UL1 TR000154), National Center for Research Resources, and National Institutes of Health, P50 HL084923, and RO1 HL114753.
We would like to acknowledge the contributions of Courtney Cassidy and Allison Sterk in data collection.
Abbreviations
- LV
Left ventricular
- %PAP
Systolic pulmonary artery pressure as a percentage of systemic pressure
- PH
Pulmonary hypertension
- RV
Right ventricular
- RV/LV ratio
Right to left ventricular diameter ratio at end-systole
- sPAP
Systolic pulmonary artery pressure
References
- 1.Rosenzweig EB, Barst RJ. Congenital heart disease and pulmonary hypertension: pharmacology and feasibility of late surgery. Prog Cardiovasc Dis. 2012;55:128–33. doi: 10.1016/j.pcad.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 2.King ME, Braun H, Goldblatt A, Liberthson R, Weyman AE. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: a cross-sectional echocardiographic study. Circulation. 1983;68:68–75. doi: 10.1161/01.cir.68.1.68. [DOI] [PubMed] [Google Scholar]
- 3.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–9. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 4.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 1985;5:918–27. doi: 10.1016/s0735-1097(85)80433-2. [DOI] [PubMed] [Google Scholar]
- 5.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–5. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 6.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 7.Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, et al. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2011;107:628–32. doi: 10.1016/j.amjcard.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Clabby ML, Canter CE, Moller JH, Bridges ND. Hemodynamic data and survival in children with pulmonary hypertension. J Am Coll Cardiol. 1997;30:554–60. doi: 10.1016/s0735-1097(97)00155-1. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Abbas AE, Khandheria BK, Lester SJ. Echocardiographic assessment of right heart hemodynamic parameters. J Am Soc Echocardiogr. 2007;20:773–82. doi: 10.1016/j.echo.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopalan N, Simon MA, Suffoletto MS, Shah H, Edelman K, Mathier MA, et al. Noninvasive estimation of pulmonary vascular resistance in pulmonary hypertension. Echocardiography. 2009;26:489–94. doi: 10.1111/j.1540-8175.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 11.Saxena N, Rajagopalan N, Edelman K, Lopez-Candales A. Tricuspid annular systolic velocity: a useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography. 2006;23:750–5. doi: 10.1111/j.1540-8175.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- 12.Seyfarth HJ, Pankau H, Hammerschmidt S, Schauer J, Wirtz H, Winkler J. Bosentan improves exercise tolerance and Tei index in patients with pulmonary hypertension and prostanoid therapy. Chest. 2005;128:709–13. doi: 10.1378/chest.128.2.709. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan F, Redington A. The right ventricle: anatomy, physiology and clinical imaging. Heart. 2008;94:1510–5. doi: 10.1136/hrt.2007.132779. [DOI] [PubMed] [Google Scholar]
- 14.Simon MA, Rajagopalan N, Mathier MA, Shroff SG, Pinsky MR, Lopez-Candales A. Tissue Doppler imaging of right ventricular decompensation in pulmonary hypertension. Congest Heart Fail. 2009;15:271–6. doi: 10.1111/j.1751-7133.2009.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yared K, Noseworthy P, Weyman AE, McCabe E, Picard MH, Baggish AL. Pulmonary artery acceleration time provides an accurate estimate of systolic pulmonary arterial pressure during transthoracic echocardiography. J Am Soc Echocardiogr. 2011;24:687–92. doi: 10.1016/j.echo.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–7. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 17.Alkon J, Humpl T, Manlhiot C, McCrindle BW, Reyes JT, Friedberg MK. Usefulness of the right ventricular systolic to diastolic duration ratio to predict functional capacity and survival in children with pulmonary arterial hypertension. Am J Cardiol. 2010;106:430–6. doi: 10.1016/j.amjcard.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Takatsuki S, Nakayama T, Jone PN, Wagner BD, Naoi K, Ivy DD, et al. Tissue Doppler imaging predicts adverse outcome in children with idiopathic pulmonary arterial hypertension. J Pediatr. 2012;161:1126–31. doi: 10.1016/j.jpeds.2012.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassem E, Humpl T, Friedberg MK. Prognostic significance of 2-dimensional, M-mode, and Doppler echo indices of right ventricular function in children with pulmonary arterial hypertension. Am Heart J. 2013;165:1024–31. doi: 10.1016/j.ahj.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Milan A, Magnino C, Veglio F. Echocardiographic indexes for the noninvasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr. 2010;23:225–39. doi: 10.1016/j.echo.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Ivy DD, Rosenzweig EB, Lemarie JC, Brand M, Rosenberg D, Barst RJ. Long-term outcomes in children with pulmonary arterial hypertension treated with bosentan in real-world clinical settings. Am J Cardiol. 2010;106:1332–8. doi: 10.1016/j.amjcard.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537–46. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurcut R, Giusca S, Ticulescu R, Popa E, Amzulescu MS, Ghiorghiu I, et al. Different patterns of adaptation of the right ventricle to pressure overload: a comparison between pulmonary hypertension and pulmonary stenosis. J Am Soc Echocardiogr. 2011;24:1109–17. doi: 10.1016/j.echo.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Popp RL, Wolfe SB, Hirata T, Feigenbaum H. Estimation of right and left ventricular size by ultrasound. A study of the echoes from the interventricular septum. Am J Cardiol. 1969;24:523–30. doi: 10.1016/0002-9149(69)90495-0. [DOI] [PubMed] [Google Scholar]
- 26.Mori S, Nakatani S, Kanzaki H, Yamagata K, Take Y, Matsuura Y, et al. Patterns of the interventricular septalmotion can predict conditions of patients with pulmonary hypertension. J Am Soc Echocardiogr. 2008;21:386–93. doi: 10.1016/j.echo.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 27.Puwanant S, Park M, Popovic ZB, Tang WH, Farha S, George D, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010;121:259–66. doi: 10.1161/CIRCULATIONAHA.108.844340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lammers AE, Haworth SG, Riley G, Maslin K, Diller GP, Marek J. Value of tissue Doppler echocardiography in children with pulmonary hypertension. J Am Soc Echocardiogr. 2012;25:504–10. doi: 10.1016/j.echo.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Barst RJ, Channick R, Ivy D, Goldstein B. Clinical perspectives with long-term pulsed inhaled nitric oxide for the treatment of pulmonary arterial hypertension. Pulm Circ. 2012;2:139–47. doi: 10.4103/2045-8932.97589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajagopalan N, Simon MA, Edelman K, Mathier MA, Lopez-Candales A. Identifying left ventricular dysfunction in pulmonary hypertension. Congest Heart Fail. 2009;15:218–21. doi: 10.1111/j.1751-7133.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 31.Ramani GV, Bazaz R, Edelman K, Lopez-Candales A. Pulmonary hypertension affects left ventricular basal twist: a novel use for speckle-tracking imaging. Echocardiography. 2009;26:44–51. doi: 10.1111/j.1540-8175.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 32.Hardegree EL, Sachdev A, Fenstad ER, Villarraga HR, Frantz RP, McGoon MD, et al. Impaired left ventricular mechanics in pulmonary arterial hypertension: identification of a cohort at high risk. Circ Heart Fail. 2013;6:748–55. doi: 10.1161/CIRCHEARTFAILURE.112.000098. [DOI] [PubMed] [Google Scholar]
- 33.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–34. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 34.van Loon RL, Roofthooft MT, Hillege HL, ten Harkel AD, van Osch-Gevers M, Delhaas T, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755–64. doi: 10.1161/CIRCULATIONAHA.110.969584. [DOI] [PubMed] [Google Scholar]